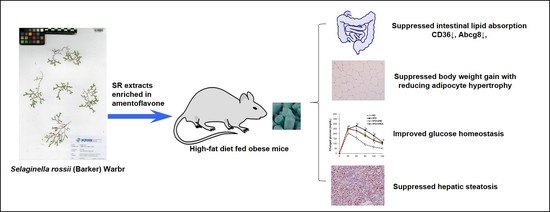
Amentoflavone-Enriched Selaginella rossii Warb. Suppresses Body Weight and Hyperglycemia by Inhibiting Intestinal Lipid Absorption in Mice Fed a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
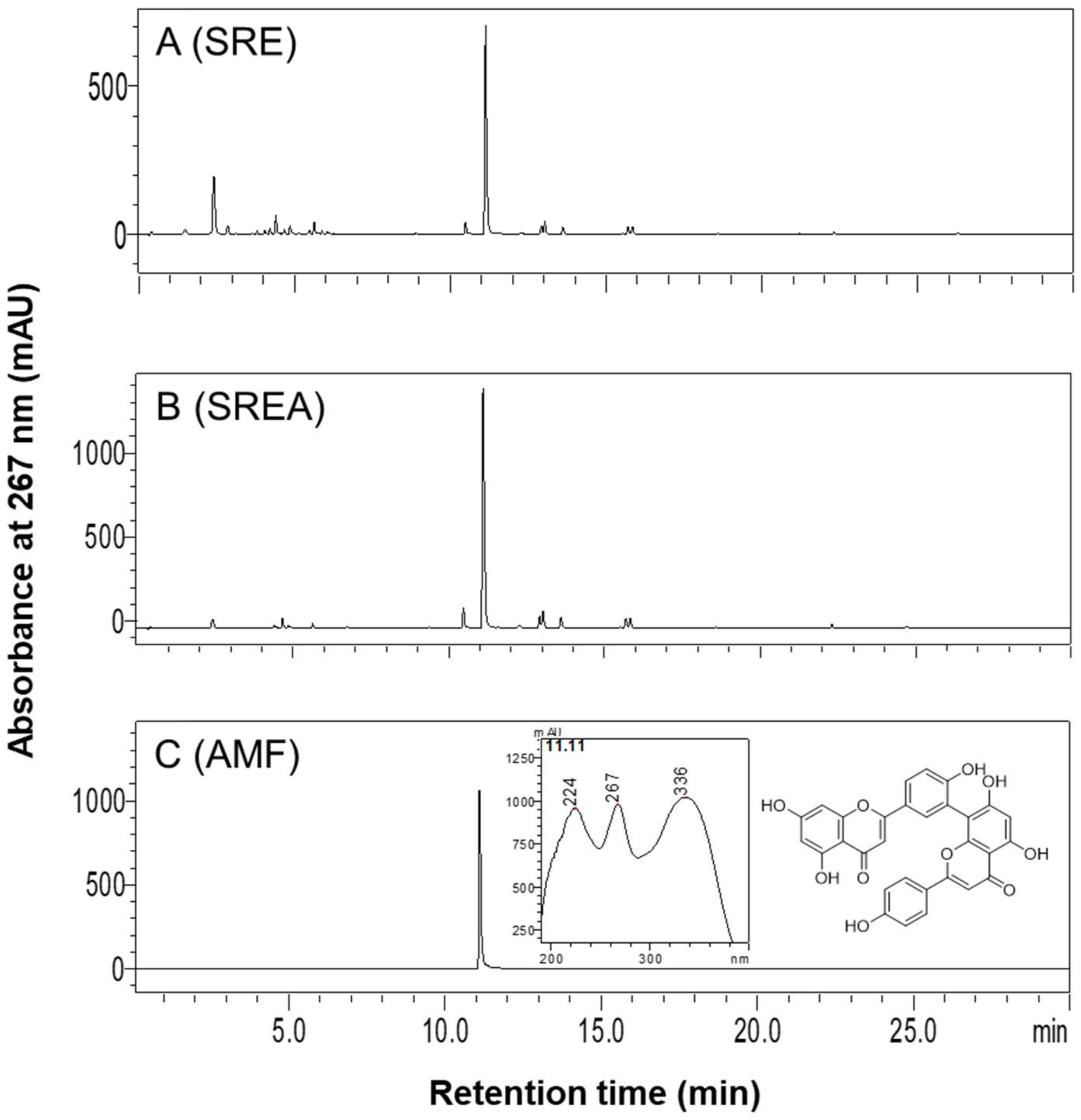
2.1. Preparation of SR Extracts and High-Performance Liquid Chromatography (HPLC) Analysis
2.2. Animals and Diets
2.2.1. Experiment 1
2.2.2. Experiment 2
2.2.3. Experiment 3
2.3. OFTT and OGTT
2.4. Plasma Parameters
2.5. Fecal Lipid Profiles
2.6. RNA Preparation and Quantitative Real-Time RT-PCR (qRT-PCR)
2.7. Histological Analysis
2.8. Western Blotting Analysis
2.9. Data Analysis
3. Results
3.1. HPLC Analysis of SR Extracts
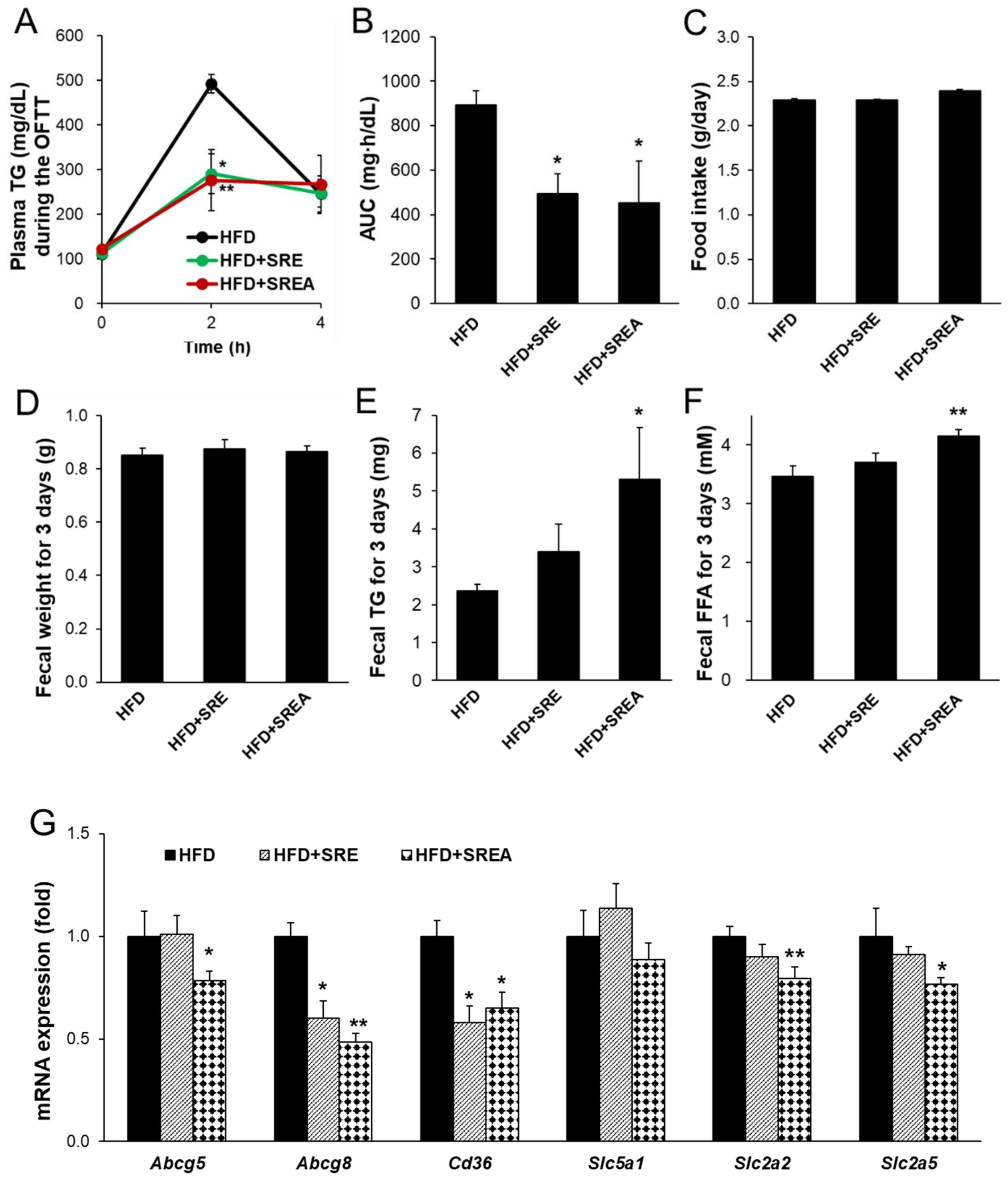
3.2. Short-Term Administration of SR Suppressed Intestinal Lipid Absorption
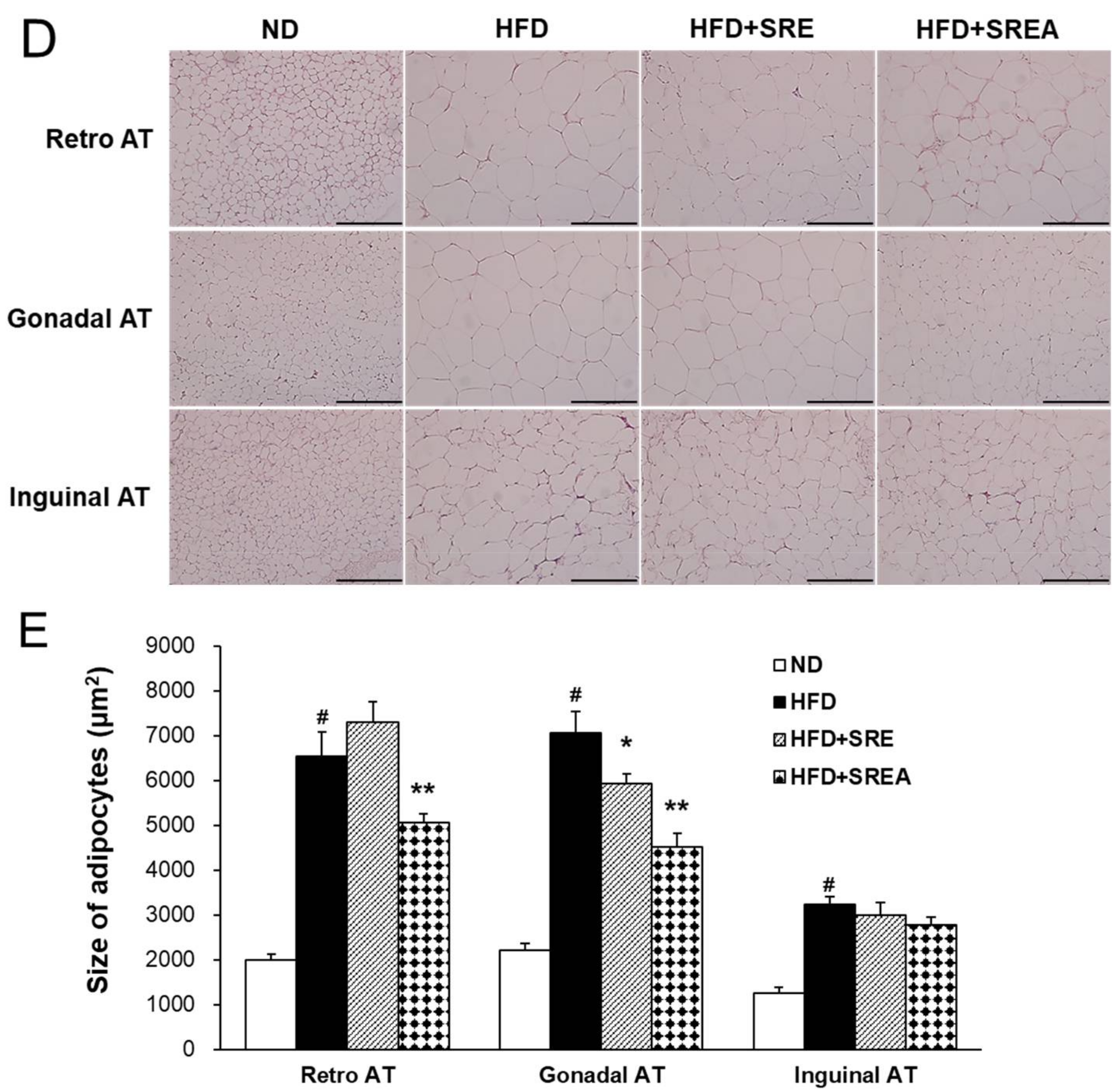
3.3. Long-Term Administration of SR Reduced HFD-Induced Obesity
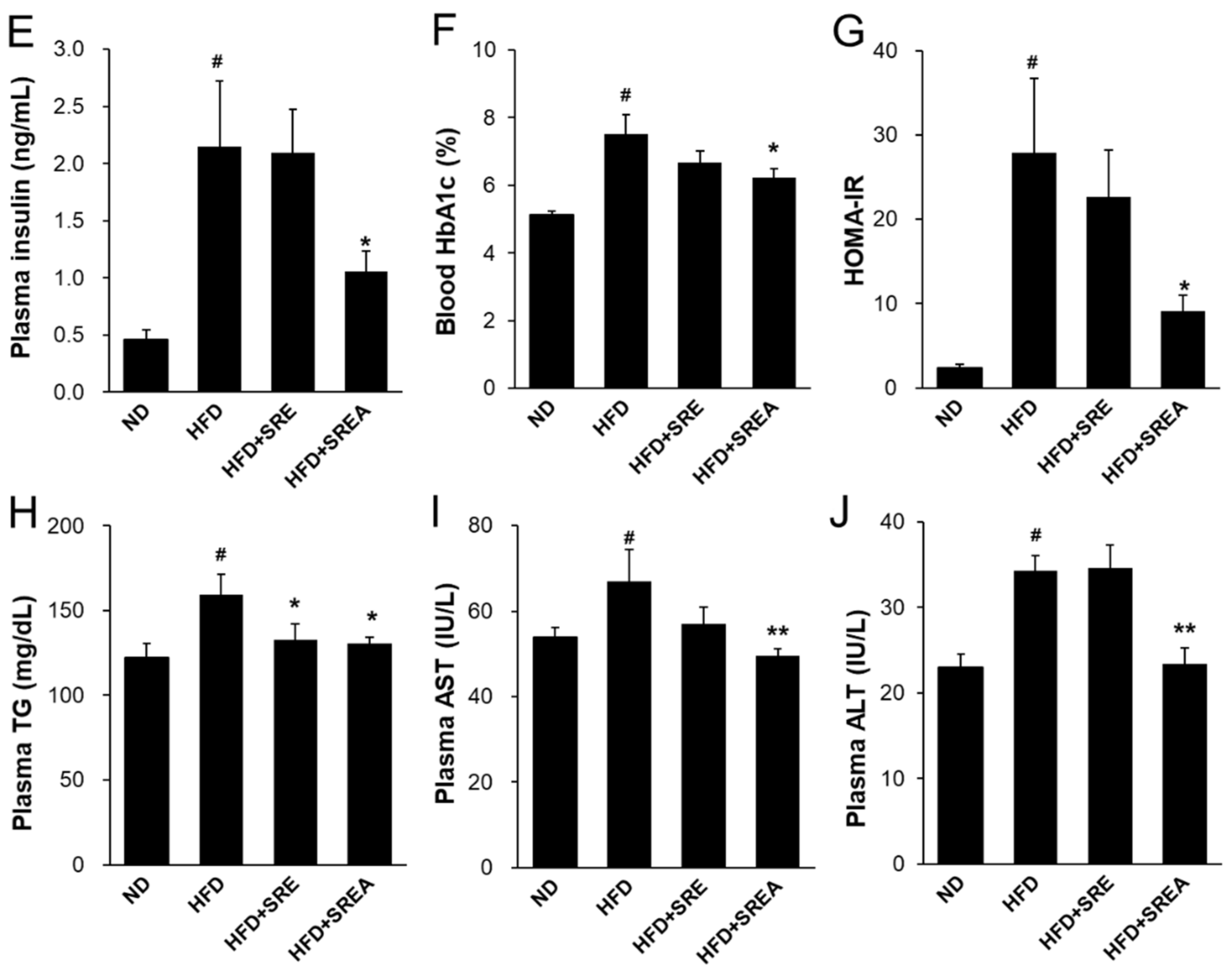
3.4. Long-Term Administration of SR Improved Metabolic Parameters in Plasma
3.5. Long-Term Administration of SR Suppressed Intestinal Lipid Absorption
3.6. Long-Term Administration of SR Protected Development of Hepatic Steatosis
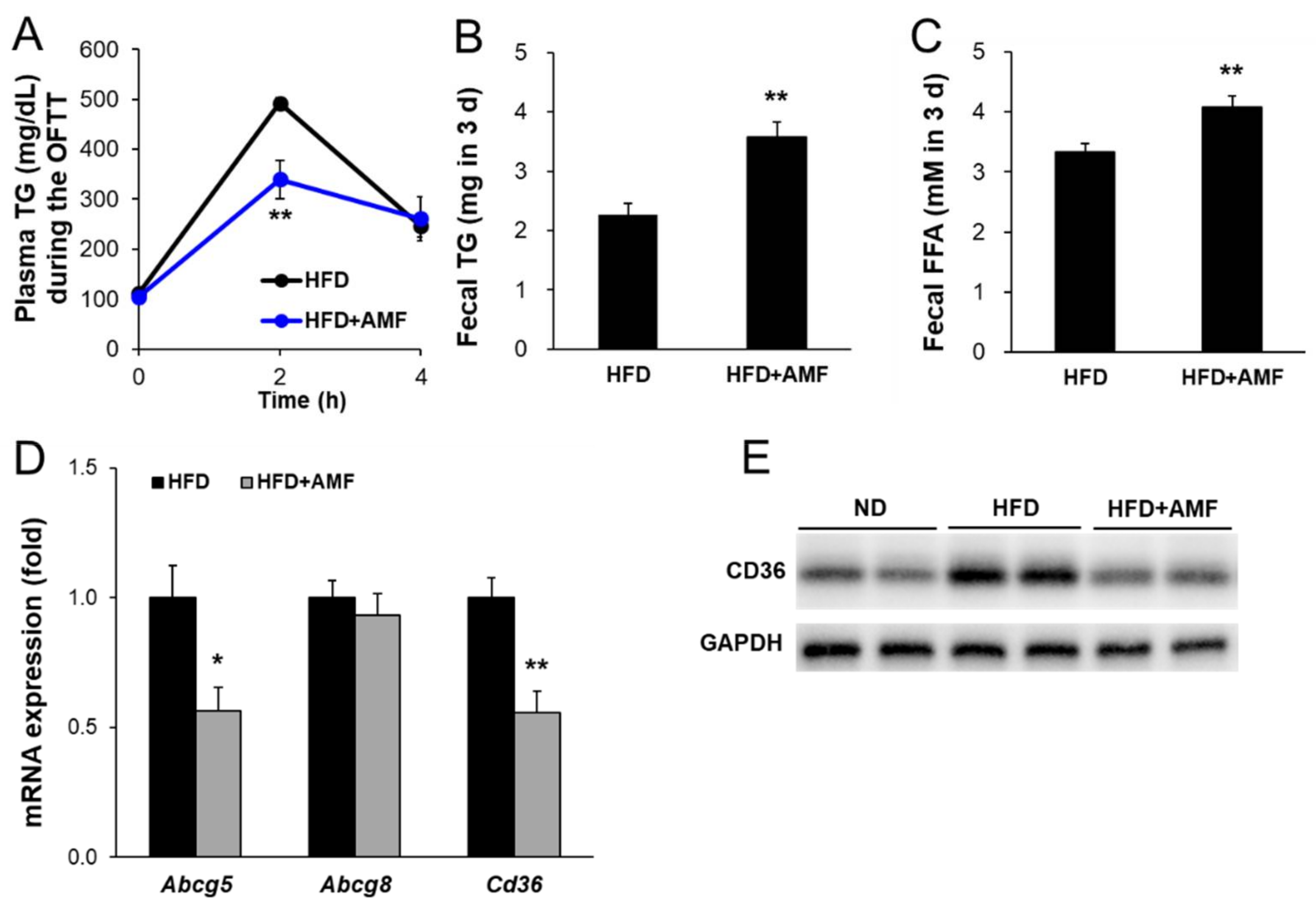
3.7. AMF Suppressed Intestinal Lipid Absorption
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; Garcia-Canaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell 2020, 183, 1848–1866.e26. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Magkos, F.; Mohammed, B.S.; Pietka, T.; Abumrad, N.A.; Patterson, B.W.; Okunade, A.; Klein, S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 15430–15435. [Google Scholar] [CrossRef] [Green Version]
- Zembroski, A.S.; Xiao, C.; Buhman, K.K. The roles of cytoplasmic lipid droplets in modulating intestinal uptake of dietary fat. Annu. Rev. Nutr. 2021, 41, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cifarelli, V.; Abumrad, N.A. Intestinal CD36 and other key proteins of lipid utilization: Role in absorption and gut homeostasis. Compr. Physiol. 2018, 8, 493–507. [Google Scholar]
- Xiao, C.; Stahel, P.; Carreiro, A.L.; Buhman, K.K.; Lewis, G.F. Recent advances in triacylglycerol mobilization by the gut. Trends Endocrinol. Metab. 2018, 29, 151–163. [Google Scholar] [CrossRef]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Kim, J.K.; Fillmore, J.J.; Chen, Y.; Yu, C.; Moore, I.K.; Pypaert, M.; Lutz, E.P.; Kako, Y.; Velez-Carrasco, W.; Goldberg, I.J.; et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc. Natl. Acad. Sci. USA 2001, 98, 7522–7527. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, R.J.; Tschop, M.H.; Wilding, J.P. Anti-obesity drugs: Past, present and future. Dis. Model. Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Borgstrom, B. Mode of action of tetrahydrolipstatin: A derivative of the naturally occurring lipase inhibitor lipstatin. Biochim. Biophys. Acta 1988, 962, 308–316. [Google Scholar] [CrossRef]
- Bakris, G.; Calhoun, D.; Egan, B.; Hellmann, C.; Dolker, M.; Kingma, I. Orlistat improves blood pressure control in obese subjects with treated but inadequately controlled hypertension. J. Hypertens. 2002, 20, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.K.; Sharma, P.; Singh, A.; Kalita, K.J.; Saha, S.; Chandra Borah, J. Adipose and non-adipose perspectives of plant derived natural compounds for mitigation of obesity. J. Ethnopharmacol. 2021, 280, 114410. [Google Scholar] [CrossRef]
- Hackett, A.; Krska, J. Is it time to regulate over-the-counter weight-loss formulations? Int. J. Pharm. Pract. 2012, 20, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.K.; Wang, W.W.; Zhang, L.; Su, C.F.; Wu, Y.Y.; Ke, Y.Y.; Hou, Q.W.; Liu, Z.Y.; Gao, A.S.; Feng, W.S. Antihyperlipidaemic and antioxidant effect of the total flavonoids in Selaginella tamariscina (Beauv.) Spring in diabetic mice. J. Pharm. Pharmacol. 2013, 65, 757–766. [Google Scholar] [CrossRef]
- Zheng, X.K.; Zhang, L.; Wang, W.W.; Wu, Y.Y.; Zhang, Q.B.; Feng, W.S. Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ. J. Ethnopharmacol. 2011, 137, 662–668. [Google Scholar] [CrossRef]
- Bailly, C. The traditional and modern uses of Selaginella tamariscina (P.Beauv.) Spring, in medicine and cosmetic: Applications and bioactive ingredients. J. Ethnopharmacol. 2021, 280, 114444. [Google Scholar] [CrossRef]
- Yu, D.; Li, X.; Yang, X.; Lu, X.; Feng, B. Anti-proliferative effect of three Selaginella plants on human monocytic leukemia U937 cell line. Nat. Prod. Res. Dev. 2016, 28, 1618–1621. [Google Scholar]
- Cho, S.; Lee, H.; Han, J.; Lee, H.; Kattia, R.O.; Nelson, Z.V.; Choi, S.; Kim, S.Y.; Park, H.Y.; Jeong, H.G.; et al. Viburnum stellato-tomentosum extract suppresses obesity and hyperglycemia through regulation of lipid metabolism in high-fat diet-fed mice. Molecules 2021, 26, 1052. [Google Scholar] [CrossRef]
- Chen, G.; Han, Y.; He, W.; Liang, F. Amentoflavone protects against high fat-induced metabolic dysfunction: Possible role of the regulation of adipogenic differentiation. Int. J. Mol. Med. 2016, 38, 1759–1767. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Cho, S.; Kang, A.; Shin, D.H.; Park, H.Y.; Jeong, T.S. Combination treatment of arazyme and soy leaf extract attenuates hyperglycemia and hepatic steatosis in high-fat diet-fed C57BL/6J mice. Life 2021, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Breneman, C.B.; Tucker, L. Dietary fibre consumption and insulin resistance—The role of body fat and physical activity. Br. J. Nutr. 2013, 110, 375–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-S.; Kwon, C.-S.; Son, K.H. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci. Biotechnol. Biochem. 2000, 64, 2458–2461. [Google Scholar] [CrossRef] [PubMed]
- Najari Beidokhti, M.; Lobbens, E.; Rasoavaivo, P.; Staerk, D.; Jäger, A. Investigation of medicinal plants from Madagascar against DPP-IV linked to type 2 diabetes. S. Afr. J. Bot. 2018, 115, 113–119. [Google Scholar] [CrossRef]
- Gok, H.N.; Deliorman Orhan, D.; Gurbuz, I.; Aslan, M. Activity-guided isolation of alpha-amylase, alpha-glucosidase, and pancreatic lipase inhibitory compounds from Rhus coriaria L. J. Food Sci. 2020, 85, 3220–3228. [Google Scholar] [CrossRef]
- Su, C.; Yang, C.; Gong, M.; Ke, Y.; Yuan, P.; Wang, X.; Li, M.; Zheng, X.; Feng, W. Antidiabetic activity and potential mechanism of amentoflavone in diabetic mice. Molecules 2019, 24, 2184. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Zhao, Y.; Zhang, B.; Li, Y. Amentoflavone improves cardiovascular dysfunction and metabolic abnormalities in high fructose and fat diet-fed rats. Food Funct. 2018, 9, 243–252. [Google Scholar] [CrossRef]
- Ma, S.C.; But, P.P.; Ooi, V.E.; He, Y.H.; Lee, S.H.; Lee, S.F.; Lin, R.C. Antiviral amentoflavone from Selaginella sinensis. Biol. Pharm. Bull. 2001, 24, 311–312. [Google Scholar] [CrossRef] [Green Version]
- Abumrad, N.A. CD36 may determine our desire for dietary fats. J. Clin. Investig. 2005, 115, 2965–2967. [Google Scholar] [CrossRef]
- Altmann, S.W.; Davis, H.R., Jr.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef] [Green Version]
- Drover, V.A.; Ajmal, M.; Nassir, F.; Davidson, N.O.; Nauli, A.M.; Sahoo, D.; Tso, P.; Abumrad, N.A. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J. Clin. Investig. 2005, 115, 1290–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, E. Insights from human congenital disorders of intestinal lipid metabolism. J. Lipid Res. 2015, 56, 945–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansbach, C.M.; Siddiqi, S. Control of chylomicron export from the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G659–G668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.M. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 2014, 25, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunham, L.R.; Kruit, J.K.; Iqbal, J.; Fievet, C.; Timmins, J.M.; Pape, T.D.; Coburn, B.A.; Bissada, N.; Staels, B.; Groen, A.K.; et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Investig. 2006, 116, 1052–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, M.; Ye, J.; Zhao, M.; Ke, X.; Huang, K.; Liu, H. Recent developments in the regulation of cholesterol transport by natural molecules. Phytother. Res. 2021, 35, 5623–5633. [Google Scholar] [CrossRef]
- Zhuang, J.-L.; Liu, Y.-Y.; Li, Z.-Z.; Zhuang, Q.-Z.; Tang, W.-Z.; Xiong, Y.; Huang, X.-Z. Amentoflavone prevents ox-LDL-induced lipid accumulation by suppressing the PPARγ/CD36 signal pathway. Toxicol. Appl. Pharmacol. 2021, 431, 115733. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D. Coupling between Na+, sugar, and water transport across the intestine. Ann. N. Y. Acad. Sci. 2000, 915, 54–66. [Google Scholar] [CrossRef]
- Burant, C.F.; Takeda, J.; Brot-Laroche, E.; Bell, G.I.; Davidson, N.O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J. Biol. Chem. 1992, 267, 14523–14526. [Google Scholar] [CrossRef]
- Liang, L.M.; Zhou, J.J.; Xu, F.; Liu, P.H.; Qin, L.; Liu, L.; Liu, X.D. Diabetes downregulates peptide transporter 1 in the rat jejunum: Possible involvement of cholate-induced FXR activation. Acta Pharmacol. Sin. 2020, 41, 1465–1475. [Google Scholar] [CrossRef]
- Hindlet, P.; Bado, A.; Farinotti, R.; Buyse, M. Long-term effect of leptin on H+-coupled peptide cotransporter 1 activity and expression in vivo: Evidence in leptin-deficient mice. J. Pharmacol. Exp. Ther. 2007, 323, 192–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, A.; Hirst, B.H. The glycine transporter GLYT1 in human intestine: Expression and function. Biol. Pharm. Bull. 2011, 34, 784–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbarino, J.; Sturley, S.L. Saturated with fat: New perspectives on lipotoxicity. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosterveer, M.H.; Van Dijk, T.H.; Tietge, U.J.F.; Boer, T.; Havinga, R.; Stellaard, F.; Groen, A.K.; Kuipers, F.; Reijngoud, D.-J. High fat feeding induces hepatic fatty acid elongation in mice. PLoS ONE 2009, 4, e6066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodis, K.; Roden, M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur. J. Clin. Investig. 2018, 48, e13017. [Google Scholar] [CrossRef] [Green Version]
- Item, F.; Konrad, D. Visceral fat and metabolic inflammation: The portal theory revisited. Obes. Rev. 2012, 13 (Suppl. S2), 30–39. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.G.; Yan, J.K.; Sun, C.P.; Li, J.X.; Ning, J.; Wang, C.; Huo, X.K.; Zhao, W.Y.; Yu, Z.L.; Feng, L.; et al. Amentoflavone from Selaginella tamariscina as a potent inhibitor of gut bacterial beta-glucuronidase: Inhibition kinetics and molecular dynamics stimulation. Chem. Biol. Interact. 2021, 340, 109453. [Google Scholar] [CrossRef]
- Li, W.; Tang, G.H.; Yin, S. Selaginellins from the genus Selaginella: Isolation, structure, biological activity, and synthesis. Nat. Prod. Rep. 2021, 38, 822–842. [Google Scholar] [CrossRef]





| Organs (g/kg body Wt.) | ND | HFD | HFD+SRE | HFD+SREA |
|---|---|---|---|---|
| Liver | 26.0 ± 1.2 | 30.1 ± 2.1 # | 27.4 ± 0.8 | 25.5 ± 0.7 * |
| Pancreas | 4.1 ± 0.2 | 4.5 ± 0.3 | 4.1 ± 0.3 | 4.0 ± 0.2 |
| WATs | ||||
| Retroperitoneal AT | 5.8 ± 1.0 | 28.1 ± 2.7 # | 22.6 ± 1.4 * | 22.4 ± 1.6 * |
| Gonadal AT | 20.8 ± 2.2 | 56.3 ± 2.9 # | 56.5 ± 3.1 | 55.3 ± 4.6 |
| Inguinal AT | 14.7 ± 1.8 | 46.3 ± 2.1 # | 36.1 ± 3.6 * | 34.5 ± 2.4 ** |
| Total WAT | 41.3 ± 4.8 | 122.7 ± 3.1 # | 112.6 ± 6.5 * | 110.9 ± 7.6 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Cho, S.; Kim, S.-Y.; Ju, J.; Lee, S.W.; Choi, S.; Li, H.; Piao, R.; Park, H.-Y.; Jeong, T.-S. Amentoflavone-Enriched Selaginella rossii Warb. Suppresses Body Weight and Hyperglycemia by Inhibiting Intestinal Lipid Absorption in Mice Fed a High-Fat Diet. Life 2022, 12, 472. https://doi.org/10.3390/life12040472
Lee H, Cho S, Kim S-Y, Ju J, Lee SW, Choi S, Li H, Piao R, Park H-Y, Jeong T-S. Amentoflavone-Enriched Selaginella rossii Warb. Suppresses Body Weight and Hyperglycemia by Inhibiting Intestinal Lipid Absorption in Mice Fed a High-Fat Diet. Life. 2022; 12(4):472. https://doi.org/10.3390/life12040472
Chicago/Turabian StyleLee, Hwa, Seona Cho, Soo-Yong Kim, Jeongha Ju, Sang Woo Lee, Sangho Choi, Hulin Li, Renzhe Piao, Ho-Yong Park, and Tae-Sook Jeong. 2022. "Amentoflavone-Enriched Selaginella rossii Warb. Suppresses Body Weight and Hyperglycemia by Inhibiting Intestinal Lipid Absorption in Mice Fed a High-Fat Diet" Life 12, no. 4: 472. https://doi.org/10.3390/life12040472
APA StyleLee, H., Cho, S., Kim, S.-Y., Ju, J., Lee, S. W., Choi, S., Li, H., Piao, R., Park, H.-Y., & Jeong, T.-S. (2022). Amentoflavone-Enriched Selaginella rossii Warb. Suppresses Body Weight and Hyperglycemia by Inhibiting Intestinal Lipid Absorption in Mice Fed a High-Fat Diet. Life, 12(4), 472. https://doi.org/10.3390/life12040472