Total Wrist Arthroplasty—A Systematic Review of the Outcome, and an Introduction of FreeMove—An Approach to Improve TWA
Abstract
1. Introduction
2. Material and Method
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Reviews
2.5. Quality Assessment and Handling of Data
2.6. General Demographic Data
2.7. Statistical Analysis
3. Results
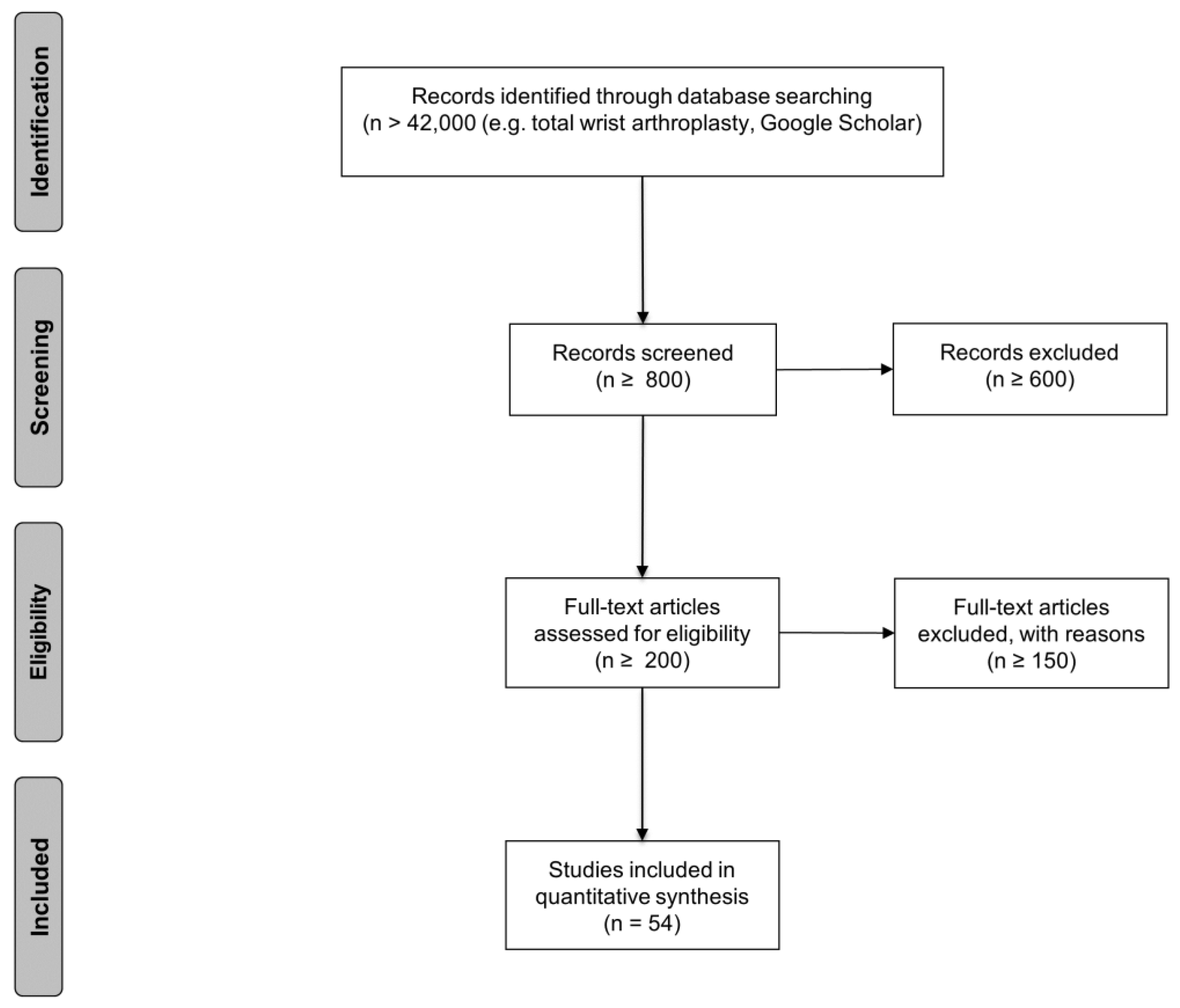
3.1. Study Selection
3.2. Selected Publications
3.3. Included Prosthesis Models
3.4. Primary Outcome—Duration of Implants
3.5. Secondary Outcome—Patient-Reported Measures of Pain
3.6. Secondary Outcome—Patient-Reported Measures of Function
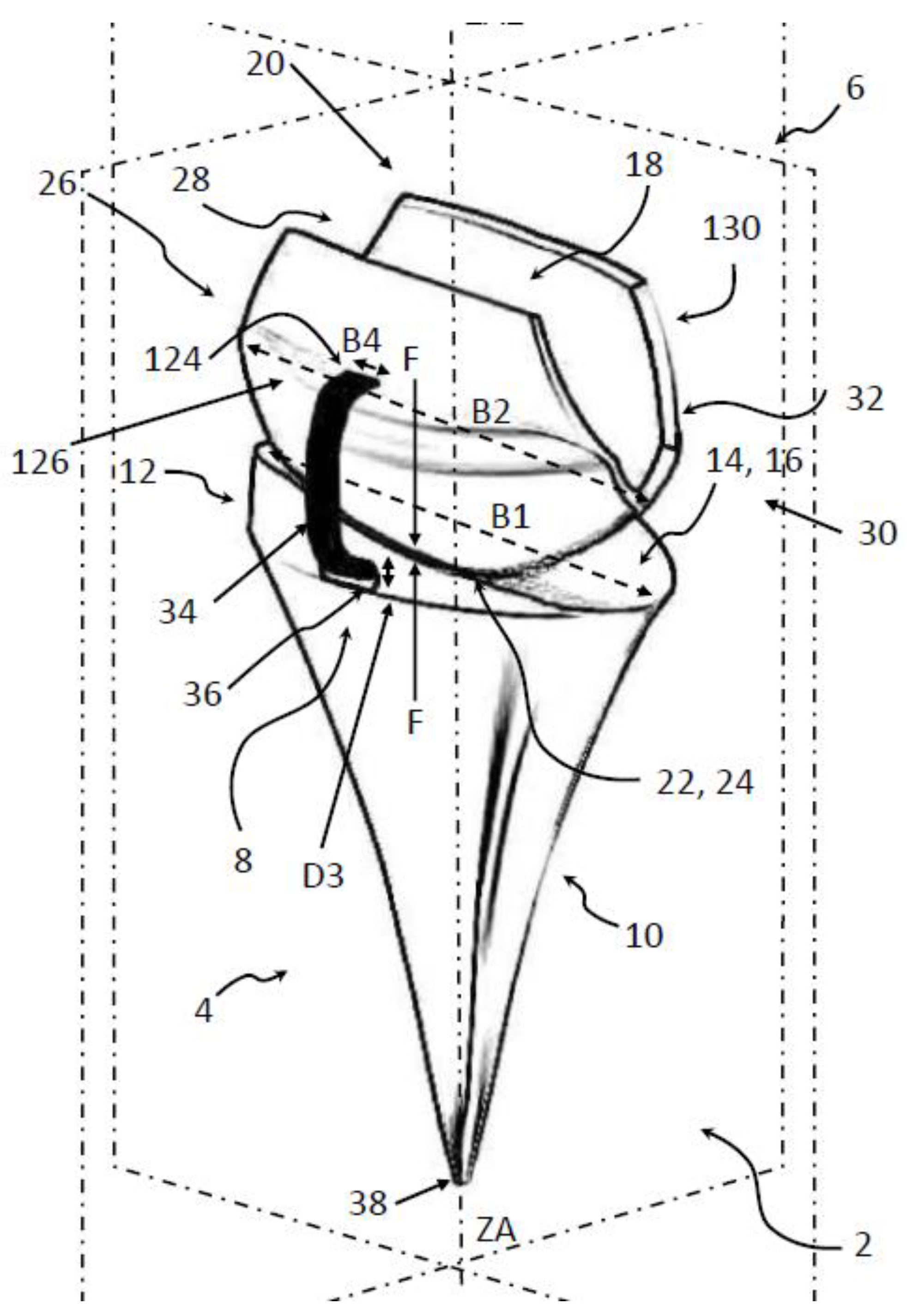
4. FreeMove—A New Approach for TWA
4.1. Introducing the Concept of FreeMove
4.2. The Principal Idea of FreeMove
4.3. Conclusion and Future Work Concerning FreeMove
5. Discussion
5.1. Duration
5.2. Pain
5.3. Disabilities of the Arm, Shoulder, and Hand (DASH)
5.4. Grip Strength
5.5. Range of Motion
5.6. Limitations in General
5.7. Methodological Quality of Included Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Trail, I.A.; Stanley, J.K. Total Wrist Arthroplasty. In Master Techniques in Orthopaedic Surgery—The Wrist, 3rd ed.; Gelberman, R.H., Ed.; Wolters Kluwer Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; pp. 457–471. [Google Scholar]
- Cavaliere, C.M.; Chung, K.C. Total wrist arthroplasty and total wrist arthrodesis in rheumatoid arthritis: A decision analysis from the hand surgeons’ perspective. J. Hand Surg. Am. 2008, 33, 1744–1755.e1-2. [Google Scholar] [CrossRef][Green Version]
- Boeckstyns, M.E.H.; Herzberg, G. Current European Practice in Wrist Arthroplasty. Hand Clin. 2017, 33, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Nydick, J.A.; Greenberg, S.M.; Stone, J.D.; Williams, B.; Polikandriotis, J.A.; Hess, A.V. Clinical outcomes of total wrist ar-throplasty. J. Hand Surg. Am. 2012, 37, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Boeckstyns, M.E.H. Wrist arthroplasty—A systematic review. Dan. Med. J. 2014, 61, 1–9. [Google Scholar]
- Nair, R. Review Article: Total Wrist Arthroplasty. J. Orthop. Surg. 2014, 22, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Nair, R. Survivorship in total wrist arthroplasty: A literature review. Curr. Orthop. Pract. 2016, 27, 93–97. [Google Scholar] [CrossRef]
- Weiss, A.P.C.; Kamal, R.N.; Shultz, P. Total wrist arthroplasty. J. Am. Acad. Orthop. Surg. 2013, 21, 140–148. [Google Scholar]
- Ritt, M.J.P.F.; Stuart, P.R.; Naggar, L.; Beckenbaugh, R.D. The Early History of Arthroplasty of the Wrist: From amputation to total wrist implant. J. Hand Surg. 1994, 19, 778–782. [Google Scholar] [CrossRef]
- Adams, B.D. Total wrist arthroplasty. J. Am. Soc. Surg. Hand 2001, 1, 236–248. [Google Scholar] [CrossRef]
- Swanson, A.B.; de Groot Swanson, G.; Maupin, B.K. Flexible implant arthroplasty of the radiocarpal joint. Surgical technique and long-term study. Clin. Orthop. Relat. Res. 1984, 187, 94–106. [Google Scholar] [CrossRef]
- Niebauer, J.J.; Shaw, J.L.; Doren, W.W. Silicone-Dacron hinge prosthesis: Design, evaluation, and application. Ann. Rheum. Dis. 1969, 28, 56. [Google Scholar]
- Chakrabarti, I. Total wrist arthroplasty—A review. J. Hand Microsurg. 2009, 1, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Weiss, A.-P.C. Total Wrist Arthroplasty. J. Hand Surg. Am. 2017, 42, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, G. Total Wrist Arthroplasty: New Perspectives. CRR 2013, 8, 239–244. [Google Scholar] [CrossRef]
- Lestienne, V. Results of interposition arthroplasty with the Amandys pyrocarbon implant in rheumatoid wrist at a mean 5 years follow-up. Hand Surg. Rehabil. 2021, 40, 579–587. [Google Scholar] [CrossRef]
- Pierrart, J.; Bourgade, P.; Mamane, W.; Rousselon, T.; Masmejean, E.H. Novel approach for posttraumatic panarthritis of the wrist using a pyrocarbon interposition arthroplasty (Amandys(®)): Preliminary series of 11 patients. Chir. Main 2012, 31, 188–194. [Google Scholar] [CrossRef]
- Bellemère, P.; Maes-Clavier, C.; Loubersac, T.; Gaisne, E.; Kerjean, Y. Amandys(®) implant: Novel pyrocarbon arthroplasty for the wrist. Chir. Main 2012, 31, 176–187. [Google Scholar] [CrossRef]
- Szalay, G.; Stigler, B.; Kraus, R.; Böhringer, G.; Schnettler, R. Die Resektion der proximalen Handwurzelreihe mit Ersatz des Kapitatum-Pols durch eine Pyrocarbonkappe (RCPI) beim fortgeschrittenen karpalen Kollaps. Handchir. Mikrochir. Plast. Chir. 2012, 44, 17–22. [Google Scholar] [CrossRef]
- Giacalone, F.; Di Summa, P.G.; Fenoglio, A.; Sard, A.; Dutto, E.; Ferrero, M.; Bertolini, M.; Garcia-Elias, M. Resurfacing Capitate Pyrocarbon Implant versus Proximal Row Carpectomy Alone: A Comparative Study to Evaluate the Role of Capitate Prosthetic Resurfacing in Advanced Carpal Collapse. Plast. Reconstr. Surg. 2017, 140, 962–970. [Google Scholar] [CrossRef]
- Marcuzzi, A.; Ozben, H.; Russomando, A. The use of a pyrocarbon capitate resurfacing implant in chronic wrist disorders. J. Hand Surg. Eur. Vol. 2014, 39, 611–618. [Google Scholar] [CrossRef]
- Yeoh, D.; Tourret, L. Total wrist arthroplasty: A systematic review of the evidence from the last 5 years. J. Hand Surg. Eur. Vol. 2015, 40, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Dellacqua, D. Total Wrist Arthroplasty. Tech. Orthop. 2009, 24, 49–57. [Google Scholar] [CrossRef]
- Nair, R. Past, Present, and Future in Total Wrist Arthroplasty: A Perspective. Curr. Orthop. Pract. 2015, 26, 318–319. [Google Scholar] [CrossRef]
- Nicoloff, M. Handgelenksprothetik–Indikation und aktueller Stand. Z. Orthopädie Unf. 2015, 153, 38–45. [Google Scholar] [CrossRef]
- Krukhaug, Y.; Lie, S.A.; Havelin, L.I.; Furnes, O.; Hove, L.M. Results of 189 wrist replacements. A report from the Norwegian Arthroplasty Register. Acta Orthop. 2011, 82, 405–409. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Ekroth, S.R.; Werner, F.W.; Palmer, A.K. Case report of long-term results of biaxial and volz total wrist arthroplasty. J. Wrist Surg. 2012, 1, 177–178. [Google Scholar] [CrossRef][Green Version]
- Jolly, S.L.; Ferlic, D.C.; Clayton, M.L.; Dennis, D.A.; Stringer, E.A. Swanson silicone arthroplasty of the wrist in rheumatoid arthritis: A long-term follow-up. J. Hand Surg. Am. 1992, 17, 142–149. [Google Scholar] [CrossRef]
- Fischer, P.; Sagerfors, M.; Jakobsson, H.; Pettersson, K. Total Wrist Arthroplasty: A 10-Year Follow-Up. J. Hand Surg. Am. 2020, 45, 780.e1–780.e10. [Google Scholar] [CrossRef]
- Sagerfors, M.; Gupta, A.; Brus, O.; Pettersson, K. Total Wrist Arthroplasty: A Single-Center Study of 219 Cases with 5-Year Follow-up. J. Hand Surg. Am. 2015, 40, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.M.; Chung, K.C. A systematic review of total wrist arthroplasty compared with total wrist arthrodesis for rheumatoid arthritis. Plast. Reconstr. Surg. 2008, 122, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Berber, O.; Garagnani, L.; Gidwani, S. Systematic Review of Total Wrist Arthroplasty and Arthrodesis in Wrist Arthritis. J. Wrist Surg. 2018, 7, 424–440. [Google Scholar] [CrossRef]
- Cobb, T.K.; Beckenbaugh, R.D. Biaxial total-wrist arthroplasty. J. Hand Surg. Am. 1996, 21, 1011–1021. [Google Scholar] [CrossRef]
- Courtman, N.H.; sochart, D.H.; Trail, I.A.; Stanley, J.K. Biaxial Wrist Replacement. J. Hand Surg. 2016, 24, 32–34. [Google Scholar] [CrossRef]
- Kretschmer, F.; Fansa, H. Die BIAX-Handgelenkprothese: Management und Erfahrungen bei 42 Patienten. Handchir. Mikrochir. Plast. Chir. 2007, 39, 238–248. [Google Scholar] [CrossRef]
- Rizzo, M.; Beckenbaugh, R.D. Results of biaxial total wrist arthroplasty with a modified (long) metacarpal stem. J. Hand Surg. Am. 2003, 28, 577–584. [Google Scholar] [CrossRef]
- Stegeman, M.; Rijnberg, W.J.; van Loon, C.J.M. Biaxial total wrist arthroplasty in rheumatoid arthritis. Satisfactory functional results. Rheumatol. Int. 2005, 25, 191–194. [Google Scholar] [CrossRef]
- Strunk, S.; Bracker, W. Handgelenksendoprothetik: Erfahrungen nach Implantation von 41 Prothesen. Handchir. Mikrochir. Plast. Chir. 2009, 41, 141–147. [Google Scholar] [CrossRef]
- Takwale, V.J.; Nuttall, D.; Trail, I.A.; Stanley, J.K. Biaxial total wrist replacement in patients with rheumatoid arthritis. J. Bone Jt. Surg. 2002, 84, 692–699. [Google Scholar] [CrossRef]
- van Harlingen, D.; Heesterbeek, P.J.C.; de Vos, M.J. High rate of complications and radiographic loosening of the biaxial total wrist arthroplasty in rheumatoid arthritis: 32 wrists followed for 6 (5–8) years. Acta Orthop. 2011, 82, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Giwa, L.; Siddiqui, A.; Packer, G. Motec Wrist Arthroplasty: 4 Years of Promising Results. J. Hand Surg. Asian Pac. Vol. 2018, 23, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, O.; Lütken, T.; Grimsgaard, C.; Bolstad, B.; Thorkildsen, R.; Røkkum, M. Promising one-to six-year results with the Motec wrist arthroplasty in patients with post-traumatic osteoarthritis. J. Bone Jt. Surg. Br. Vol. 2012, 94, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, O.; Holm-Glad, T.; Bolstad, B.; Grimsgaard, C.; Thorkildsen, R.; Røkkum, M. Five- to 10-Year Prospective Follow-Up of Wrist Arthroplasty in 56 Nonrheumatoid Patients. J. Hand Surg. Am. 2017, 42, 788–796. [Google Scholar] [CrossRef]
- Levadoux, M.; Legré, R. Total wrist arthroplasty with Destot prostheses in patients with posttraumatic arthritis. J. Hand Surg. Am. 2003, 28, 405–413. [Google Scholar] [CrossRef][Green Version]
- Meuli, H.C.; Fernandez, D.L. Uncemented total wrist arthroplasty. J. Hand Surg. Am. 1995, 20, 115–122. [Google Scholar] [CrossRef]
- Meuli, H.C. Meuli total wrist arthroplasty. Clin. Orthop. Relat. Res. 1984, 187, 107–111. [Google Scholar] [CrossRef]
- Radmer, S.; Andresen, R.; Sparmann, M. Total wrist arthroplasty in patients with rheumatoid arthritis. J. Hand Surg. Am. 2003, 28, 789–794. [Google Scholar] [CrossRef]
- Rahimtoola, Z.O.; Rozing, P.M. Preliminary Results of Total Wrist Arthroplasty Using the RWS Prosthesis. J. Hand Surg. 2003, 28, 54–60. [Google Scholar] [CrossRef]
- Badge, R.; Kailash, K.; Dickson, D.R.; Mahalingam, S.; Raza, A.; Birch, A.; Nuttall, D.; Murali, S.R.; Hayton, M.J.; Talwalkar, S.; et al. Medium-term outcomes of the Universal-2 total wrist arthroplasty in patients with rheumatoid arthritis. Bone Jt. J. 2016, 98, 1642–1647. [Google Scholar] [CrossRef]
- Ferreres, A.; Lluch, A.; Del Valle, M. Universal total wrist arthroplasty: Midterm follow-up study. J. Hand Surg. Am. 2011, 36, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.W.; Ross, A.; Wright, J.; Martin, D.J.; Bransby-Zachary, M.; MacDonald, D.J. Universal 2 total wrist arthroplasty: High satisfaction but high complication rates. J. Hand Surg. Eur. Vol. 2018, 43, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Pfanner, S.; Munz, G.; Guidi, G.; Ceruso, M. Universal 2 Wrist Arthroplasty in Rheumatoid Arthritis. J. Wrist Surg. 2017, 6, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Zijlker, H.J.A.; Ritt, M.J.P.F.; IJsselstein, C.B. Long-Term Results of Universal 2 Total Wrist Arthroplasty. J. Wrist Surg. 2019, 8, 317–320. [Google Scholar] [CrossRef]
- Bidwai, A.S.C.; Cashin, F.; Richards, A.; Brown, D.J. Short to medium results using the remotion total wrist replacement for rheumatoid arthritis. Hand Surg. 2013, 18, 175–178. [Google Scholar] [CrossRef]
- Boeckstyns, M.E.H.; Herzberg, G.; Merser, S. Favorable results after total wrist arthroplasty: 65 wrists in 60 patients followed for 5–9 years. Acta Orthop. 2013, 84, 415–419. [Google Scholar] [CrossRef]
- Cooney, W.; Manuel, J.; Froelich, J.; Rizzo, M. Total wrist replacement: A retrospective comparative study. J. Wrist Surg. 2012, 1, 165–172. [Google Scholar] [CrossRef][Green Version]
- Froschauer, S.M.; Zaussinger, M.; Hager, D.; Behawy, M.; Kwasny, O.; Duscher, D. Re-motion total wrist arthroplasty: 39 non-rheumatoid cases with a mean follow-up of 7 years. J. Hand Surg. Eur. Vol. 2019, 44, 946–950. [Google Scholar] [CrossRef]
- Herzberg, G.; Boeckstyns, M.; Sorensen, A.I.; Axelsson, P.; Kroener, K.; Liverneaux, P.; Obert, L.; Merser, S. “Remotion” total wrist arthroplasty: Preliminary results of a prospective international multicenter study of 215 cases. J. Wrist Surg. 2012, 1, 17–22. [Google Scholar] [CrossRef]
- Honecker, S.; Igeta, Y.; Al Hefzi, A.; Pizza, C.; Facca, S.; Liverneaux, P.A. Survival Rate on a 10-Year Follow-Up of Total Wrist Replacement Implants: A 23-Patient Case Series. J. Wrist Surg. 2019, 8, 24–29. [Google Scholar] [CrossRef]
- Chevrollier, J.; Strugarek-Lecoanet, C.; Dap, F.; Dautel, G. Results of a unicentric series of 15 wrist prosthesis implantations at a 5.2 year follow-up. Acta Orthop. Belg. 2016, 82, 31–42. [Google Scholar] [PubMed]
- Divelbiss, B.J.; Sollerman, C.; Adams, B.D. Early results of the Universal total wrist arthroplasty in rheumatoid arthritis. J. Hand Surg. Am. 2002, 27, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.A.; Kamal, R.N.; Cone, E.; Weiss, A.-P.C. High Survivorship and Few Complications with Cementless Total Wrist Ar-throplasty at a Mean Followup of 9 Years. Clin. Orthop. Relat. Res. 2017, 475, 3082–3087. [Google Scholar] [CrossRef] [PubMed]
- Menon, J. Universal total wrist implant. J. Arthroplast. 1998, 13, 515–523. [Google Scholar] [CrossRef]
- van Winterswijk, P. Promising Clinical Results of the Universal Total Wrist Prosthesis in Rheumatoid Arthritis. TOORTHJ 2010, 4, 67–70. [Google Scholar] [CrossRef][Green Version]
- Ward, C.M.; Kuhl, T.; Adams, B.D. Five to ten-year outcomes of the Universal total wrist arthroplasty in patients with rheu-matoid arthritis. J. Bone Jt. Surg. Am. 2011, 93, 914–919. [Google Scholar] [CrossRef]
- Rahimtoola, Z.O.; Hubach, P. Total modular wrist prosthesis: A new design. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2004, 38, 160–165. [Google Scholar] [CrossRef]
- Biehl, C.; Stoll, M.; Heinrich, M.; Biehl, L.; Jung, J.; Heiss, C.; Szalay, G. Long-Term Results of the Modular Physiological Wrist Prosthesis (MPW\textregistered) in Patients with Inflammatory Diseases. Life 2021, 11, 355. [Google Scholar] [CrossRef]
- Bosco, J.A.; Bynum, D.K.; Bowers, W.H. Long-term outcome of Volz total wrist arthroplasties. J. Arthroplast. 1994, 9, 25–31. [Google Scholar] [CrossRef]
- Gellman, H.; Hontas, R.; Brumfield, R.H.; Tozzi, J.; Conaty, J.P. Total wrist arthroplasty in rheumatoid arthritis. A long-term clinical review. Clin. Orthop. Relat. Res. 1997, 342, 71–76. [Google Scholar] [CrossRef]
- Lamberta, F.J.; Ferlic, D.C.; Clayton, M.L. Volz total wrist arthroplasty in rheumatoid arthritis: A preliminary report. J. Hand Surg. Am. 1980, 5, 245–252. [Google Scholar] [CrossRef]
- Figgie, M.P.; Ranawat, C.S.; Inglis, A.E.; Sobel, M.; Figgie III, H.E. Trispherical total wrist arthroplasty in rheumatoid arthritis. J. Hand Surg. Am. 1990, 15, 217–223. [Google Scholar] [CrossRef]
- Kistler, U.; Weiss, A.-P.C.; Simmen, B.R.; Herren, D.B. Long-term results of silicone wrist arthroplasty in patients with rheu-matoid arthritis. J. Hand Surg. Am. 2005, 30, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Swanson, A.B. Silicone Rubber Implants for Replacement of Arthritic or Destroyed Joints in the Hand. Surg. Clin. N. Am. 1968, 48, 1113–1127. [Google Scholar] [CrossRef]
- Matsui, Y.; Minami, A.; Kondo, M.; Ishikawa, J.; Motomiya, M.; Iwasaki, N. A minimum 5-year longitudinal study of a new total wrist arthroplasty in patients with rheumatoid arthritis. J. Hand Surg. Am. 2020, 45, 255.e1–255.e7. [Google Scholar] [CrossRef]
- Aicher, B.; Peil, B. Pain measurement: Visual Analogue Scale (VAS) and Verbal Rating Scale (VRS) in clinical trials with OTC analgesics in headache. Cephalalgia 2012, 32, 185–197. [Google Scholar] [CrossRef]
- Crichton, N. Visual analogue scale (VAS). J. Clin. Nurs. 2001, 10, 706. [Google Scholar]
- Rhee, H.; Belyea, M.; Mammen, J. Visual analogue scale (VAS) as a monitoring tool for daily changes in asthma symptoms in adolescents: A prospective study. Allergy Asthma Clin. Immunol. 2017, 13, 24. [Google Scholar] [CrossRef]
- Hudak, P.L.; Amadio, P.C.; Bombardier, C.; Beaton, D.; Cole, D.; Davis, A.; Hawker, G.; Katz, J.N.; Makela, M.; Marx, R.G.; et al. Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder, and head). Am. J. Ind. Med. 1996, 29, 602–608. [Google Scholar] [CrossRef]
- Morapudi, S.P.K.; Marlow, W.J.; Withers, D.; Ralte, P.; Gabr, A.; Waseem, M. Total wrist arthroplasty using the Universal 2 prosthesis. J. Orthop. Surg. 2012, 20, 365–368. [Google Scholar] [CrossRef]
- Friedel, R.; Lenz, M.; Dönicke, T.; Hofmann, G.O. Handgelenksendoprothetik. Obere Extremität 2008, 3, 58–63. [Google Scholar] [CrossRef]
- Kennedy, C.D.; Huang, J.I. Prosthetic Design in Total Wrist Arthroplasty. Orthop. Clin. N. Am. 2016, 47, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Grosland, N.M.; Rogge, R.D.; Adams, B.D. Influence of Articular Geometry on Prosthetic Wrist Stability. Clin. Orthop. Relat. Res. 2004, 421, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.E.T.; Johnstone, A. A new design concept for wrist arthroplasty. Proc. Inst. Mech. Eng. H 2005, 219, 43–52. [Google Scholar] [CrossRef]
- Reigstad, A.; Reigstad, O.; Grimsgaard, C.; Røkkum, M. New concept for total wrist replacement. J. Plast. Surg. Hand Surg. 2011, 45, 148–156. [Google Scholar] [CrossRef]
- Leven, S.; Eschweiler, J.; Tingart, M.; Rath, B. Handgelenksendoprothese. PCT/ EP2020/ 064595; RWTH Aachen University. 28 May 2019. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2020239777 (accessed on 10 February 2022).
- Kaufmann, R.A.; Pfaeffle, H.J.; Blankenhorn, B.D.; Stabile, K.; Robertson, D.; Goitz, R. Kinematics of the midcarpal and radi-ocarpal joint in flexion and extension: An in vitro study. J. Hand Surg. Am. 2006, 31, 1142–1148. [Google Scholar] [CrossRef]
- Akhbari, B.; Morton, A.M.; Shah, K.N.; Molino, J.; Moore, D.C.; Weiss, A.-P.C.; Wolfe, S.W.; Crisco, J.J. Proximal-distal shift of the center of rotation in a total wrist arthroplasty is more than twice of the healthy wrist. J. Orthop. Res. 2020, 38, 1575–1586. [Google Scholar] [CrossRef]
- Neu, C.P.; Crisco, J.J., 3rd; Wolfe, S.W. In vivo kinematic behavior of the radio-capitate joint during wrist flexion-extension and radio-ulnar deviation. J. Biomech. 2001, 34, 1429–1438. [Google Scholar] [CrossRef]
- Ogunro, S.; Ahmed, I.; Tan, V. Current indications and outcomes of total wrist arthroplasty. Orthop. Clin. N. Am. 2013, 44, 371–379. [Google Scholar] [CrossRef]
- Schill, S.; Thabe, H.; Mohr, W. Langzeitergebnisse nach Swanson-Prothesenversorgung des rheumatischen Handgelenks. Handchir. Mikrochir. Plast. Chir. 2001, 33, 198–206. [Google Scholar] [CrossRef]
- Schill, S.; Thabe, H. Modularphysiologische Handgelenkprothese. Orthopade 2003, 32, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Brigstocke, G.; Hearnden, A.; Holt, C.A.; Whatling, G. The functional range of movement of the human wrist. J. Hand Surg. 2013, 38, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Brumfield, R.H.; Champoux, J.A. A biomechanical study of normal functional wrist motion. Clin. Orthop. Relat. Res. 1984, 187, 23–25. [Google Scholar] [CrossRef]
- Palmer, A.K.; Werner, F.W.; Murphy, D.; Glisson, R. Functional wrist motion: A biomechanical study. J. Hand Surg. Am. 1985, 10, 39–46. [Google Scholar] [CrossRef]
- Ryu, J.; Cooney III, W.P.; Askew, L.J.; An, K.-N.; Chao, E.Y.S. Functional ranges of motion of the wrist joint. J. Hand Surg. Am. 1991, 16, 409–419. [Google Scholar] [CrossRef]
| Total Number of Procedures/Prosthesis | 2286 |
|---|---|
| Number of different prostheses | 20 |
| Mean follow-up ranges from (month) | 11 [17] |
| Mean follow-up ranges to (month) | 213.6 [30] |
| Average age ranges from (years) | 47 [31] |
| Average age ranges to (years) | 68.3 [30] |
| Youngest patient (years) | 17 [26] |
| Oldest patient (years) | 88 [32,33] |
| Male:Female (ratio) | 65.5%:34.5% |
| Rheumatoid arthritis (%) | 59.5% |
| Prosthesis | Manufacturer | Short Description |
|---|---|---|
| Biaxial prosthesis [26,32,36,37,38,39,40,41,42,43] | DePuy, Warsaw, IN, USA | The Biaxial prosthesis:
|
| Elos prosthesis [26] | Swemac, Linkoping, Sweden | The Elos prosthesis:
|
| Gibbon prosthesis [26] | Swemac, Linkoping, Sweden | The Gibbon prosthesis:
|
| Motec prosthesis [44,45,46] | Swemac, Linköping, Sweden | The Motec prosthesis:
|
| Destot implant [47] | The Destot implant:
| |
| Meuli Wrist Prosthesis (third revised implant) [41,48,49] | The prosthesis MWP III (Meuli Wrist Prosthesis/third revised implant):
| |
| Anatomic physiologic wrist prosthesis (APH) [50] | Implant-Service Vertriebs-GmbH, Hamburg, Germany | The Anatomic physiologic wrist prosthesis
|
| RWS Prosthesis [51] | HowmedicaTM, Pfizer Hospital Products Group, The Netherlands | The RWS Prosthesis
|
| Universal-prosthesis (second generation) (UWP-2) [32,33,41,52,53,54,55,56] | Integra Life Sciences, Plainsboro, NJ, USA; (previously manufactured by Kinetikos Medical Inc.) | The Universal 2 prosthesis
|
| RE-MOTION [32,33,57,58,59,60,61,62] | Small Bone Innovations Inc; Morrisville, PA, USA | The RE-MOTION (formally AVANTA) TWR:
|
| Universal prosthesis, first-generation [30,53,59,63,64,65,66,67,68] | Kinetikos Medical Inc.,4115 Sorrento ValleyBlvd., San Diego, CA, USA | The Universal Wrist Implant:
|
| Maestro [4,32,33,59] | Biomet, Warsaw, IN, USA | The Maestro prosthesis
|
| Total modular wrist prosthesis [69] | Micromed, Germany | The Total modular wrist prosthesis
|
| Modular Physiological Wrist prosthesis (MPW) [70] | Link Company™, Hamburg, Germany | The Modular Physiological Wrist prosthesis:
|
| Resurfacing Capitate Pyrocarbon Implant (RCPI) [20,21] | Tornier, Grenoble, France | The Resurfacing Capitate Pyrocarbon Implant:
|
| Volz prosthesis [71,72,73] | Stryker, Mahwah, NJ, USA Howmedica Company, Rutherford, NJ, USA | The Volz prosthesis:
|
| Trispherical total wrist prosthesis [74] | The trispherical total wrist prosthesis:
| |
| Amandys [16,17,18] | Tornier, Bioprofile | The Amandys implant:
|
| Swanson wrist implant (Silicone implant) [31,75,76] | Wright Medical, Memphis, TN, USA | The Swanson Wrist Joint Implant
|
| DARTS-Total Wrist System [77] | Teijin Nakashima Medical Co., Ltd., Okayama, Japan | The DARTS—Total Wrist System:
|
| No. | Reference | Year of Publication | Kaplan-Meier | Time-point | 95% Confidence Intervals | Type of Implant | |
|---|---|---|---|---|---|---|---|
| [%] | [years] | Range from | Range to | ||||
| 1 | Jolly [31] | 1992 | 42.0 | 7.0 | ./. | ./. | Swanson |
| 2 | Cobb [36] | 1996 | 83.0 | * | 72.0 | 93.0 | Biaxial |
| 3 | Takwale [42] | 2002 | 83.0 | 8.0 | 68.0 | 98.0 | Biaxial |
| 4 | Levadoux [47] | 2003 | 85.0 | 4.0 | ./. | ./. | Destot |
| 5 | Kurkhaug [26] | 2011 | 85.0 | 5.0 | 78.0 | 93.0 | Biaxial |
| 57.0 | 5.0 | 33.0 | 81.0 | Elos | |||
| 77.0 | 4.0 | 30.0 | 90.0 | Gibbon | |||
| 6 | van Harlingen [43] | 2011 | 81.0 | 7.0 | 64.0 | 91.0 | Biaxial |
| 7 | Ward [68] | 2011 | 75.0 | 5.0 | ./. | ./. | UWP-1 |
| 60.0 | 7.0 | ./. | ./. | ||||
| 8 | Boeckstyns [58] | 2013 | 90.0 | 6.0 | ./. | ./. | Remotion |
| 9 | Sagerfors [33] | 2015 | 84.0 | 5.0 | ./. | ./. | Biaxial |
| 81.0 | 8.0 | ./. | ./. | ||||
| 78.0 | 12.0 | ./. | ./. | ||||
| 99.0 | 5.0 | ./. | ./. | Remotion | |||
| 94.0 | 8.0 | ./. | ./. | ||||
| 95.0 | 8.0 | ./. | ./. | Maestro | |||
| 10 | Badge [52] | 2016 | 91.0 | 7.8 | 84.0 | 91.0 | UWP-1 |
| 11 | Gil [65] | 2017 | 78.0 | 15.0 | 62.0 | 91.0 | UWP-1 |
| 12 | Honecker [62] | 2017 | 95.7 | 4.0 | ./. | ./. | Remotion |
| 91.3 | 6.0 | ./. | ./. | ||||
| 69.0 | 8.0 | ./. | ./. | ||||
| 69.0 | 10.0 | ./. | ./. | ||||
| 13 | Fischer [32] | 2020 | 94.0 | 10.0 | ./. | ./. | Remotion |
| 86.0 | 10.0 | ./. | ./. | Biax | |||
| 83.0 | 10.0 | ./. | ./. | UWP-2 | |||
| 93.0 | 10.0 | ./. | ./. | Maestro | |||
| 14 | Biehl [70] | 2021 | 33.0 | 6.9 | ./. | ./. | MPW |
| Reference | Year of Publication | the Life Span of the TWA | Range from | Range to | Type of Implant |
|---|---|---|---|---|---|
| [Month] | [Month] | [Month] | |||
| Ekroth [30] | 2012 | 93.6 | 36.0 | 132,0 | UWP-1 |
| No. | Reference | Year of Publication | Number Included for Follow Up | Worst Pain Reported by Visual Analog Score (VAS) (0–10) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperatively (n) | Mean | Range from | Range to | Postoperatively (n) | Mean | Range from | Range to | ||||
| 1 | Kistler [75] | 2005 | 27.0 | 27.0 | 6.5 | ./. | ./. | 27.0 | 1.8 | ./. | ./. |
| 2 | Bidawi [57] | 2012 | 10.0 | 10.0 | 8.5 | ./. | ./. | 10.0 | 3.2 | ./. | ./. |
| 3 | Cooney [59] | 2012 | 39.0 | 39.0 | 7.0 | ./. | ./. | 39.0 | 2.3 | ./. | ./. |
| 4 | Ekroth [30] | 2012 | 12.0 | 12.0 | ./. | ./. | ./. | 7.0 | 0.3 | ./. | ./. |
| 5 | Nydick [4] | 2012 | 23.0 | 23.0 | 8.0 | ./. | ./. | 23.0 | 2.0 | ./. | ./. |
| 6 | Badge [52] | 2016 | 85.0 | 47.0 | 8.1 | 3.0 | 10.0 | 61.0 | 5.4 | 0.0 | 10.0 |
| 7 | Chevrollier [63] | 2016 | 15.0 | 15.0 | ./. | ./. | ./. | 15.0 | 2.0 | 0.0 | 7.0 |
| 8 | Gil [65] | 2017 | 39.0 | 39.0 | 8.6 | ./. | ./. | 39.0 | 0.4 | ./. | ./. |
| 9 | Honecker [62] | 2017 | 23.0 | 23.0 | 6.8 | ./. | ./. | 23.0 | 2.8 | ./. | ./. |
| 10 | Pfanner [55] | 2017 | 23.0 | 23.0 | 9.0 | ./. | ./. | 23.0 | 0.8 | ./. | ./. |
| 11 | Giacalone [20] | 2017 | 25.0 | 25.0 | ./. | ./. | ./. | 25.0 | 2.0 | ./. | ./. |
| 12 | Bellemere [18] | 2019 | 51.0 | 51.0 | 6.5 | ./. | ./. | 51.0 | 2.3 | ./. | ./. |
| 13 | Froschauer [60] | 2019 | 39.0 | 39.0 | 7.0 | ./. | ./. | 39.0 | 2.0 | ./. | ./. |
| 14 | Biehl [70] | 2021 | 34.0 | 34.0 | 7.0 | ./. | ./. | 34.0 | 1.8 | ./. | ./. |
| 15 | Lestienne [16] | 2021 | 28.0 | 28.0 | 6.0 | 1.0 | 8.0 | 28.0 | 2.0 | 0.0 | 7.0 |
| No. | Reference | Year of Publication | Number of Procedures Included | Number Included for Follow Up | Disabilities of the Arm, Shoulder, and Hand (DASH) (0–100) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | (n) | Preoperatively (n) | Mean | Range from | Range to | Postoperatively (n) | Mean | Range from | Range to | |||
| 1 | Divelbiss [64] | 2002 | 8.0 | 8.0 | 8.0 | ./. | ./. | ./. | 8.0 | 22.4 | ./. | ./. |
| 2 | Strunk [41] | 2009 | 34.0 | 34.0 | ./. | ./. | ./. | ./. | 34.0 | 60.8 | 20.0 | 97.5 |
| 3 | van Winterswijk [67] | 2010 | 17.0 | 17.0 | 17.0 | 91.0 | ./. | ./. | 17.0 | 65.0 | ./. | ./. |
| 4 | Ward [68] | 2011 | 20.0 | 20.0 | 10.0 | 62.0 | 42.0 | 80.0 | 10.0 | 40.0 | 18.0 | 80.0 |
| 5 | van Harlingen [43] | 2011 | 32.0 | 32.0 | 31.0 | 66.0 | ./. | ./. | 31.0 | 34.0 | ./. | ./. |
| 6 | Cooney [59] | 2012 | 46.0 | 30.0 | ./. | ./. | ./. | ./. | 30.0 | 35.0 | ./. | ./. |
| 7 | Ekroth [30] | 2012 | 12.0 | 12.0 | 12.0 | ./. | ./. | ./. | 7.0 | 60.7 | ./. | ./. |
| 8 | Herzberg [61] | 2012 | 112.0 | 112.0 | ./. | ./. | ./. | ./. | 112.0 | 20.5 | ./. | ./. |
| 9 | Morapudi [82] | 2012 | 21.0 | 21.0 | 21.0 | 55.1 | 22.5 | 87.0 | 21.0 | 44.8 | 4.3 | 83.3 |
| 10 | Nydick [4] | 2012 | 23.0 | 23.0 | 23.0 | ./. | ./. | ./. | 23.0 | 31.0 | ./. | ./. |
| 11 | Reigstad [45] | 2012 | 27.0 | 27.0 | 30.0 | 43.0 | ./. | ./. | 27.0 | 19.2 | ./. | ./. |
| 12 | Pierrat [17] | 2012 | 11.0 | 11.0 | 11.0 | 61.6 | ./. | ./. | 11.0 | 42.9 | ./. | ./. |
| 13 | Boeckstyns [58] | 2013 | 65.0 | 52.0 | 52.0 | 58.0 | 14.0 | 89.0 | 28.0 | 42.0 | 0.0 | 84.0 |
| 14 | Marcuzzi [21] | 2014 | 35.0 | 35.0 | 35.0 | 56.9 | 16.7 | 95.0 | 35.0 | 11.4 | 1.0 | 50.8 |
| 15 | Badge [52] | 2016 | 85.0 | 85.0 | 40.0 | 61.3 | 16.0 | 91.0 | 59.0 | 45.8 | 0.0 | 89.0 |
| 16 | Chevrollier [63] | 2016 | 17.0 | 15.0 | 15.0 | ./. | ./. | ./. | 15.0 | 29.0 | 2.3 | 65.9 |
| 17 | Reigstad [46] | 2017 | 37.0 | 37.0 | 48.0 | 38.0 | ./. | ./. | 48.0 | 25.0 | ./. | ./. |
| 18 | Honecker [62] | 2017 | 23.0 | 23.0 | 23.0 | 57.9 | ./. | ./. | 23.0 | 37.9 | ./. | ./. |
| 19 | Giacalone [20] | 2017 | 25.0 | 25.0 | 25.0 | ./. | ./. | ./. | 25.0 | 20.0 | ./. | ./. |
| 20 | Giwa [44] | 2018 | 25.0 | 25.0 | 25.0 | 57.6 | ./. | ./. | 25.0 | 21.1 | ./. | ./. |
| 21 | Kennedy [54] | 2018 | 48.0 | 48.0 | 48.0 | 58.2 | ./. | ./. | 48.0 | 25.4 | ./. | ./. |
| 22 | Bellemere [18] | 2019 | 51.0 | 51.0 | 51.0 | 63.0 | ./. | ./. | 51.0 | 34.0 | ./. | ./. |
| 23 | Friedel [83] | 2019 | 9.0 | 9.0 | 9.0 | ./. | ./. | ./. | 9.0 | 48.0 | ./. | ./. |
| 24 | Froschauer [60] | 2019 | 39.0 | 39.0 | 39.0 | 63.0 | ./. | ./. | 39.0 | 29.0 | ./. | ./. |
| 25 | Matsui [77] | 2019 | 20.0 | 20.0 | 20.0 | 61.2 | ./. | ./. | 20.0 | 36.1 | ./. | ./. |
| 26 | Zijlker [56] | 2019 | 26.0 | 26.0 | 26.0 | ./. | ./. | ./. | 26.0 | 41.0 | ./. | ./. |
| 27 | Biehl [70] | 2021 | 34.0 | 34.0 | 34.0 | ./. | ./. | ./. | 34.0 | 47.1 | 1.7 | 88.8 |
| 28 | Lestienne [16] | 2021 | 28.0 | 28.0 | 28.0 | 62.0 | 34.0 | 100.0 | 28.0 | 36.0 | 0.0 | 75.0 |
| No. | Reference | Year of Publication | Number of Procedures Included | Number Included for Follow Up | Grip Strength (kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | (n) | Preoperatively (n) | Mean | Range from | Range to | Postoperatively (n) | Mean | Range from | Range to | |||
| 1 | Meuli [48] | 1995 | 49.0 | 49.0 | 10.0 | 15.0 | 10.0 | 25.0 | 10.0 | 25.0 | 10.0 | 25.0 |
| 2 | Levadoux [47] | 2003 | 28.0 | 28.0 | 28.0 | 20.0 | 5.0 | 35.0 | 28.0 | 32.0 | 10.0 | 70.0 |
| 3 | Rizzo [39] | 2003 | 17.0 | 17.0 | 17.0 | 5.6 | ./. | ./. | 17.0 | 9.8 | ./. | ./. |
| 4 | Bidawi [57] | 2012 | 10.0 | 10.0 | 10.0 | 2.1 | ./. | ./. | 10.0 | 7.9 | ./. | ./. |
| 5 | Cooney [59] | 2012 | 46.0 | 30.0 | 30.0 | 10.0 | ./. | ./. | 30.0 | 13.0 | ./. | ./. |
| 6 | Herzberg [61] | 2012 | 112.0 | 112.0 | 112.0 | ./. | ./. | ./. | 112.0 | 29.5 | ./. | ./. |
| 7 | Pierrart [17] | 2012 | 11.0 | 11.0 | 11.0 | 20.4 | ./. | ./. | 11.0 | 8.3 | ./. | ./. |
| 8 | Reigstad [45] | 2012 | 27.0 | 27.0 | 30.0 | 22.6 | ./. | ./. | 27.0 | 22.8 | ./. | ./. |
| 9 | Boeckstyns et al. | 2013 | 65.0 | 52.0 | 52.0 | 10.0 | ./. | ./. | 52.0 | 15.0 | ./. | ./. |
| 10 | Marcuzzi [21] | 2014 | 35.0 | 35.0 | 35.0 | 10.1 | 2.0 | 29.3 | 35.0 | 16.5 | 2.6 | 42.8 |
| 11 | Badge [52] | 2016 | 85.0 | 85.0 | 46.0 | 4.8 | 1.7 | 11.5 | 37.0 | 10.2 | 0.0 | 28.0 |
| 12 | Chevrollier [63] | 2016 | 17.0 | 15.0 | 15.0 | ./. | ./. | 15.0 | 17.3 | 8.0 | 27.0 | |
| 13 | Reigstad [46] | 2017 | 37.0 | 37.0 | 48.0 | 21.0 | ./. | ./. | 48.0 | 24.0 | ./. | ./. |
| 14 | Honecker [62] | 2017 | 23.0 | 23.0 | 23.0 | 7.6 | ./. | ./. | 23.0 | 13.9 | ./. | ./. |
| 15 | Giwa [44] | 2018 | 25.0 | 25.0 | 25.0 | 12.3 | ./. | ./. | 25.0 | 27.8 | ./. | ./. |
| 16 | Lestienne [16] | 2021 | 28.0 | 28.0 | 28.0 | 10.0 | 4.0 | 23.0 | 28.0 | 17.0 | 8.0 | 27.0 |
| No. | Reference | Year of Publication | Preoperatively | Postoperatively | Additional Information | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flexion | Extension | Overall FE | Radial | Ulnar | Overall RUD | Flexion | Extension | Overall FE | Radial | Ulnar | Overall RUD | ||||
| 1 | Figgie [74] | 1983 | ./. | ./. | 35.0 | ./. | ./. | ./. | ./. | ./. | 50.0 | 10.0 | 10.0 | 20.0 | |
| 2 | Bosco [71] | 1994 | ./. | ./. | ./. | ./. | ./. | ./. | 17.0 | 32.0 | 49.0 | 2.0 | 23.0 | 25.0 | Active Range of Motion |
| 3 | Meuli [48] | 1995 | ./. | ./. | ./. | ./. | ./. | ./. | 30.0 | 40.0 | 70.0 | 10.0 | 10.0 | 20.0 | |
| 4 | Cobb [36] | 1996 | 34.0 | 23.0 | 57.0 | 5.0 | 16.0 | 21.0 | 29.0 | 36.0 | 65.0 | 10.0 | 20.0 | 30.0 | Last follow up |
| 5 | Gellman [72] | 1997 | 9.6 | 13.9 | 23.5 | 3.2 | 5.0 | 8.2 | 10.3 | 18.2 | 28.5 | 7.8 | 13.2 | 21.0 | |
| 6 | Menon [66] | 1998 | 20.0 | 37.0 | 57.0 | 4.0 | 12.0 | 16.0 | 36.0 | 41.0 | 77.0 | 7.0 | 13.0 | 20.0 | |
| 7 | Courtman [37] | 1999 | ./. | ./. | 50.0 | ./. | ./. | 17.0 | ./. | ./. | 36.0 | ./. | ./. | 32.0 | |
| 8 | Divelbiss [64] | 2002 | ./. | ./. | ./. | ./. | ./. | ./. | 41.0 | 35.0 | 76.0 | 9.0 | 19.0 | 28.0 | after 2 years |
| 9 | Takwale [42] | 2002 | ./. | ./. | ./. | ./. | ./. | ./. | 28.8 | 17.4 | 46.2 | 6.0 | 13.6 | 19.6 | |
| 10 | Levadoux [47] | 2003 | 26.0 | 20.0 | 46.0 | 7.0 | 25.0 | 32.0 | 48.0 | 41.0 | 89.0 | 12.0 | 22.0 | 34.0 | |
| 11 | Radmer [50] | 2003 | ./. | ./. | ./. | ./. | ./. | ./. | 35.0 | 34.0 | 69.0 | 7.0 | 17.0 | 24.0 | |
| 12 | Rahimtoola [51] | 2003 | 26.0 | 7.0 | 33.0 | 2.0 | 10.0 | 12.0 | 35.0 | 24.0 | 59.0 | 10.0 | 15.0 | 25.0 | |
| 13 | Rizzo [39] | 2003 | 20.0 | 29.0 | 49.0 | 4.0 | 22.0 | 26.0 | 23.0 | 34.0 | 57.0 | 9.0 | 25.0 | 34.0 | |
| 14 | Rahimtoola [69] | 2004 | 23.0 | 23.0 | 46.0 | 6.0 | 11.0 | 17.0 | 32.0 | 31.0 | 63.0 | 8.0 | 16.0 | 24.0 | |
| 15 | Stegeman [40] | 2005 | 17.0 | 17.0 | 34.0 | 3.0 | 6.0 | 9.0 | 41.0 | 41.0 | 82.0 | 14.0 | 31.0 | 45.0 | |
| 16 | Kistler [75] | 2005 | ./. | ./. | ./. | ./. | ./. | ./. | 28.0 | 15.0 | 43.0 | 7.0 | 14.0 | 21.0 | |
| 17 | Kretschmer [38] | 2007 | 29.0 | 31.0 | 60.0 | 12.0 | 18.0 | 30.0 | 32.0 | 36.0 | 68.0 | 13.0 | 20.0 | 33.0 | |
| 18 | Strunk [41] | 2009 | ./. | ./. | ./. | ./. | ./. | ./. | 25.6 | 24.5 | 50.1 | 8.0 | 13.0 | 21.0 | |
| 19 | van Winterswijk [67] | 2010 | 21.0 | 30.0 | 51.0 | 5.0 | 12.0 | 17.0 | 29.0 | 38.0 | 67.0 | 7.0 | 17.0 | 24.0 | |
| 20 | Ferreres [53] | 2011 | ./. | ./. | ./. | ./. | ./. | ./. | 42.0 | 26.0 | 68.0 | 1.0 | 26.0 | 27.0 | |
| 21 | Ward [68] | 2011 | 32.0 | 16.0 | 48.0 | 6.0 | 15.0 | 21.0 | 42.0 | 20.0 | 62.0 | 8.0 | 17.0 | 25.0 | |
| 22 | van Harlingen [43] | 2011 | 21.0 | 18.0 | 39.0 | 5.0 | 4.0 | 9.0 | 29.0 | 28.0 | 57.0 | 10.0 | 19.0 | 29.0 | |
| 23 | Bidawi [57] | 2012 | ./. | ./. | ./. | ./. | ./. | ./. | 22.5 | 34.5 | 57.0 | 6.8 | 15.5 | 22.3 | |
| 24 | Cooney [59] | 2012 | ./. | ./. | ./. | ./. | ./. | ./. | 30.0 | 38.0 | 68.0 | 8.0 | 20.0 | 28.0 | |
| 25 | Ekroth [30] | 2012 | ./. | ./. | ./. | ./. | ./. | ./. | ./. | ./. | 54.5 | ./. | ./. | 28.0 | |
| 26 | Herzberg [61] | 2012 | ./. | ./. | ./. | ./. | ./. | ./. | 33.0 | 23.5 | 65.6 | 7.5 | 26.0 | 33.5 | mean Non RA and RA |
| 27 | Morapudi [82] | 2012 | 16.7 | 20.9 | 37.6 | ./. | ./. | ./. | 22.4 | 30.5 | 52.9 | ./. | ./. | ./. | |
| 28 | Nydick [4] | 2012 | 45.0 | 40.0 | 85.0 | 8.0 | 27.0 | 35.0 | 43.0 | 47.0 | 90.0 | 14.0 | 29.0 | 43.0 | |
| 29 | Pierrart [17] | 2012 | 44.1 | 34.5 | 78.6 | 13.7 | 17.5 | 31.2 | 35.0 | 36.5 | 71.5 | 10.0 | 25.6 | 35.6 | Last follow up |
| 30 | Reigstad [45] | 2012 | ./. | ./. | 104.0 | ./. | ./. | ./. | ./. | ./. | 120.0 | ./. | ./. | ./. | after 1 year |
| 31 | Boeckstyns [58] | 2013 | 31.0 | 30.0 | 61.0 | 8.0 | 16.0 | 24.0 | 31.0 | 29.0 | 60.0 | 6.0 | 22.0 | 28.0 | all cases |
| 32 | Marcuzzi [21] | 2014 | 25.0 | 25.0 | 50.0 | 4.7 | 12.0 | 16.7 | 33.0 | 34.0 | 67.0 | 5.3 | 19.0 | 24.3 | |
| 33 | Badge [52] | 2016 | 19.1 | 20.8 | 39.9 | 6.1 | 14.7 | 20.8 | 29.1 | 30.7 | 59.8 | 4.0 | 14.2 | 18.2 | |
| 34 | Chevrollier [63] | 2016 | ./. | ./. | ./. | ./. | ./. | ./ | ./. | ./. | 33.0 | ./. | ./. | 20.0 | |
| 35 | Gil [65] | 2017 | ./. | ./. | ./. | ./. | ./. | ./. | 37.0 | 29.0 | 66.0 | ./. | ./. | ./. | |
| 36 | Honecker [62] | 2017 | 35.4 | 34.3 | 69.7 | ./. | ./. | ./. | 38.7 | 44.7 | 83.4 | ./. | ./. | ./. | |
| 37 | Pfanner [55] | 2017 | ./. | ./. | ./. | ./. | ./. | ./. | ./. | ./. | 53.4 | ./. | ./. | 18.4 | mean of all cases |
| 38 | Giacalone [20] | 2017 | ./. | ./. | ./. | ./. | ./. | ./. | 27.0 | 33.0 | 60.0 | 12.0 | 27.0 | 39.0 | |
| 39 | Giwa [44] | 2018 | ./. | ./. | 78.4 | ./. | ./. | 35.2 | ./. | ./. | 112.3 | ./. | ./. | 40.4 | |
| 40 | Kennedy [54] | 2018 | ./. | ./. | ./. | ./. | ./. | ./. | 33.0 | 24.0 | 57.0 | ./. | ./. | ./. | |
| 41 | Bellemere [18] | 2019 | ./. | ./. | 66.0 | ./. | ./. | ./. | ./. | 75.0 | ./. | ./. | ./. | ||
| 42 | Friedel [83] | 2019 | ./. | ./. | ./. | ./. | ./. | ./. | 31.0 | 29.0 | 60.0 | ./. | ./. | ./. | |
| 43 | Froschauer [60] | 2019 | 20.0 | 20.0 | 40.0 | 5.0 | 15.0 | 20.0 | 40.0 | 35.0 | 75.0 | 15.0 | 30.0 | 45.0 | |
| 44 | Matsui [77] | 2019 | ./. | ./. | 42.3 | ./. | ./. | ./. | ./. | ./. | 48.2 | ./. | ./. | ./. | Last follow up |
| 45 | Biehl [70] | 2021 | 26.8 | 20.8 | 47.6 | 12.0 | 16.9 | 28.9 | 26.5 | 12.3 | 38.8 | 25.3 | 9.2 | 34.5 | |
| 46 | Lestienne [16] | 2021 | ./. | ./. | ./. | ./. | ./. | ./. | 33.0 | 33.0 | 66.0 | 10.0 | 20.0 | 30.0 | Last follow up |
| No. | Reference | Year of Publication | Preoperatively | Postoperatively | ||||
|---|---|---|---|---|---|---|---|---|
| Pronation | Supination | Overall | Pronation | Supination | Overall | |||
| 1 | Cobb [36] | 1996 | 69.0 | 65.0 | 134.0 | 73.0 | 67.0 | 140.0 |
| 2 | Divelbiss [64] | 2002 | ./. | ./. | ./. | 88.0 | 80.0 | 168.0 |
| 3 | Levadoux [47] | 2003 | 60.0 | 45.0 | 105.0 | 90.0 | 77.0 | 167.0 |
| 4 | Rahimtoola [51] | 2003 | 77.0 | 46.0 | 123.0 | 83.0 | 57.0 | 140.0 |
| 5 | Rizzo [39] | 2003 | 68.0 | 61.0 | 129.0 | 75.0 | 66.0 | 141.0 |
| 6 | Rahimtoola [69] | 2004 | 73.0 | 66.0 | 139.0 | 88.0 | 82.0 | 170.0 |
| 7 | Strunk [41] | 2008 | ./. | ./. | ./. | 82.0 | 71.0 | 153.0 |
| 8 | Ward [68] | 2011 | 54.0 | 50.0 | 104.0 | 83.0 | 71.0 | 154.0 |
| 9 | van Harlingen [43] | 2011 | 80.0 | 70.0 | 150.0 | 85.0 | 90.0 | 175.0 |
| 10 | Cooney [59] | 2012 | ./. | ./. | ./. | 75.0 | 70.0 | 145.0 |
| 11 | Pierrart [17] | 2012 | 81.5 | 72.5 | 154.0 | 83.5 | 88.0 | 171.5 |
| 12 | Reigstad [45] | 2012 | 87.0 | 83.0 | 170.0 | 82.0 | 85.0 | 167.0 |
| 13 | Boeckstyns [58] | 2013 | 79.0 | 71.0 | 150.0 | 81.0 | 83.0 | 164.0 |
| 14 | Reigstad [46] | 2017 | 82.0 | 81.0 | 163.0 | 83.0 | 83.0 | 166.0 |
| 15 | Honecker [62] | 2017 | 72.3 | 68.3 | 140.6 | 75.1 | 77.8 | 152.9 |
| 16 | Giwa [44] | 2018 | ./. | ./. | 136.7 | ./. | ./. | 137.2 |
| 17 | Biehl [70] | 2021 | 60.0 | 65.0 | 125.0 | 58.4 | 79.0 | 137.4 |
| 18 | Lestienne [16] | 2021 | 66.0 | 64.0 | 130.0 | 73.0 | 75.0 | 148.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eschweiler, J.; Li, J.; Quack, V.; Rath, B.; Baroncini, A.; Hildebrand, F.; Migliorini, F. Total Wrist Arthroplasty—A Systematic Review of the Outcome, and an Introduction of FreeMove—An Approach to Improve TWA. Life 2022, 12, 411. https://doi.org/10.3390/life12030411
Eschweiler J, Li J, Quack V, Rath B, Baroncini A, Hildebrand F, Migliorini F. Total Wrist Arthroplasty—A Systematic Review of the Outcome, and an Introduction of FreeMove—An Approach to Improve TWA. Life. 2022; 12(3):411. https://doi.org/10.3390/life12030411
Chicago/Turabian StyleEschweiler, Jörg, Jianzhang Li, Valentin Quack, Björn Rath, Alice Baroncini, Frank Hildebrand, and Filippo Migliorini. 2022. "Total Wrist Arthroplasty—A Systematic Review of the Outcome, and an Introduction of FreeMove—An Approach to Improve TWA" Life 12, no. 3: 411. https://doi.org/10.3390/life12030411
APA StyleEschweiler, J., Li, J., Quack, V., Rath, B., Baroncini, A., Hildebrand, F., & Migliorini, F. (2022). Total Wrist Arthroplasty—A Systematic Review of the Outcome, and an Introduction of FreeMove—An Approach to Improve TWA. Life, 12(3), 411. https://doi.org/10.3390/life12030411









