Metabolic Bone Disorders in Children with Inflammatory Bowel Diseases
Abstract
1. Introduction
2. Background
2.1. IBD—Characteristics, Etiology, and Treatment
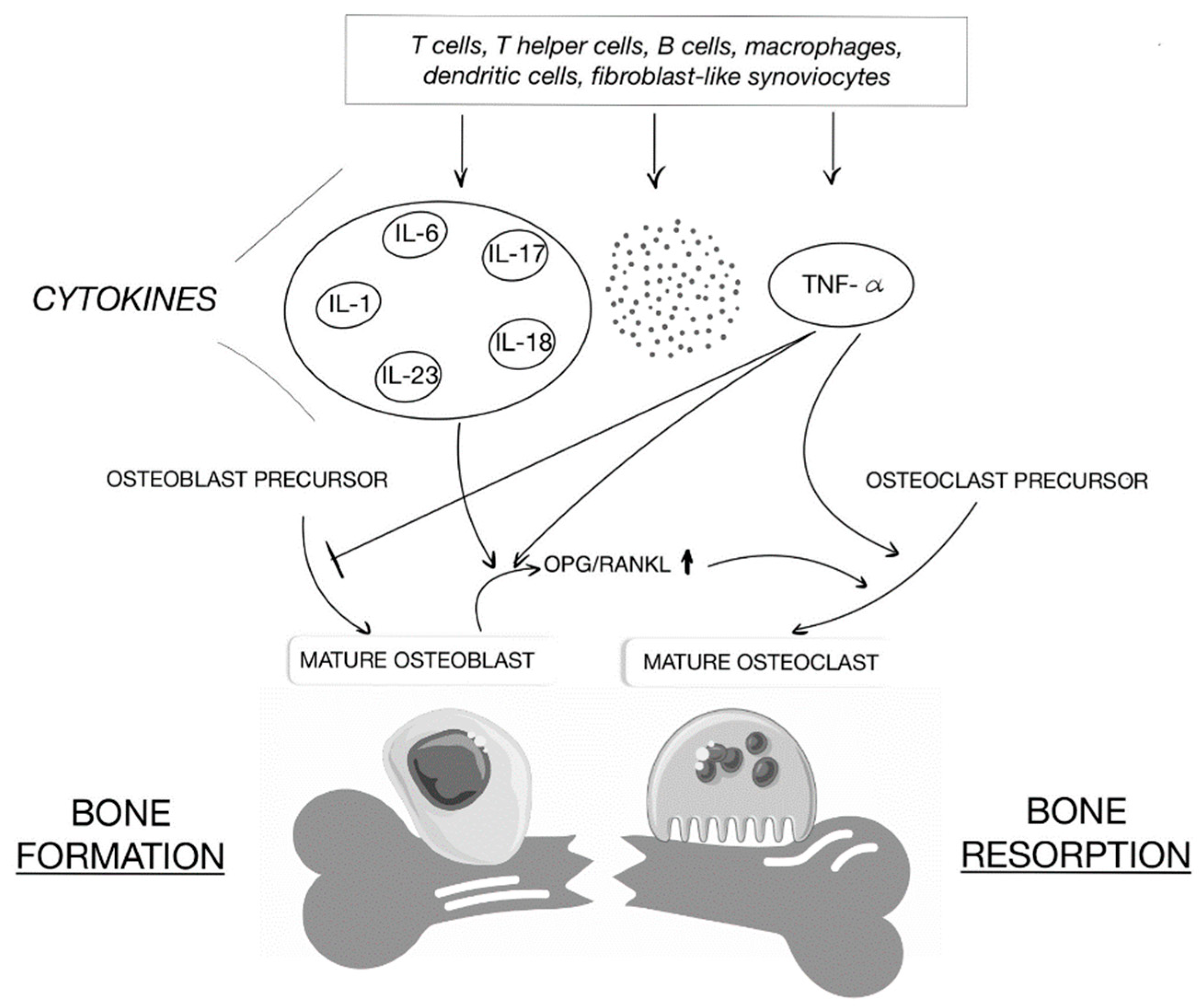
2.2. IBD and Metabolic Bone Disorders—Potential Connections
2.3. CD and UC—Does One Type Affect Bones More Than the Other?
2.4. Sex Differences, Growth, Puberty, and Bone Health
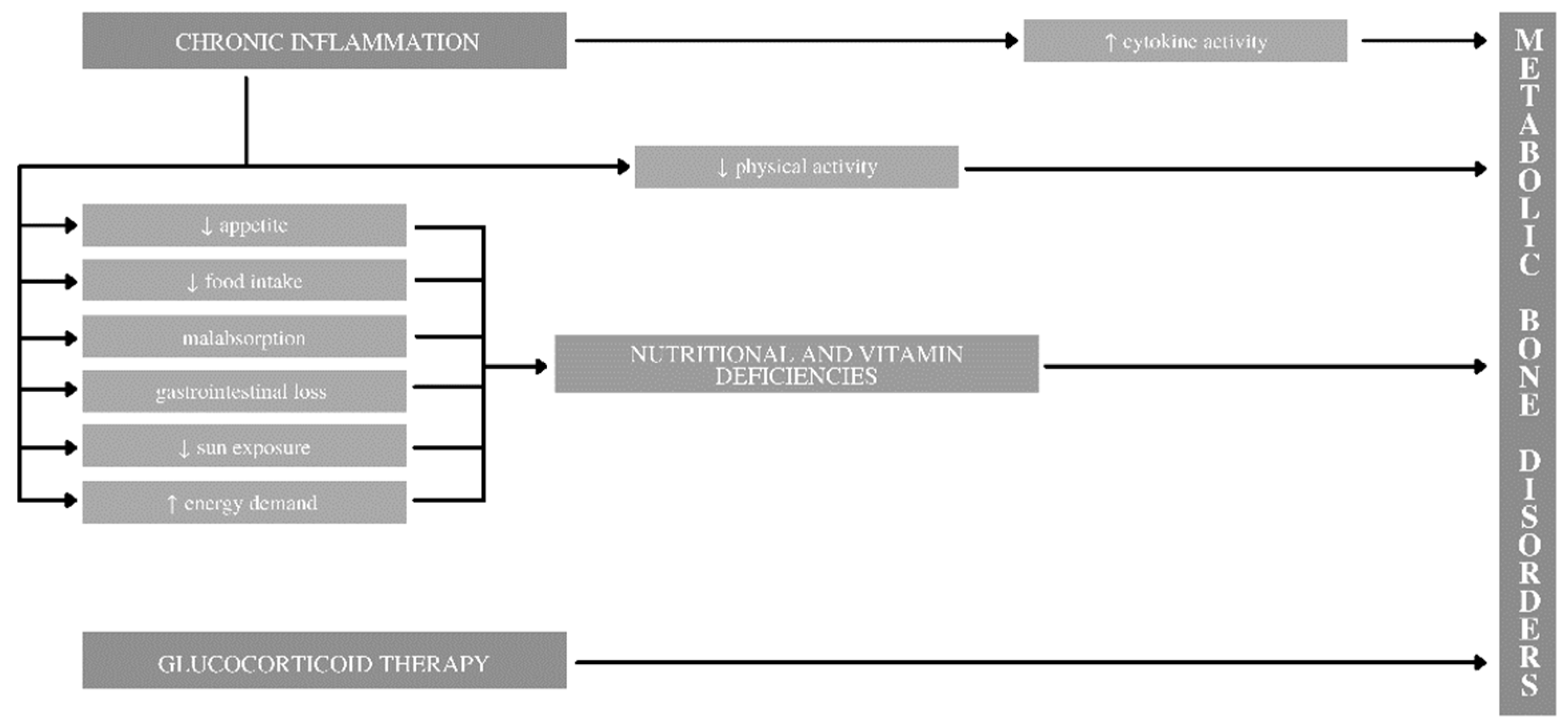
2.5. Chronic Inflammation
2.6. Treatment Methodology
2.7. Physical Activity
2.8. Altered Body Composition and Musculoskeletal Deficits
2.9. Vitamin D
2.10. Other Aspects
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krzesiek, E.; Kofla-Dlubacz, A.; Akutko, K.; Stawarski, A. The Incidence of Inflammatory Bowel Disease in the Paediatric Population in the District of Lower Silesia, Poland. J. Clin. Med. 2021, 10, 3994. [Google Scholar] [CrossRef] [PubMed]
- Langholz, E.; Munkholm, P.; Krasilnikoff, P.A.; Binder, V. Inflammatory bowel diseases with onset in childhood. Clinical features, morbidity, and mortality in a regional cohort. Scand. J. Gastroenterol. 1997, 32, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Rabau, M.Y.; Haboubi, N.Y. Indeterminate colitis. Technol. Coloproctol. 2007, 11, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Manne, S.; Treem, W.R.; Bennett, D. Prevalence of Inflammatory Bowel Disease in Pediatric and Adult Populations: Recent Estimates from Large National Databases in the United States, 2007–2016. Inflamm. Bowel Dis. 2020, 26, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Jevon, G.P.; Madhur, R. Endoscopic and histologic findings in pediatric inflammatory bowel disease. Gastroenterol. Hepatol. 2010, 6, 174–180. [Google Scholar]
- Kamińska, B.; Landowski, P. Rola wybranych czynników środowiskowych w etiopatogenezie nieswoistych zapaleń jelit. Forum Med. Rodz. 2009, 3, 43–48. [Google Scholar]
- D’Arcangelo, G.; Distante, M.; Raso, T.; Rossetti, D.; Catassi, G.; Aloi, M. Safety of Biological Therapy in Children with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Roodman, G.D. Cell biology of the osteoclast. Exp. Hematol. 1999, 27, 1229–1241. [Google Scholar] [CrossRef]
- Sims, N.A.; Walsh, N.C. GP130 cytokines and bone remodeling in health and disease. BMB Rep. 2010, 43, 513–523. [Google Scholar] [CrossRef]
- Sigursdsson, G.V.; Schmidt, S.; Mellström, D.; Ohlsson, C.; Karlsson, M.; Lorentzon, M.; Saalman, R. Physical exercise is associated with beneficial bone mineral density and body composition in young adults with childhood-onset inflammatory bowel disease. Scand. J. Gastroenterol. 2021, 56, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Masip, E.; Donat, E.; Miquel, B.P.; Ribes-Koninckx, C. Bone minreal density in spanish children at the diagnosis of inflammatory bowel disease. Arch. Osteoporos. 2021, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Trivić, I.; Sila, S.; Batoš, A.T.; Mišak, Z.; Kolaček, S.; Hojsak, I. Moderate-to-Vigorous Physical Activity is Associated with Higher Bone Mineral Density in Children with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 74, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Klibanski, A.; Adams-Campbell, L.; Bassford, T.; Blair, S.N.; Boden, S.D.; Dickersin, K.; Gifford, D.R.; Glasse, L.; Goldring, S.R.; Hruska, K.; et al. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar]
- Garcia, R.; Klein, M.; Schiller, A. Rubin’s Pathology: Clinicopathologic Foundations of Medicine, “Metabolic Bone Diseases”; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 1225–1250. [Google Scholar]
- Bernstein, C.N.; Benchimol, E.I.; Bitton, A.; Murthy, S.K.; Nguyen, G.C.; Lee, K.; Cooke-Lauder, J.; Kaplan, G.G. The Impact of Inflammatory Bowel Disease in Canada 2018: Extra-intestinal Diseases in IBD. J. Can. Assoc. Gastroenterol. 2019, 2 (Suppl. 1), S73–S80. [Google Scholar] [CrossRef]
- Werkstetter, K.J.; Pozza, S.B.D.; Filipiak-Pitroff, B.; Schatz, S.B.; Prell, C.; Bufler, P.; Koletzko, B.; Koletzko, S. Long-term development of bone geometry and muscle in pediatric inflammatory bowel disease. Am. J. Gastroenerol. 2011, 106, 988–998. [Google Scholar] [CrossRef]
- Levy-Shraga, Y.; Megnazi, O.; Modan-Moses, D.; Tripto-Shkolnik, L.; Gruber, N.; Haberman, Y.; Shouval, D.S.; Weiss, B. Trabecular Bone Score in Children and Adolescents with Inflammatory Bowel Diseases. J. Clin. Densitom. 2021, 24, 243–251. [Google Scholar] [CrossRef]
- Su, H.Y.; Gupta, V.; Day, A.S.; Gearry, R.B. Rising Incidence of Inflammatory Bowel Disease in Canterbury, New Zealand. Inflamm. Bowel Dis. 2016, 22, 2238–2244. [Google Scholar] [CrossRef]
- Sigursdsson, G.V.; Schmidt, S.; Mellström, D.; Ohlsson, C.; Kindblom, J.M.; Lorentzon, M.; Saalman, R. Bone Mass Development from Childhood into Young Adulthood in Patients with Childhood-onset Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2215–2226. [Google Scholar] [CrossRef][Green Version]
- Gokhale, R.; Favus, M.J.; Karrison, T.; Sutton, M.M.; Rich, B.; Kirschner, B.S. Bone mineral density assessment in children with inflammatory bowel disease. Gastroenterology 1998, 114, 902–911. [Google Scholar] [CrossRef]
- Rozes, S.; Guilmin-Crepon, S.; Alison, M.; Thomas, E.; Hugot, J.P.; Viala, J.; Martinez-Vinson, C. Bone Health in Pediatric Patients with Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 231–235. [Google Scholar] [PubMed]
- Jin, H.Y.; Lim, J.S.; Lee, Y.; Choi, Y.; Oh, S.H.; Kim, K.M.; Yoo, H.W.; Choi, J.H. Growth, puberty, and bone health in children and adolescents with inflammatory bowel disease. BMC Pediatr. 2021, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Ricciuto, A.; Aardoom, M.; Orlanski-Meyer, E.; Navon, D.; Carman, N.; Aloi, M.; Bronsky, J.; Däbritz, J.; Dubinsky, M.; Hussey, S.; et al. Predicting Outcomes in Pediatric Crohn’s Disease for Management Optimization: Systematic Review and Consensus Statements from the Pediatric Inflammatory Bowel Disease-Ahead Program. Gastroenterology 2021, 160, 403–436.e26. [Google Scholar] [CrossRef] [PubMed]
- Sawczenko, A.; Ballinger, A.B.; Savage, M.O.; Sanderson, I.R. Clinical features affecting final adult height in patients with pediatric-onset Crohn’s disease. Pediatrics 2006, 118, 124–129. [Google Scholar] [CrossRef]
- Levy-Shraga, Y.; Shenkar, A.; Modan-Moses, D.; Assa, A.; Haberman, Y.; Shouval, D.; Guz-Mark, A.; Lahad, A.; Weiss, B. Longitudinal changes in bone mineral density in children with inflammatory bowel diseases. Acta Paediatr. 2020, 109, 1026–1032. [Google Scholar] [CrossRef]
- Gupta, N.; Liu, C.; King, E.; Sylvester, F.; Lee, D.; Boyle, B.; Trauernicht, A.; Chen, S.; Colletti, R.; ImproveCareNow Network. Continued Statural Growth in Older Adolescents and Young Adults with Crohn’s Disease and Ulcerative Colitis Beyond the Time of Expected Growth Plate Closure. Inflamm. Bowel Dis. 2020, 26, 1880–1889. [Google Scholar] [CrossRef]
- Paganelli, M.; Albanese, C.; Borrelli, O.; Civitelli, F.; Canitano, N.; Viola, F.; Passariello, R.; Cucchiara, S. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 416–423. [Google Scholar] [CrossRef]
- Ronel, N.; Tzion, R.L.; Orlanski-Meyer, E.; Shteyer, E.; Guz-Mark, A.; Assa, A.; Strich, D.; Turner, D.; Ledder, O. Clinical Criteria Can Identify Children with Osteopenia in Newly Diagnosed Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 270–275. [Google Scholar] [CrossRef]
- Conrad, M.A.; Kelsen, J.R. The Treatment of Pediatric Inflammatory Bowel Disease with Biologic Therapies. Curr. Gastroenterol. Rep. 2020, 22, 36. [Google Scholar] [CrossRef]
- Pichler, J.; Hanslik, A.; Huber, W.D.; Aufricht, C.; Bidmon-Fliegenschnee, B. Paediatric patients with inflammatory bowel disease who received infliximab experienced improved growth and bone health. Acta Paediatr. 2014, 103, e69–e75. [Google Scholar] [CrossRef]
- Thayu, M.; Leonard, M.B.; Hyams, J.S.; Crandall, W.V.; Kugathasan, S.; Otley, A.R.; Olson, A.; Johanns, J.; Marano, C.W.; Heuschkel, R.B.; et al. Improvement in biomarkers of bone formation during infliximab therapy in pediatric Crohn’s disease: Results of the REACH study. Clin. Gastroenterol. Hepatol. 2008, 6, 1378–1384. [Google Scholar] [CrossRef]
- Veerappan, S.G.; Healy, M.; Walsh, B.; O’Morain, C.A.; Daly, J.S.; Ryan, B.M. A 1-year prospective study of the effect of infliximab on bone metabolism in inflammatory bowel disease patients. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Zavatta, G.; Clarke, B.L. Glucocorticoid- and Transplantation-Induced Osteoporosis. Endocrinol. Metab. Clin. N. Am. 2021, 50, 251–273. [Google Scholar] [CrossRef] [PubMed]
- Sigursdsson, G.V.; Schmidt, S.; Mellström, D.; Ohlsson, C.; Karlsson, M.; Lorentzon, M.; Saalman, R. Altered body composition profiles in young adults with childhood-onset inflammatory bowel disease. Scand. J. Gastroenterol. 2020, 55, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Vihinen, M.K.; Kolho, K.L.; Ashorn, M.; Verkasalo, M.; Raivio, T. Bone turnover and metabolism in paediatric patients with inflammatory bowel disease treated with systemic glucocorticoids. Eur. J. Endocrinol. 2008, 159, 693–698. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D.; Lee, A.M.; Herbert, K.; Long, J.; Thayu, M.; Griffin, L.M.; Baldassano, R.N.; Denson, L.A.; Zemel, B.S.; Denburg, M.R.; et al. Increases in IGF-1 After Anti-TNF-α Therapy Are Associated with Bone and Muscle Accrual in Pediatric Crohn Disease. J. Clin. Endocrinol. Metab. 2018, 103, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Maratova, K.; Hradsky, O.; Matyskova, J.; Copova, I.; Soucek, O.; Sumnik, Z.; Bronsky, J. Musculoskeletal system in children and adolescents with inflammatory bowel disease: Normal muscle force, decreased trabecular bone mineral density and low prevalence of vertebral fractures. Eur. J. Pediatr. 2017, 176, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, R.H.; Hashmi, H.; Baker, R.D.; Gelfond, D.; Baker, S.S. Vitamin and mineral status in patients with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 89–92. [Google Scholar] [CrossRef]
- Ward, L.M.; Ma, J.; Rauch, F.; Benchimol, E.; Hay, J.; Leonard, M.B.; Matzinger, M.A.; Shenouda, N.; Lentle, B.; Cosgrove, H.; et al. Musculoskeletal health in newly diagnosed children with Crohn’s disease. Osteoporos. Int. 2017, 28, 3169–3177. [Google Scholar] [CrossRef]
- Bechtold, S.; Alberer, M.; Arenz, T.; Putzker, S.; Filipiak-Pittroff, B.; Schwarz, H.P.; Koletzko, S. Reduced Muscle Mass and Bone Size in Pewarddiatric Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2010, 16, 216–225. [Google Scholar] [CrossRef]
- Werkstetter, K.J.; Schatz, S.B.; Alberer, M.; Filipiak-Pittroff, B.; Koletzko, S. Influence of Exclusive Enteral Nutrition Therapy on Bone Density and Geometry in Newly Diagnosed Pediatric Crohn’s Disease Patients. Ann. Nutr. Metab. 2013, 63, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.M.; Thayu, M.; Baldassano, R.N.; DeBoer, M.D.; Zemel, B.S.; Denburg, M.R.; Denson, L.A.; Shults, J.; Herskovitz, R.; Long, J.; et al. Improvements in Bone Density and Structure during Anti-TNF-alpha Therapy in Pediatric Crohn’s Disease. J. Clin. Endocrinol. Metab. 2015, 100, 2630–2639. [Google Scholar] [CrossRef]
- Steell, L.; Johnston, B.A.; Dewantoro, D.; Foster, J.E.; Gaya, D.R.; Macdonald, J.; McMillan, M.; Russell, R.K.; Seenan, J.P.; Ahmed, S.F.; et al. Muscle deficits with normal bone microarchitecture and geometry in young adults with well-controlled childhood-onset Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Rigterink, T.; Appleton, L.; Day, A.S. Vitamin D therapy in children with inflammatory bowel disease: A systematic review. World J. Clin. Pediatr. 2019, 8, 1–14. [Google Scholar] [CrossRef]
- Jasielska, M.; Grzybowska-Chlebowczyk, U. Hypocalcemia and Vitamin D Deficiency in Children with Inflammatory Bowel Diseases and Lactose Intolerance. Nutrients 2021, 13, 2583. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 2012, 97, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Amrousy, D.E.; Ashry, H.E.; Hodeib, H.; Hassan, S. Vitamin D in Children with Inflammatory Bowel Disease: A Randomized Controlled Clinical Trial. J. Clin. Gastroenterol. 2021, 55, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H. Osteoimmunology: The Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol. Rev. 2017, 97, 1295–1349. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, C.; Chen, H.; Ma, J.; Zhu, Y.; Wang, P.; Zhang, Y.; Ma, H.; Zhang, H. Efficacy and safety of medical therapy for low bone mineral density in patients with Crohn disease: A systematic review with network meta-analysis. Medicine 2017, 96, e6378. [Google Scholar] [CrossRef]
- Laakso, S.; Valta, H.; Verkasalo, M.; Toiviainen-Salo, S.; Viljakainen, H.; Mäkitie, O. Impaired bone health in inflammatory bowel disease: A case-control study in 80 pediatric patients. Calcif. Tissue Res. 2012, 91, 121–130. [Google Scholar] [CrossRef]
- Wasserman, H.; Gordon, C.M. Bone Mineralization and Fracture Risk Assessment in the Pediatric Population. J. Clin. Densitom. 2017, 20, 389–396. [Google Scholar] [CrossRef]
- Wallace, T.C.; Marzorati, M.; Spence, L.; Weaver, C.M.; Williamson, P.S. New Frontiers in Fibers: Innovative and Emerging Research on the Gut Microbiome and Bone Health. J. Am. Coll. Nutr. 2017, 36, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Misof, B.M.; Roschger, P.; Klaushofer, K.; Rauch, F.; Ma, J.; Mack, D.R.; Ward, L.M. Increased bone matrix mineralization in treatment-naïve children with inflammatory bowel disease. Bone 2017, 105, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Pappa, H.; Thayu, M.; Sylvester, F.; Leonard, M.; Zemel, B.; Gordon, C. Skeletal Health of Children and Adolescents with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 11–25. [Google Scholar] [CrossRef]
- Zemel, B.S.; Leonard, M.B.; Kelly, A.; Lappe, J.M.; Gilsanz, V.; Oberfield, S.; Mahboubi, S.; Shepherd, J.A.; Hangartner, T.N.; Frederick, M.M.; et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J. Clin. Endocrinol. Metab. 2010, 95, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Zemel, B.S.; Kalkwarf, H.J.; Gilsanz, V.; Lappe, J.M.; Oberfield, S.; Shepherd, J.A.; Frederick, M.M.; Huang, X.; Lu, M.; Mahboubi, S.; et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: Results of the bone mineral density in childhood study. J. Clin. Endocrinol. Metab. 2011, 96, 3160–3169. [Google Scholar] [CrossRef] [PubMed]
- Smyczyńska, J.; Smyczyńska, U.; Stawerska, R.; Domagalska-Nalewajek, H.; Lewiński, A.; Hilczer, M. Seasonality of vitamin D concentrations and the incidence of vitamin D deficiency in children and adolescents from central Poland. Pediatr. Endocrinol. Diabetes Metab. 2019, 25, 54–59. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Haynes, K.; Denburg, M.R.; Zemel, B.S.; Leonard, M.B.; Abrams, J.A.; Yang, Y.X. Use of proton pump inhibitors is associated with fractures in young adults: A population-based study. Osteoporos. Int. 2015, 26, 2501–2507. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Steell, L.; Gray, S.R.; Russell, R.K.; MacDonald, J.; Seenan, J.P.; Wong, S.C.; Gaya, D.R. Pathogenesis of Musculoskeletal Deficits in Children and Adults with Inflammatory Bowel Disease. Nutrients 2021, 13, 2899. [Google Scholar] [CrossRef]
- Van der Eerden, B.C.; Karperien, M.; Wit, J.M. Systemic and local regulation of the growth plate. Endocr. Rev. 2003, 24, 782–801. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Design | Study Group Age (Years) | Key Findings | Comments |
|---|---|---|---|---|
| Sigursdsson et al. (2021) [11] | Cross-sectional cohort | 72 IBD, 1341 non-IBD 22.7 (21.3–24.5) | Physical exercise beneficial for BMD | Young adults with childhood-onset IBD |
| Masip et al. (2021) [12] | Retrospective | 57 IBD (27 CD) 11.18 ± 2.24 | Suboptimal BMD at diagnosis No difference in BMD between patients with CD and UC ↓ weight and height associated with a ↓ BMD | |
| Trivić et al. (2022) [13] | Cross-sectional cohort | 40 IBD (20 CD, 18 UC, 2 IBD-U) 15.3 ± 0.4 | Strong positive relationship between MVPA, LBM and BMD | |
| Werkstetter et al. (2011) [17] | Prospective cohort | 102 IBD (82 CD) New IBD: 30 | ↓ trabecular BMD, ↓ cortical thickness, and ↑ cortical BMD | Parameters measured by pQCT at the forearm |
| Levy-Shraga et al. (2021) [18] | Retrospective | 50 IBD (35 CD) 13.8 ± 3.0 | ↓ TBS only in CD | BMD measurements by DXA |
| Sigurdsson et al. (2017) [20] | Prospective cohort | 74 IBD (25 CD) 22.9 ± 2.4 | ↓ BMD Z-score (lumbar spine and total hip) No difference in BMD between patients with CD and UC | Young adults with childhood-onset IBD |
| Gokhale et al. (1998) [21] | Prospective cohort | 99 IBD | ↓ BMD Z-score Cumulative corticosteoroid dose as a predictor of ↓ BMD | BMD measurements by DXA |
| Rozes et al. (2021) [22] | Retrospective | 193 CD | ↓ BMD Z-score Long-term glucocorticoid therapy as a predictor of ↓ BMD | |
| Jin et al. (2021) [23] | Retrospective | 127 IBD (117 CD) 13.5 ± 2.5 | ↓ Vitamin D, ↓ weight-SDS, ↓ IGF-1-SDS, ↓ testosterone, osteoporosis | |
| Sawczenko et al. [25] | Retrospective | 123 CD | ↓ Final height in comparison with target height | |
| Levy-Shraga et al. [26] | Retrospective | 41 IBD 12.1 ± 3.5 | ↑ BMD more pronounced in children who gained weight | Two BMD measurements by DXA, mean interval between the scans 3.4 ± 2.0 years |
| Gupta et al. [27] | Retrospective | 3007 IBD (76% CD) | Growing beyond the time of expected growth plate closure | |
| Paganelli et al. [28] | Retrospective | 56 IBD (35 CD) | Inverse correlation between BMAD and IL-6 in patients with UC Disease activity indexes inversely correlated with BMAD Beneficial effect of IFX on bone density | Cumulative dose of corticosteroids and duration of therapy with no correlation with BMAD |
| Ronel et al. [29] | Retrospective | 116 CD | Osteopenia in nearly half of children with newly onset CD | |
| Pilcher et al. [31] | Retrospective | 33 IBD 13.5 * | After treatment with IFX: ↑ weight, positive catch-up growth, ↑ vitamin D, ↔ bone mass | |
| Thayu et al. [32] | Multicenter, randomized controlled trial | 101 CD 13.3 ± 2.5 | IFX therapy associated with ↑ BSAP and ↑ P1NP, inhibition of TNF–α effects on osteoblasts ↑ CTX-1 and ↑ DPD reflect coupling of bone formation and resorption, ↑ linear growth ↑ Risk for having altered body composition traits | |
| Sigursdsson et al. (2020) [35] | Cross-sectional cohort | 94 IBD (29 CD) 18–27 years | Myopenic and myopenic-obese body composition profiles associated with ↓ BMD | Young adults with childhood-onset IBD |
| Vihinen et al. (2008) [36] | Prospective cohort study | 22 IBD 12.3 ** | ↓ Bone formation in children with active IBD ↓ Bone turnover due to glucocorticoid treatment | |
| DeBoer et al. (2018) [37] | Prospective cohort study | 63 CD | IGF-1 Z-scores predicted recovery of bone and muscle outcomes following initiation of anti-TNF-α therapy | |
| Maratova et al. (2017) [38] | Prospective cohort study | 70 IBD 13.8 * | Altered bone density and geometry but normal dynamic muscle functions | Parameters measured by pQCT |
| Alkhouri et al. (2013) [39] | Retrospective study | 61 IBD (46 CD) 12.3 ± 2.5 | ↓ Vitamin D | |
| Ward et al. (2017) [40] | Prospective cohort study | 73 CD 7.0–17.7 | Profound muscle and bone deficits in children with newly diagnosed CD | Parameters measured by DXA and pQCT |
| Bechtold et al. (2010) [41] | Cross-sectional study | 143 IBD (98 CD) 13.9 ± 3.5 | Bone disease in children with IBD seems to be secondary to muscle wasting With longer disease duration, bone adapts to the lower muscle CSA | Parameters measured by pQCT |
| Werkstetter et al. (2013) [42] | Prospective cohort study | Newly diagnosed CD 10.6–17.7 | Disturbed bone remodeling and severely impaired muscle mass in newly diagnosed CD children Bone metabolism and muscle mass improved after starting EEN | |
| Griffin et al. (2015) [43] | Prospective cohort study | 74 CD | Anti-TNF-α therapy associated with ↑ trabecular BMD and ↑ cortical structure | |
| Steell et al. (2020) [44] | Prospective cohort study | 27 CD 23.2 * | Muscle deficits, no abnormal bone microarchitecture or geometry at the distal femur | Young adults with childhood-onset IBD |
| Jasielska et al. (2021) [46] | Prospective cohort study | 74 IBD (43 CD) 14.07 ± 3.58 | Low-lactose diet with no effect on BMD | |
| Amrousy et al. (2021) [48] | Randomized double-blind controlled clinical trial | 120 IBD | Vitamin D supplementation decreased the IBD activity score | |
| Laakso et al. (2012) [51] | Cross-sectional study | 80 IBD 14.9 * | ↓ BA-adjusted lumbar spine and ↓ whole-body aBMD and ↓ whole-body BMC adjusted for height | |
| Misof et al. (2017) [54] | Prospective cohort study | 20 IBD 14.5 ± 2.3 | Children with treatment-naïve IBD: ↓ bone turnover leading to a higher bone matrix mineralization density |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olczyk, M.; Czkwianianc, E.; Socha-Banasiak, A. Metabolic Bone Disorders in Children with Inflammatory Bowel Diseases. Life 2022, 12, 423. https://doi.org/10.3390/life12030423
Olczyk M, Czkwianianc E, Socha-Banasiak A. Metabolic Bone Disorders in Children with Inflammatory Bowel Diseases. Life. 2022; 12(3):423. https://doi.org/10.3390/life12030423
Chicago/Turabian StyleOlczyk, Mariusz, Elżbieta Czkwianianc, and Anna Socha-Banasiak. 2022. "Metabolic Bone Disorders in Children with Inflammatory Bowel Diseases" Life 12, no. 3: 423. https://doi.org/10.3390/life12030423
APA StyleOlczyk, M., Czkwianianc, E., & Socha-Banasiak, A. (2022). Metabolic Bone Disorders in Children with Inflammatory Bowel Diseases. Life, 12(3), 423. https://doi.org/10.3390/life12030423






