Abstract
Malaria, caused by Plasmodium species (spp.), is a deadly parasitic disease that results in approximately 400,000 deaths per year globally. Autophagy pathways play a fundamental role in the developmental stages of the parasite within the mammalian host. They are also involved in the production of Plasmodium-derived extracellular vesicles (EVs), which play an important role in the infection process, either by providing nutrients for parasite growth or by contributing to the immunopathophysiology of the disease. For example, during the hepatic stage, Plasmodium-derived EVs contribute to parasite virulence by modulating the host immune response. EVs help in evading the different autophagy mechanisms deployed by the host for parasite clearance. During cerebral malaria, on the other hand, parasite-derived EVs promote an astrocyte-mediated inflammatory response, through the induction of a non-conventional host autophagy pathway. In this review, we will discuss the cross-talk between Plasmodium-derived microvesicles and autophagy, and how it influences the outcome of infection.
1. Introduction
Malaria, a parasitic disease caused by Plasmodium spp., resulted in more than 229 million clinical cases globally in 2019 [1]. The infection starts with a bite from an infected female Anopheles mosquito, which injects thousands of sporozoites into the mammalian host derma. The sporozoites rapidly travel to the liver, via the bloodstream, to initiate the pre-erythrocytic stage of the infection. They infect the hepatocytes, transform into trophozoites, and, eventually, give rise to thousands of merozoites. The released merozoites invade red blood cells (RBCs) to initiate the erythrocytic stage of the infection, responsible for clinical manifestations of malaria [2,3]. The adhesion and sequestration of the infected RBCs (iRBCs) in various organs is responsible for the severe manifestations of the disease [4]. From the asymptomatic liver stage to the clinical erythrocytic stage, intracellular Plasmodium parasites are always enclosed in the parasitophorous vacuole (PV). This PV is essential for growth and nourishment of the parasite. PV is also important in evading the host immune system, as well as escaping clearance by host autophagy mechanisms [5,6].
Autophagy is an intracellular vesicular-related process that regulates the cell environment against pathological conditions [7,8]. It consists of a highly conserved and synchronized network of autophagy-related (ATG) genes and proteins that promotes the formation of the microtubule-associated protein 1 light chain 3 (MAP1LC3/LC3)-positive phagophore, which becomes a double-membrane structured autophagosome through the acquisition of lipids [8,9,10]. Autophagy actively participates in cellular homeostasis, through the degradation, clearance, and recycling of damaged proteins and organelles from the cytoplasm to autophagosomes, and then to lysosomes [11,12,13].
The parasites release a large number of extracellular vesicles (EVs) during infection, and these contribute to inflammatory processes associated with malaria [14]. EVs are small, lipid bilayer membrane-bound vesicles that are naturally released from almost all types of cells, and they contain proteins, lipids, nucleic acids, and other metabolites [14,15]. They are formed under natural or pathological states during the process of endocytosis and/or autophagy [7,8,16]. They are classified into several subtypes, based on their size, mode of release, origin, and composition. Exosomes (40 to 120 nm in diameter) are the smallest vesicles, generated from endosome invagination [14,17,18]. Microvesicles (50 to 1000 nm in diameter) are formed by the budding of the plasma membrane [14,17,18]. Apoptotic bodies (2000 to 5000 nm in diameter) are the largest vesicles, derived from cells undergoing apoptosis [14,17]. EVs are involved in a wide range of biological processes, including cell-to-cell communication and transport, and the exchange of genetic information, cytosolic proteins, or lipids [18].
EVs have emerged as key players in most parasitic diseases, including malaria. They play an important role in infection biology and immunopathogenesis [14,15,19]. They are implicated in the infectious process, as they are involved not only in providing nutrients for parasite differentiation and multiplication, but are also important players in host–parasite interactions and the activation of the immune response [6,20]. In malaria, EVs are often referred to as microparticles (MPs), and can be of host or parasite origin [14]. Both infected and uninfected endothelial cells, reticulocytes, and platelets release MPs upon interaction with parasites [14,21,22]. EVs were first described in 2004 in Malawian children with cerebral malaria (CM), and, since then, several studies have described not only the presence of EVs during disease, but have also suggested their potential involvement in disease severity. EVs are, therefore, suggested to be a potential biomarker for the severity of the disease [14,23,24]. Plasmodium-derived microvesicles (pMVs) are thought to promote parasite virulence and influence pathogenesis, by modulating host immune responses through intercellular communication [25]. However, both the nature of the cargo and the mechanisms of actions of pMVs remain undefined.
In this paper, we will discuss the cross-talk between pMVs and autophagy, and how this influences the outcome of the infection. We will first focus on the role of autophagy and pMVs in parasite growth and multiplication, before discussing the importance of autophagy in the transfer of pMVs to astrocytes and their role in the pathogenesis of CM.
2. Materials and Methods
2.1. Mice and Parasites
We used female C57BL/6 mice, 8–10 weeks old (Janvier laboratories, C57BL/6JRjFEMELLESPF8). C57BL/6 TLR3-deficient mice lines [26] and C57BL/6.WLA-Berr2 congenic mice ((B6.WLA-Berr1) ECM resistant CMR) [27] were bred and maintained under specific pathogen-free (SPF) conditions at Institute Pasteur Lille animal facility. Experiments were performed in agreement with the ethics of animal experimentation, and were approved by the French animal welfare committee “Ministère de l’Agriculture et de la Pêche” n°A 75485. All experiments carried out on CMR and TLR3KO mice were conducted as described in the previous work published in CMS [28]. Primary cultures of astrocytes and GFP-PbA (1.49L clone, gift of Dr D. Walliker, Institute of Genetics, Edinburgh, UK) were used as previously described in [28].
2.2. Confocal and Transmission Electron Microscopy
Purified astrocytes from CMR and TLR3KO mice were stimulated with GFP-PbA-iRBCs (10:1) at 37 °C for 6 h and removed, then further incubated for 24 h or 48 h. For confocal microscopy, cells were plated on glass slides, and for TEM, on coverslips in 35 mm glass bottom dishes (No 1.5, MatTek, P35G-1.5-10-C), as previously described [28].
2.3. Quantification of Gene Expression
Gene expression quantification was performed using RT-qPCR RT-qPCR on 1 × 106 cells. Cell treatment and processing were conducted as described by Leleu et al. [28].
3. Results
3.1. Host Autophagy Pathways in Malaria
The following two types of autophagy pathways are known to be engaged during the development of Plasmodium in the mammalian host: (a) Non-selective autophagy, which is an adaptive response for cellular remodeling upon unfavorable stress conditions. It is characterized by a double-membrane-bound autophagosome, decorated by microtubule-associated protein 1 light chain 3 beta (LC3-II) molecules. These autophagosomes engulf the parasite, and also support its nourishment during the intrahepatic stage [12,29]. (b) selective autophagy, which is initiated by unc-51-like autophagy-activating kinase-1 (Ulk) and class III phosphatidylinositol 3-kinase (PI3K) complexes. Selective autophagy specifically targets intracellular parasites and promotes their elimination through ubiquitination [8,30,31].
Recently, we have demonstrated the involvement of an unconventional LC3-mediated autophagy pathway (LAP), independent of Ulk1, in the transfer and degradation of P. berghei ANKA (PbA)-MVs inside astrocytes. LAP is an intermediary pathway between autophagy and phagocytosis, involving ATG proteins and the recruitment of LC3-II directly on the single-membrane LAPosome formed around the engulfed microbe, prior to its fusion with lysosomes [32]. The LAP pathway was also found to be an inducer of astrocyte pro-inflammatory responses that play a major role in the pathogenesis of CM [28].
3.2. pMVs and Autophagy-Related Responses during Pre-Erythrocytic Development
During the liver stage of development, packed inside a transient vacuole (TV), the sporozoites injure and traverse several hepatocytes, before finally settling in one to initiate infection [2]. The invading sporozoite is contained in the PV, wherein it first differentiates into a trophozoite, before switching on replicative processes to form a schizont, containing thousands of merozoites. These merozoites egress the hepatocyte in a merosome (see below), invade erythrocytes, and start the blood-stage infection [2,33,34]. The two vacuolar organelles, TV and PV, constitute the first Plasmodium-derived vesicles during the parasitic life cycle. They allow the parasite (i) to escape hepatocyte elimination strategies, and (ii) to use host cell nutrients and develop alternative scavenging pathways for its survival [5,6,35,36]. As summarized in Figure 1A, the following two host cell pathways for clearing the parasite are triggered during PbA sporozoite development in the liver: (1) in the early intrahepatic stage, parasites inside a TV, or in a deficient PV, are cleared by the induction of a PI3P-associated sporozoite elimination (PASE) process that differs from autophagy, but induces a lysosomal acidification pathway; (2) in the late intrahepatic stage, the PV membrane (PVM) engulfed parasite is targeted by LAP-like non-conventional autophagy that occurs independently of PI3K, RB1-inducible coiled-coil 1 (Rb1cc1), and Ulk1, and without the formation of reactive oxygen species (ROS) (Figure 1A) [37,38,39,40]. The absence of PI3K and Becn1 (beclin 1, autophagy related) in this process argues in favor of the involvement of alternative non-canonical autophagy, distinct from LAP, in the elimination of the parasite during this late hepatic stage [37,41]. Interestingly, prolonged expression of LC3-II molecules has been observed on the PVM [41]. The lipidation and incorporation of LC3-II at the PVM is promoted by Atg5, and is necessary for the subsequent binding of ubiquitin, sequestosome 1 (Sqstm11/p62), and neighbor of BRCA1 gene 1 (Nbr1) at the membrane [42]. Boonhok et al. (2016) have described an interferon-gamma (IFN-γ)-mediated non-canonical autophagy machinery for the elimination of P. vivax sporozoites in primary human hepatocyte cultures, called Plasmodium-associated autophagy-related (PAAR) responses (Figure 1A) [43]. PAAR is characterized by the expression of MAPLC3/LC3 on the PVM, and is dependent upon Becn1, PI3K, and Atg5, but not Ulk1 [43]. Although some intra-hepatocyte parasites are eliminated by LAP-like autophagy, others can escape cellular autodigestive elimination [41]. Indeed, to control and avoid elimination by autophagy, mature schizonts remodel LC3+PVM by shedding autophagic proteins trapped in the tubovesicular network (TVN), and by redirecting the lysosome to the TVN [36,39]. In addition, the interaction of PbA, upregulated in infective sporozoites gene 3 (UIS3) protein, with host MAP1LC3/LC3 inhibits the autophagy function, and allows parasite replication [38,44]. In parallel, PbA is able to divert the non-selective host autophagy and exploits it as a nutritive source for supporting parasite growth [41,45,46]. Together, these mechanisms favor the egress of hepatic merozoites, which are released from infected hepatocytes as merosomes, another pMV that consists of hundreds of parasites surrounded by a host cell membrane [47,48,49,50]. During this process, Plasmodium seems to use vesicle encapsulation as a mechanism to hide from host immune mechanisms, and to escape elimination.
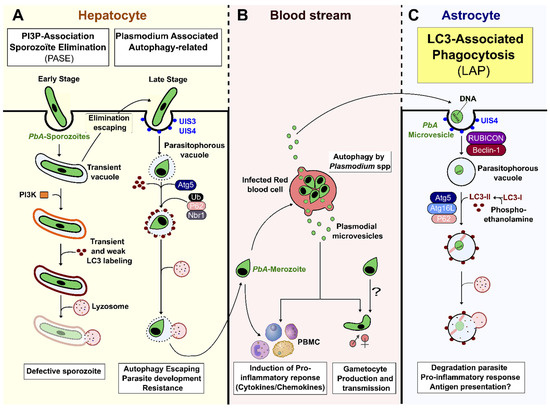
Figure 1.
Host autophagy pathways and Plasmodium-derived microvesicles. (A) Plasmodium sporozoite infects a hepatocyte by invagination of the host cell membrane, thus forming a transient vacuole (TV) or parasitophorous vacuole (PV). If parasites are unable to discard their unnecessary organelles, or correctly remodel their vacuole, the PI3K complex is formed at the vacuole membrane, and leads to parasite elimination by cytosolic lysosomes. Although LC3 is observed transiently at the vacuole membrane, it is not essential for efficient Plasmodium degradation. This elimination, called PI3P-associated sporozoite elimination (PASE), occurs during the early intrahepatic stage. During the late intrahepatic stage, when the parasite starts its differentiation and multiplication, the PVM enclosing the parasite can be labelled by LC3 promoted by Atg5, resulting in the host Plasmodium-associated autophagy-related (PAAR) response. This pathway, associated with ubiquitin, sqstm1/p62, and Nbr1, is independent of PI3k, Rb1cc1, and Ulk complexes, and does not necessarily lead to clearance of the parasite, as it can avoid fusion with lysosomes by remodeling its vacuolar membrane. (B) After leaving the liver, merozoites invade RBCs. The parasite is able to produce Plasmodium-derived microvesicles by autophagy, using PbAATG, in order to transport genetic information and promote gametocytogenesis. However, pMVs can also induce a pro-inflammatory response. (C) PbA-MVs are transferred from iRBCs to astrocytes inside a PVM directly targeted by LC3-II to form a LAPosome. This then fuses with lysosomes, resulting in parasite clearance. This LC3-associated phagocytosis (LAP) pathway, an unconventional autophagy pathway, induces a pro-inflammatory response in astrocytes, leading to ECM.
3.3. Autophagy and pMV Crosstalk during Blood-Stage Infection
Merozoites eventually released from merosomes invade RBCs to initiate the blood stage of infection [3,51]. The parasite uses its autophagy machinery (PfATG) to recycle unnecessary proteins to support its nourishment and biogenesis (Figure 1B). PfATG also allows for survival in the case of nutrient starvation, especially in the intraerythrocytic stage [52]. However, unlike hepatocytes, RBCs do not generate intracellular autophagic host defense mechanisms for parasite clearance [52,53,54]. Interestingly, merosomes are potent immunoregulators. They induce macrophage activation via Toll-like receptor 4 (TLR4) and myeloid differentiation primary response 88 (MyD88) pathways, resulting in an increase in CD40 expression and tumor necrosis factor alpha (TNF-α) secretion [55]. Blood-stage P. falciparum-derived MVs (PfMVs) express parasite antigens capable of stimulating human peripheral blood mononuclear cells (PBMCs) and macrophages, upregulating pro-inflammatory cytokine secretion and activating neutrophil migration [20]. In P. vivax-infected patients, circulating pMVs can adhere to human splenic cells by interacting with intercellular adhesion molecule-1 (ICAM-1) [56]. Recently, PfMVs have been shown to induce natural killer (NK) cell responses during malaria, after interacting with the melanoma differentiation-associated protein 5 (MDA5), a RIG-I-like receptor (RLR) [57]. PMVs isolated from P. falciparum- and P. vivax-infected patients have been shown to transport miRNA and parasite proteins [14,58]. Such vesicles can be internalized by iRBCs, resulting in the transfer of genetic information inside exosome-like vesicles, and these vesicles have been found to promote gametocytogenesis [20,59]. In addition, pMVs derived from human brain endothelial cells stimulate the proliferation/activation of T cells [60,61]. These later observations reinforce the hypothesis of a role for pMVs in the pathogenesis of CM.
3.4. Role of Autophagy-Dependent pMVs in CM
CM is a lethal complication of Plasmodium infection. It causes around 0.4 million deaths per year, principally in children <5 years of age and in immunosuppressed individuals [1]. The pathophysiology is a consequence of the sequestration of iRBCs in brain microvessels and the detrimental immune response, characterized by neuroinflammation and the lymphocyte migration that results from it [62,63,64,65]. In C57BL/6 CM-susceptible (CMS) mice infected with PbA, astrocytes are activated early during infection, and release pro-inflammatory factors that contribute to neuroinflammation and death [66,67,68]. We have previously described the in vitro transfer of PbA-MVs to astrocytes after contact with iRBCs (Figure 1C) [68]. We propose that iRBCs located in the perivascular space could interact with pseudopodia from activated astrocytes during neuropathogenesis [66,69]. As shown in Figure 2A, the transfer of PbA-MVs occurs at a contact point between the astrocytic foot and PbA-iRBCs in CMS_derived primary astrocyte cultures stimulated for 6 h. This transfer of PbA-MVs to astrocytes occurs via the unconventional LAP autophagy pathway, which is independent of Ulk1 and calcium binding and coiled-coil domain 2 (Calcoco2/Ndp52) [28] (Figure 1C). The LAP pathway is activated via Becn1, the RUN domain, and the cysteine-rich domain, containing Beclin 1-interacting protein (Rubcn), Atg16L1, Atg5, and Sqstm1/p62. The parasite material transferred to the astrocyte is contained in PV-expressing LC3-II molecules after activation. LAP mediates the degradation of PbA-MVs in astrocytes (Figure 2B,C). Eventually, an organelle called LAPosome fuses with lysosomes to degrade PbA-MVs (Figure 2B,C). The treatment of PbA CMS mice with bafilomycin A1, an autophagy inhibitor, has been shown to prevent ECM [28]. Confocal microscopy revealed that, in contrast, in CMS-derived astrocytes in CMR-derived cells, PbA-MVs transferred from iRBCs were not internalized, but, instead, remained at the astrocyte extracellular membrane (Figure 2B). Indeed, RT-qPCR only detected a small quantity of P. berghei 18S ribosomal (PB18S) gene in these astrocytes (Figure 2C). Unlike in the case of CMS-derived astrocytes, increased expression of MAP1LC3/LC3, ATG5 and ATG16L1 LAP autophagy genes was not observed in CMR-derived cells (Figure 2D).
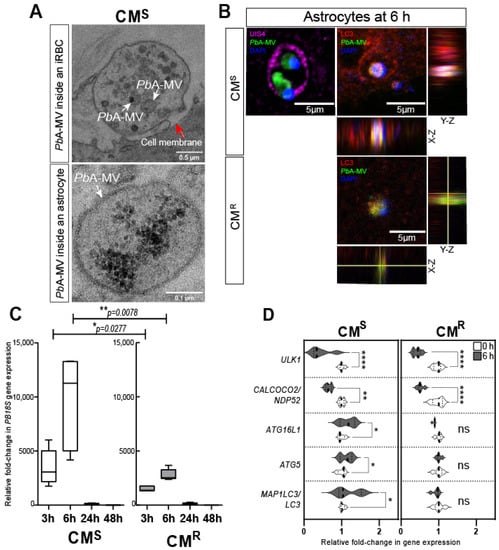
Figure 2.
PbA-MVs transfer to astrocytes from ECM-sensitive (CMS) and -resistant (CMR) mice upon 6-h contact with iRBCs. Primary astrocyte cultures derived from neonatal CMS or CMR mice were stimulated for 6 h with GFP-PbA-iRBCs, washed, and followed for 48 h post iRBC contact, as previously described [28]. (A) Transmission electron microscopy revealed that PbA-MVs were transferred to CMS-derived astrocytes at the contact point with iRBCs (top micrograph, white arrows) and were observed intracellularly (bottom micrograph, white arrows) at the 6-h time point. The red arrow shows the cell membrane of the astrocyte. (B) GFP-PbA-MV (green) and parasite DNA (blue) are enclosed inside a PV labelled by UIS4 (pink; left panel) or LC3-II (red; right panel) to form a LAPosome inside CMS-derived astrocytes. By contrast, PbA-MVs remained at the cell membrane of CMR-derived astrocytes. (C) The quantity of PB18S gene detected in CMR-derived astrocytes was significantly lower than that found in CMS-derived cells, confirming reduced transfer of PbA-MVs in these cells. (D) LAP-related gene expression did not increase in CMR-derived astrocytes, as compared to CMS-derived cells, 6 h after PbA-iRBC contact. Student’s t-test was used to compare median fold change in gene expression in panels C and D (n = 5). * p < 0.05; ** p < 0.01; **** p < 0.0001.
Astrocytes are key players in the innate immune response in the brain [68,70,71]. During malaria, they secrete pro-inflammatory cytokines/chemokines, and express major histocompatibility complex (MHC) class I and II molecules [66,67,68]. We demonstrated that the secretion of chemokine (C-X-C motif) ligand 10 (CXCL10), chemokine (C-C motif) ligand 2 (CCL2), and TNF-α by astrocytes is dependent on the transfer and degradation of PbA-MVs by the LAP pathway [28]. It is known that the production of CXCL10 in the brain is a prerequisite for the recruitment of effector CD8+ T cells expressing the chemokine (C-X-C motif) receptor 3 (CXCR3) involved in ECM [65,67,72,73]. We also evidenced that astrocytes from CMR mice produced significantly lower levels of pro-inflammatory cytokines/chemokines than CMS-derived cells after stimulation with PbA-iRBCs (Figure 3A). This is probably due to the smaller number of PbA-MVs transferred to astrocytes from CMR mice. However, we also observed upregulation of leukaemia inhibitory factor (LIF), transforming growth factor beta (TGF-β), and interleukin 10 (IL10) gene expressions in CMS-derived astrocytes after contact with PbA-iRBCs (Figure 3A).
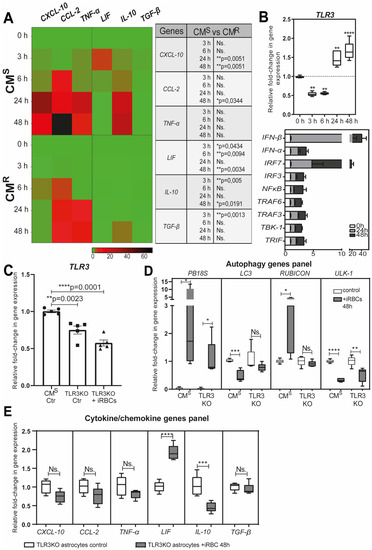
Figure 3.
Immune response of astrocytes after PbA-MV transfer and TLR3 engagement. Primary astrocyte cultures, derived from CMS, CMR, or TLR3KO neonatal mice, were stimulated for 6 h with GFP-PbA-iRBCs. The cells were then washed and followed up for 48 h post iRBC contact. (A) Pro-inflammatory CXCL-10, CCL-2, and TNF-α genes and anti-inflammatory cytokine LIF, IL10, and TGF-β genes were highly expressed in CMS-derived astrocytes, as compared to CMR-derived cells. (B) TLR3 pathway genes were significantly upregulated in CMS-derived astrocytes after 24 h of contact with PbA-iRBCs. (C) TLR3 gene expression was totally abolished in TLR3KO-derived astrocytes. (D) Decreased PB18S gene expression and downregulation of autophagy-related genes (LC3, RUBCN, and ULK1) were observed in TLR3KO-derived, but not CMS-derived, astrocytes at 48 h after iRBC stimulation. White bars indicate 0-h stimulation and grey bars indicate 48-h stimulation. (E) The absence of CXCL-10, CCL-2, TNF-α, IL-10, and TGF-β gene expression in TLR3KO-derived astrocytes suggests involvement of the TLR3 pathway in the astrocyte immune response. Student’s t-test (except for (B), where one-way ANOVA was used) was used to compare median fold change (n = 5). * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Activated astrocytes express pattern recognition receptors (PRRs), such as TLRs, which are key sensors of danger, and are involved in the initiation of the brain’s innate immune response [74,75,76]. After 24 h of parasite contact, we observed upregulation of TLR3 gene expression in CMS-derived astrocytes (Figure 3B). The expression of genes of pro-inflammatory signaling molecules, such as TIR domain-containing adapter-inducible interferon-β (TRIF), TNF receptor-associated factor 6 (TRAF6), TRAF3, TANK-binding kinase 1 (TBK1), interferon regulatory factor 3 (IRF3), IRF7, and IFN-β, was also increased (Figure 3B). In contrast to what was observed in astrocytes derived from CMS mice, in ECM-resistant TLR3 knockout (KO) mice, the expression of PB18S and autophagy genes was significantly decreased following PbA-MVs transfer in astrocytes (Figure 3C,D). In addition, the production of CXCL10, CCL2, TNF-α, IL-10, and TGF-β was totally abolished in astrocytes from TLR3KO mice, unlike in cells from CMS mice (Figure 3E). These observations strongly suggest a link between the LAP pathway and TLR3-dependent pathway in the transfer of PbA-MVs and the induction of pro-inflammatory cytokine and chemokine responses in astrocytes from CMS mice. Therefore, a TLR3-TRIF-dependent pathway could also participate in neuroinflammation during ECM, similar to what has been reported for the intrahepatic stage [77].
4. Discussion
This review examines the role of autophagy in the different developmental stages of the Plasmodium parasite. Autophagy is involved in a multitude of parasite biological processes, such as the regulation of intracellular cytoplasmic protein turnover, organelle differentiation, parasite growth, gametogenesis, and infection dissemination. By virtue of its involvement in the biogenesis and function of Plasmodium-derived EVs, autophagy also influences the stimulation of pro-inflammatory responses of innate immune cells and, hence, the severity of the disease.
Our recent description of the involvement of LAP in the transfer of PbA-derived MVs to astrocytes, and the resulting induction of pro-inflammatory factors, exemplifies the importance of MVs in host–parasite interactions and infection outcomes. We also found that a TLR3-mediated anti-inflammatory response is induced in astrocytes after contact with parasite MVs. These observations suggest a dual, but contrasting, role for autophagy in parasite–astrocyte interactions. Autophagy can favor either (i) detrimental neuroinflammation, through the production of CXCL-10 that exacerbates the inflammatory process, or (ii) a protective outcome, resulting from the production of neuroimmunomodulators, such as LIF, which downregulate the exacerbated inflammation and prevent T cells from infiltrating the brain [78]. Through the genesis of parasite-derived EVs, LAP may also influence antigen presentation by astrocytes to pathogenic CD8+ T cells that have migrated to the brain. It is important to note that the astrocytes’ production of pro-/anti-inflammatory factors is regulated by crucial molecular events that are responsible for neuropathology during malaria. These events need to be explored further (Figure 4).
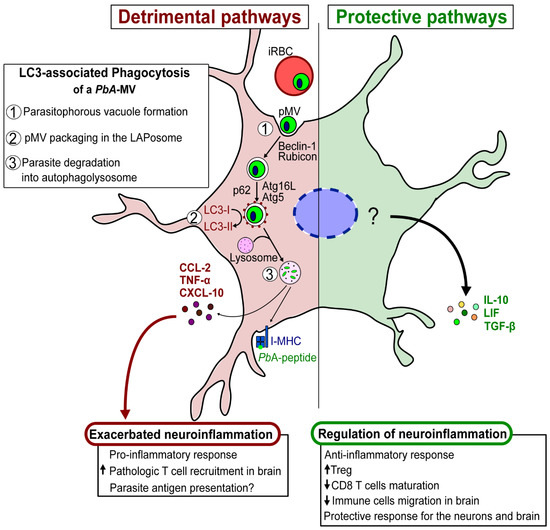
Figure 4.
Schematic hypothesis of detrimental/protective pathways of astrocytes involved in the pathogenesis of ECM.
In summary, Plasmodium-derived MVs interact with autophagy pathways to contribute to protection/pathology during malaria. On the one hand, they promote parasite clearance, stimulate a pro-inflammatory innate immune response, and contribute to the downregulation of brain inflammation during CM. On the other hand, they participate in the dissemination of the parasite within the host, as well as in its differentiation to sexual forms. They also contribute to the activation of pro-inflammatory innate responses. Besides this, they can precipitate severe disease by promoting antigen presentation to pathological CD8+ T cells by astrocytes that infiltrate the brain during CM.
Author Contributions
Conceptualization: S.P.; Methodology: I.L., P.-A.C., J.R. and S.P.; Formal analysis: I.L.; Investigation: I.L.; Writing—Original Draft Preparation: I.L., J.A. and S.P.; Writing—Review & Editing: I.L., J.A., P.-A.C., J.R. and S.P.; Supervision: S.P.; Funding Acquisition: S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the LabEx PARAFRAP: ANR-11-LABX-0024 and the Grand Projet Fédérateur-GPF “Brain and Infection” Institute Pasteur Paris.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We gratefully acknowledge Sulabha Pathak and Corine Glineur for discussions and comments during the writing of the manuscript; the PLETHA platform of Pasteur Institute of Lille for assistance and animal maintenance; the BiCell platform for confocal microscopy facility at the Pasteur Institute of Lille; Shabdda communications for content editing.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ATG: autophagy related; Becn1: beclin 1, autophagy related; CCL2: chemokine (C-C motif) ligand 2; CMS/R: cerebral malaria sensitive/resistant; CXCL10: chemokine (C-X-C motif) ligand 10; ECM: experimental cerebral malaria; EV: extracellular vesicles; GFP: green fluorescent protein; IL10: interleukin 10; iRBCs: infected red blood cells; IRF3/7: interferon regulatory factor 3/7; KO: knockout; LAP: LC3-associated phagocytosis; LIF: leukemia inhibitory factor; MAP1LC3/LC3: microtubule-associated protein 1 light chain 3; PAAR: Plasmodium-associated autophagy related; PB18S: Plasmodium berghei 18S ribosomal gene; PbA/P. berghei ANKA: Plasmodium berghei ANKA; PbA-MVs: P. berghei ANKA-microvesicles; Pf: Plasmodium falciparum; PI3K: phosphatidylinositol 3-kinase; pMV: Plasmodium-derived MV; PV: parasitophorous vacuole; PV/M: parasitophorous vacuole membrane; Rubcn/rubicon: RUN domain and cysteine-rich domain containing beclin 1-interacting protein; Sqstm1/p62: sequestosome 1; TGF-β: transforming growth factor beta; TLR: Toll-like receptors; TNF-α: tumor necrosis factor alpha; TV: transient vacuole; ULK1: unc-51-like kinase 1.
References
- World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001579-1. [Google Scholar]
- Frischknecht, F.; Matuschewski, K. Plasmodium Sporozoite Biology. Cold Spring Harb. Perspect. Med. 2017, 7, a025478. [Google Scholar] [CrossRef] [Green Version]
- Shears, M.J.; Sekhar Nirujogi, R.; Swearingen, K.E.; Renuse, S.; Mishra, S.; Jaipal Reddy, P.; Moritz, R.L.; Pandey, A.; Sinnis, P. Proteomic Analysis of Plasmodium Merosomes: The Link between Liver and Blood Stages in Malaria. J. Proteome Res. 2019, 18, 3404–3418. [Google Scholar] [CrossRef] [PubMed]
- Franke-Fayard, B.; Fonager, J.; Braks, A.; Khan, S.M.; Janse, C.J. Sequestration and Tissue Accumulation of Human Malaria Parasites: Can We Learn Anything from Rodent Models of Malaria? PLoS Pathog. 2010, 6, e1001032. [Google Scholar] [CrossRef] [Green Version]
- Lüder, C.G.K.; Stanway, R.R.; Chaussepied, M.; Langsley, G.; Heussler, V.T. Intracellular Survival of Apicomplexan Parasites and Host Cell Modification. Int. J. Parasitol. 2009, 39, 163–173. [Google Scholar] [CrossRef]
- Nyboer, B.; Heiss, K.; Mueller, A.-K.; Ingmundson, A. The Plasmodium Liver-Stage Parasitophorous Vacuole: A Front-Line of Communication between Parasite and Host. Int. J. Med. Microbiol. 2018, 308, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Levine, B. Autophagy, Immunity, and Microbial Adaptations. Cell Host Microbe 2009, 5, 527–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinsztein, D.C.; Bento, C.F.; Deretic, V. Therapeutic Targeting of Autophagy in Neurodegenerative and Infectious Diseases. J. Exp. Med. 2015, 212, 979–990. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The Role of Atg Proteins in Autophagosome Formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and Medical Implications of Mammalian Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- He, C.; Klionsky, D.J. Regulation Mechanisms and Signaling Pathways of Autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in Immunity and Inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Saitoh, T.; Akira, S. Autophagy in Infection, Inflammation and Immunity. Nat. Rev. Immunol. 2013, 13, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, N.G.; Cheng, L.; Eriksson, E.M. The Role of Extracellular Vesicles in Malaria Biology and Pathogenesis. Malar. J. 2017, 16, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Wang, L.; Li, J.; Wang, L.; Wu, Z.; Sun, X. Extracellular Vesicle-Mediated Communication Within Host-Parasite Interactions. Front. Immunol. 2019, 9, 3066. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Debs, S.; Cohen, A.; Hosseini-Beheshti, E.; Chimini, G.; Hunt, N.H.; Grau, G.E.R. Interplay of Extracellular Vesicles and Other Players in Cerebral Malaria Pathogenesis. Biochim. Biophys. Acta BBA-Gen. Subj. 2019, 1863, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mantel, P.-Y.; Hoang, A.N.; Goldowitz, I.; Potashnikova, D.; Hamza, B.; Vorobjev, I.; Ghiran, I.; Toner, M.; Irimia, D.; Ivanov, A.R.; et al. Malaria-Infected Erythrocyte-Derived Microvesicles Mediate Cellular Communication within the Parasite Population and with the Host Immune System. Cell Host Microbe 2013, 13, 521–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combes, V.; Coltel, N.; Faille, D.; Wassmer, S.C.; Grau, G.E. Cerebral Malaria: Role of Microparticles and Platelets in Alterations of the Blood–Brain Barrier. Int. J. Parasitol. 2006, 36, 541–546. [Google Scholar] [CrossRef]
- Faille, D.; Combes, V.; Mitchell, A.J.; Fontaine, A.; Juhan-Vague, I.; Alessi, M.-C.; Chimini, G.; Fusaï, T.; Grau, G.E. Platelet Microparticles: A New Player in Malaria Parasite Cytoadherence to Human Brain Endothelium. FASEB J. 2009, 23, 3449–3458. [Google Scholar] [CrossRef] [PubMed]
- Combes, V.; Coltel, N.; Alibert, M.; van Eck, M.; Raymond, C.; Juhan-Vague, I.; Grau, G.E.; Chimini, G. ABCA1 Gene Deletion Protects against Cerebral Malaria. Am. J. Pathol. 2005, 166, 295–302. [Google Scholar] [CrossRef]
- El-Assaad, F.; Wheway, J.; Hunt, N.H.; Grau, G.E.R.; Combes, V. Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria. PLoS Pathog. 2014, 10, e1003839. [Google Scholar] [CrossRef] [Green Version]
- Babatunde, K.A.; Yesodha Subramanian, B.; Ahouidi, A.D.; Martinez Murillo, P.; Walch, M.; Mantel, P.-Y. Role of Extracellular Vesicles in Cellular Cross Talk in Malaria. Front. Immunol. 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of Double-Stranded RNA and Activation of NF-ΚB by Toll-like Receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Keswani, T.; Roland, J.; Herbert, F.; Delcroix-Genete, D.; Bauderlique-Le Roy, H.; Gaayeb, L.; Cazenave, P.-A.; Pied, S. Expression of CD300lf by Microglia Contributes to Resistance to Cerebral Malaria by Impeding the Neuroinflammation. Genes Immun. 2020, 21, 45–62. [Google Scholar] [CrossRef]
- Leleu, I.; Genete, D.; Desnoulez, S.S.; Saidi, N.; Brodin, P.; Lafont, F.; Tomavo, S.; Pied, S. A Noncanonical Autophagy Is Involved in the Transfer of Plasmodium-Microvesicles to Astrocytes. Autophagy 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Coppens, I. Metamorphoses of Malaria: The Role of Autophagy in Parasite Differentiation. Essays Biochem. 2011, 51, 127–136. [Google Scholar] [CrossRef]
- Tsukada, M.; Ohsumi, Y. Isolation and Characterization of Autophagy-Defective Mutants of Saccharomyces cerevisiae. FEBS Lett. 1993, 333, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Hara, T.; Takamura, A.; Kishi, C.; Iemura, S.; Natsume, T.; Guan, J.-L.; Mizushima, N. FIP200, a ULK-Interacting Protein, Is Required for Autophagosome Formation in Mammalian Cells. J. Cell Biol. 2008, 181, 497–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, S.; Arasteh, M.; Jefferson, M.; Pearson, T.; Wang, Y.; Zhang, W.; Bicsak, B.; Divekar, D.; Powell, P.P.; Naumann, R.; et al. The ATG5-Binding and Coiled Coil Domains of ATG16L1 Maintain Autophagy and Tissue Homeostasis in Mice Independently of the WD Domain Required for LC3-Associated Phagocytosis. Autophagy 2019, 15, 599–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, M.M. Migration of Plasmodium Sporozoites Through Cells Before Infection. Science 2001, 291, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Loubens, M.; Vincensini, L.; Fernandes, P.; Briquet, S.; Marinach, C.; Silvie, O. Plasmodium Sporozoites on the Move: Switching from Cell Traversal to Productive Invasion of Hepatocytes. Mol. Microbiol. 2021, 115, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Latré de Laté, P.; Pineda, M.; Harnett, M.; Harnett, W.; Besteiro, S.; Langsley, G. Apicomplexan Autophagy and Modulation of Autophagy in Parasite-Infected Host Cells. Biomed. J. 2017, 40, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Agop-Nersesian, C.; De Niz, M.; Niklaus, L.; Prado, M.; Eickel, N.; Heussler, V.T. Shedding of Host Autophagic Proteins from the Parasitophorous Vacuolar Membrane of Plasmodium Berghei. Sci. Rep. 2017, 7, 2191. [Google Scholar] [CrossRef] [Green Version]
- Wacker, R.; Eickel, N.; Schmuckli-Maurer, J.; Annoura, T.; Niklaus, L.; Khan, S.M.; Guan, J.-L.; Heussler, V.T. LC3-Association with the Parasitophorous Vacuole Membrane of Plasmodium Berghei Liver Stages Follows a Noncanonical Autophagy Pathway. Cell. Microbiol. 2017, 19, e12754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Real, E.; Rodrigues, L.; Cabal, G.G.; Enguita, F.J.; Mancio-Silva, L.; Mello-Vieira, J.; Beatty, W.; Vera, I.M.; Zuzarte-Luís, V.; Figueira, T.N.; et al. Plasmodium UIS3 Sequesters Host LC3 to Avoid Elimination by Autophagy in Hepatocytes. Nat. Microbiol. 2018, 3, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niklaus, L.; Agop-Nersesian, C.; Schmuckli-Maurer, J.; Wacker, R.; Grünig, V.; Heussler, V.T. Deciphering Host Lysosome-Mediated Elimination of Plasmodium Berghei Liver Stage Parasites. Sci. Rep. 2019, 9, 7967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bindschedler, A.; Wacker, R.; Egli, J.; Eickel, N.; Schmuckli-Maurer, J.; Franke-Fayard, B.M.; Janse, C.J.; Heussler, V.T. Plasmodium Berghei Sporozoites in Nonreplicative Vacuole Are Eliminated by a PI3P-mediated Autophagy-independent Pathway. Cell. Microbiol. 2021, 23, e13271. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.; Eickel, N.; De Niz, M.; Heitmann, A.; Agop-Nersesian, C.; Wacker, R.; Schmuckli-Maurer, J.; Caldelari, R.; Janse, C.J.; Khan, S.M.; et al. Long-Term Live Imaging Reveals Cytosolic Immune Responses of Host Hepatocytes against Plasmodium Infection and Parasite Escape Mechanisms. Autophagy 2015, 11, 1561–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmuckli-Maurer, J.; Reber, V.; Wacker, R.; Bindschedler, A.; Zakher, A.; Heussler, V.T. Inverted Recruitment of Autophagy Proteins to the Plasmodium Berghei Parasitophorous Vacuole Membrane. PLoS ONE 2017, 12, e0183797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonhok, R.; Rachaphaew, N.; Duangmanee, A.; Chobson, P.; Pattaradilokrat, S.; Utaisincharoen, P.; Sattabongkot, J.; Ponpuak, M. LAP-like Process as an Immune Mechanism Downstream of IFN-γ in Control of the Human Malaria Plasmodium vivax Liver Stage. Proc. Natl. Acad. Sci. USA 2016, 113, E3519–E3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Z.; Klionsky, D.J. Plasmodium Protein UIS3 Protects the Parasite from Autophagy Clearance. Autophagy 2018, 14, 1291–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes da Silva, M.; Thieleke-Matos, C.; Cabrita-Santos, L.; Ramalho, J.S.; Wavre-Shapton, S.T.; Futter, C.E.; Barral, D.C.; Seabra, M.C. The Host Endocytic Pathway Is Essential for Plasmodium Berghei Late Liver Stage Development: Plasmodium Interaction with Host Endocytic Pathway. Traffic 2012, 13, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Thieleke-Matos, C.; Lopes da Silva, M.; Cabrita-Santos, L.; Portal, M.D.; Rodrigues, I.P.; Zuzarte-Luis, V.; Ramalho, J.S.; Futter, C.E.; Mota, M.M.; Barral, D.C.; et al. Host Cell Autophagy Contributes to Plasmodium Liver Development. Cell. Microbiol. 2016, 18, 437–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graewe, S.; Rankin, K.E.; Lehmann, C.; Deschermeier, C.; Hecht, L.; Froehlke, U.; Stanway, R.R.; Heussler, V. Hostile Takeover by Plasmodium: Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress. PLoS Pathog. 2011, 7, e1002224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burda, P.-C.; Caldelari, R.; Heussler, V.T. Manipulation of the Host Cell Membrane during Plasmodium Liver Stage Egress. mBio 2017, 8, e00139-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prudêncio, M.; Rodriguez, A.; Mota, M.M. The Silent Path to Thousands of Merozoites: The Plasmodium Liver Stage. Nat. Rev. Microbiol. 2006, 4, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A. Manipulation of Host Hepatocytes by the Malaria Parasite for Delivery into Liver Sinusoids. Science 2006, 313, 1287–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowman, A.F.; Crabb, B.S. Invasion of Red Blood Cells by Malaria Parasites. Cell 2006, 124, 755–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervantes, S.; Bunnik, E.M.; Saraf, A.; Conner, C.M.; Escalante, A.; Sardiu, M.E.; Ponts, N.; Prudhomme, J.; Florens, L.; Le Roch, K.G. The Multifunctional Autophagy Pathway in the Human Malaria Parasite, Plasmodium falciparum. Autophagy 2014, 10, 80–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlins, A.M.; Ben-Rached, F.; Williams, R.A.; Proto, W.R.; Coppens, I.; Ruch, U.; Gilberger, T.W.; Coombs, G.H.; Mottram, J.C.; Müller, S.; et al. Plasmodium Falciparum ATG8 Implicated in Both Autophagy and Apicoplast Formation. Autophagy 2013, 9, 1540–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, P.G.T.; Griffin, J.T.; Cairns, M.; Rogerson, S.J.; van Eijk, A.M.; ter Kuile, F.; Ghani, A.C. A Model of Parity-Dependent Immunity to Placental Malaria. Nat. Commun. 2013, 4, 1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couper, K.N.; Barnes, T.; Hafalla, J.C.R.; Combes, V.; Ryffel, B.; Secher, T.; Grau, G.E.; Riley, E.M.; de Souza, J.B. Parasite-Derived Plasma Microparticles Contribute Significantly to Malaria Infection-Induced Inflammation through Potent Macrophage Stimulation. PLoS Pathog. 2010, 6, e1000744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toda, H.; Diaz-Varela, M.; Segui-Barber, J.; Roobsoong, W.; Baro, B.; Garcia-Silva, S.; Galiano, A.; Gualdrón-López, M.; Almeida, A.C.G.; Brito, M.A.M.; et al. Plasma-Derived Extracellular Vesicles from Plasmodium Vivax Patients Signal Spleen Fibroblasts via NF-KB Facilitating Parasite Cytoadherence. Nat. Commun. 2020, 11, 2761. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chew, M.; Hou, J.; Lai, F.; Leopold, S.J.; Loo, H.L.; Ghose, A.; Dutta, A.K.; Chen, Q.; Ooi, E.E.; et al. Microvesicles from Malaria-Infected Red Blood Cells Activate Natural Killer Cells via MDA5 Pathway. PLoS Pathog. 2018, 14, e1007298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketprasit, N.; Cheng, I.S.; Deutsch, F.; Tran, N.; Imwong, M.; Combes, V.; Palasuwan, D. The Characterization of Extracellular Vesicles-Derived MicroRNAs in Thai Malaria Patients. Malar. J. 2020, 19, 285. [Google Scholar] [CrossRef] [PubMed]
- Regev-Rudzki, N.; Wilson, D.W.; Carvalho, T.G.; Sisquella, X.; Coleman, B.M.; Rug, M.; Bursac, D.; Angrisano, F.; Gee, M.; Hill, A.F.; et al. Cell-Cell Communication between Malaria-Infected Red Blood Cells via Exosome-like Vesicles. Cell 2013, 153, 1120–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheway, J.; Obeid, S.; Couraud, P.-O.; Combes, V.; Grau, G.E.R. The Brain Microvascular Endothelium Supports T Cell Proliferation and Has Potential for Alloantigen Presentation. PLoS ONE 2013, 8, e52586. [Google Scholar] [CrossRef]
- Wheway, J.; Latham, S.L.; Combes, V.; Grau, G.E.R. Endothelial Microparticles Interact with and Support the Proliferation of T Cells. J. Immunol. 2014, 193, 3378–3387. [Google Scholar] [CrossRef] [Green Version]
- Bagot, S.; Nogueira, F.; Collette, A.; do Rosario, V.; Lemonier, F.; Cazenave, P.-A.; Pied, S. Comparative Study of Brain CD8+ T Cells Induced by Sporozoites and Those Induced by Blood-Stage Plasmodium Berghei ANKA Involved in the Development of Cerebral Malaria. Infect. Immun. 2004, 72, 2817–2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collette, A.; Bagot, S.; Ferrandiz, M.E.; Cazenave, P.-A.; Six, A.; Pied, S. A Profound Alteration of Blood TCRB Repertoire Allows Prediction of Cerebral Malaria. J. Immunol. 2004, 173, 4568–4575. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.G.; Pamplona, A.; Pena, A.C.; Mota, M.M.; Pied, S.; Vigário, A.M. Accumulation of Plasmodium berghei -Infected Red Blood Cells in the Brain Is Crucial for the Development of Cerebral Malaria in Mice. Infect. Immun. 2010, 78, 4033–4039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigário, A.M.; Gorgette, O.; Dujardin, H.C.; Cruz, T.; Cazenave, P.-A.; Six, A.; Bandeira, A.; Pied, S. Regulatory CD4+CD25+ Foxp3+ T Cells Expand during Experimental Plasmodium Infection but Do Not Prevent Cerebral Malaria. Int. J. Parasitol. 2007, 37, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.N.; Stewart-Hutchinson, P.J.; Strangward, P.; Dandamudi, D.B.; Coles, J.A.; Villegas-Mendez, A.; Gallego-Delgado, J.; van Rooijen, N.; Zindy, E.; Rodriguez, A.; et al. Perivascular Arrest of CD8+ T Cells Is a Signature of Experimental Cerebral Malaria. PLoS Pathog. 2015, 11, e1005210. [Google Scholar] [CrossRef] [PubMed]
- Dalko, E.; Genete, D.; Auger, F.; Dovergne, C.; Lambert, C.; Herbert, F.; Cazenave, P.-A.; Roland, J.; Pied, S. Heme Dampens T-Cell Sequestration by Modulating Glial Cell Responses during Rodent Cerebral Malaria. Brain. Behav. Immun. 2016, 58, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.K.; Dalko, E.; Delcroix-Genete, D.; Herbert, F.; Cazenave, P.-A.; Pied, S. Uptake of Parasite-Derived Vesicles by Astrocytes and Microglial Phagocytosis of Infected Erythrocytes May Drive Neuroinflammation in Cerebral Malaria: Plasmodium Interaction with Glial Cells. Glia 2017, 65, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Strangward, P.; Haley, M.J.; Shaw, T.N.; Schwartz, J.-M.; Greig, R.; Mironov, A.; de Souza, J.B.; Cruickshank, S.M.; Craig, A.G.; Milner, D.A.; et al. A Quantitative Brain Map of Experimental Cerebral Malaria Pathology. PLoS Pathog. 2017, 13, e1006267. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Trojanowski, P.J.; Villanueva, E.; Navarro, E.; Godbout, J.P. Sequential Activation of Microglia and Astrocyte Cytokine Expression Precedes Increased Iba-1 or GFAP Immunoreactivity Following Systemic Immune Challenge: Iba1 and GFAP Are Unreliable Activation Markers. Glia 2016, 64, 300–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liddelow, S.A.; Marsh, S.E.; Stevens, B. Microglia and Astrocytes in Disease: Dynamic Duo or Partners in Crime? Trends Immunol. 2020, 41, 820–835. [Google Scholar] [CrossRef] [PubMed]
- Belnoue, E.; Kayibanda, M.; Vigario, A.M.; Deschemin, J.-C.; van Rooijen, N.; Viguier, M.; Snounou, G.; Rénia, L. On the Pathogenic Role of Brain-Sequestered Aβ CD8 + T Cells in Experimental Cerebral Malaria. J. Immunol. 2002, 169, 6369–6375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofield, L.; Grau, G.E. Immunological Processes in Malaria Pathogenesis. Nat. Rev. Immunol. 2005, 5, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes Are Active Players in Cerebral Innate Immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Huda, S.; Sinha Babu, S.P. Toll-like Receptor Polymorphism in Host Immune Response to Infectious Diseases: A Review. Scand. J. Immunol. 2019, 90, e12771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Acioglu, C.; Heary, R.F.; Elkabes, S. Role of Astroglial Toll-like Receptors (TLRs) in Central Nervous System Infections, Injury and Neurodegenerative Diseases. Brain. Behav. Immun. 2021, 91, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Keswani, T.; Delcroix-Genete, D.; Herbert, F.; Leleu, I.; Lambert, C.; Draheim, M.; Salome-Desnoulez, S.; Saliou, J.M.; Cazenave, P.-A.; Silvie, O.; et al. Plasmodium Yoelii Uses a TLR3-Dependent Pathway to Achieve Mammalian Host Parasitism. J. Immunol. 2020, 205, 3071–3082. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.M.; Collier, L.A.; Winford, E.D.; Leonardo, C.C.; Ajmo, C.T.; Foran, E.A.; Kopper, T.J.; Gensel, J.C.; Pennypacker, K.R. Leukemia Inhibitory Factor Modulates the Peripheral Immune Response in a Rat Model of Emergent Large Vessel Occlusion. J. Neuroinflamm. 2018, 15, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).