Application of Orthobiologics in Achilles Tendinopathy: A Review
Abstract
:1. Introduction
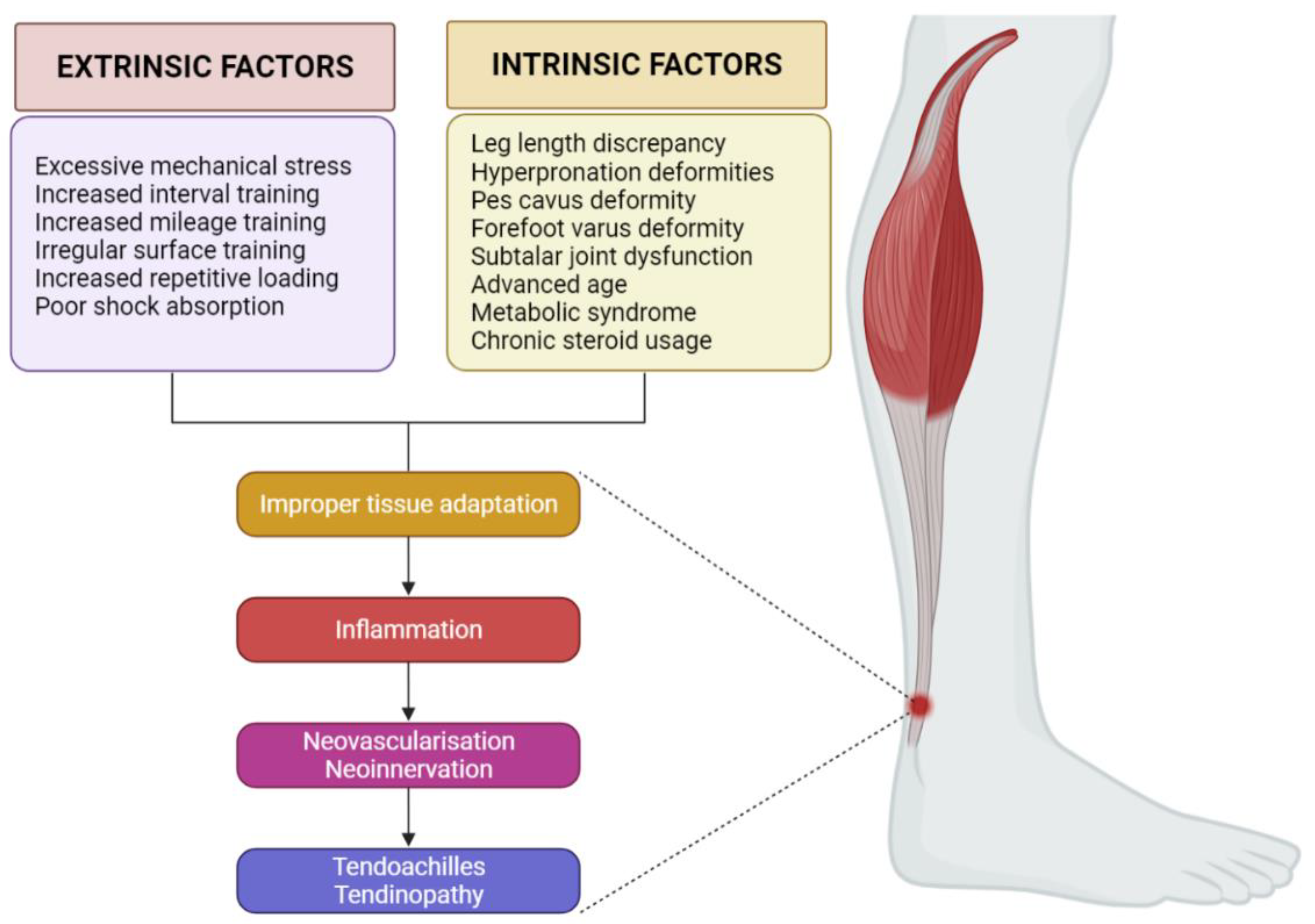
2. Etiopathogenesis of AT
2.1. Biomechanics
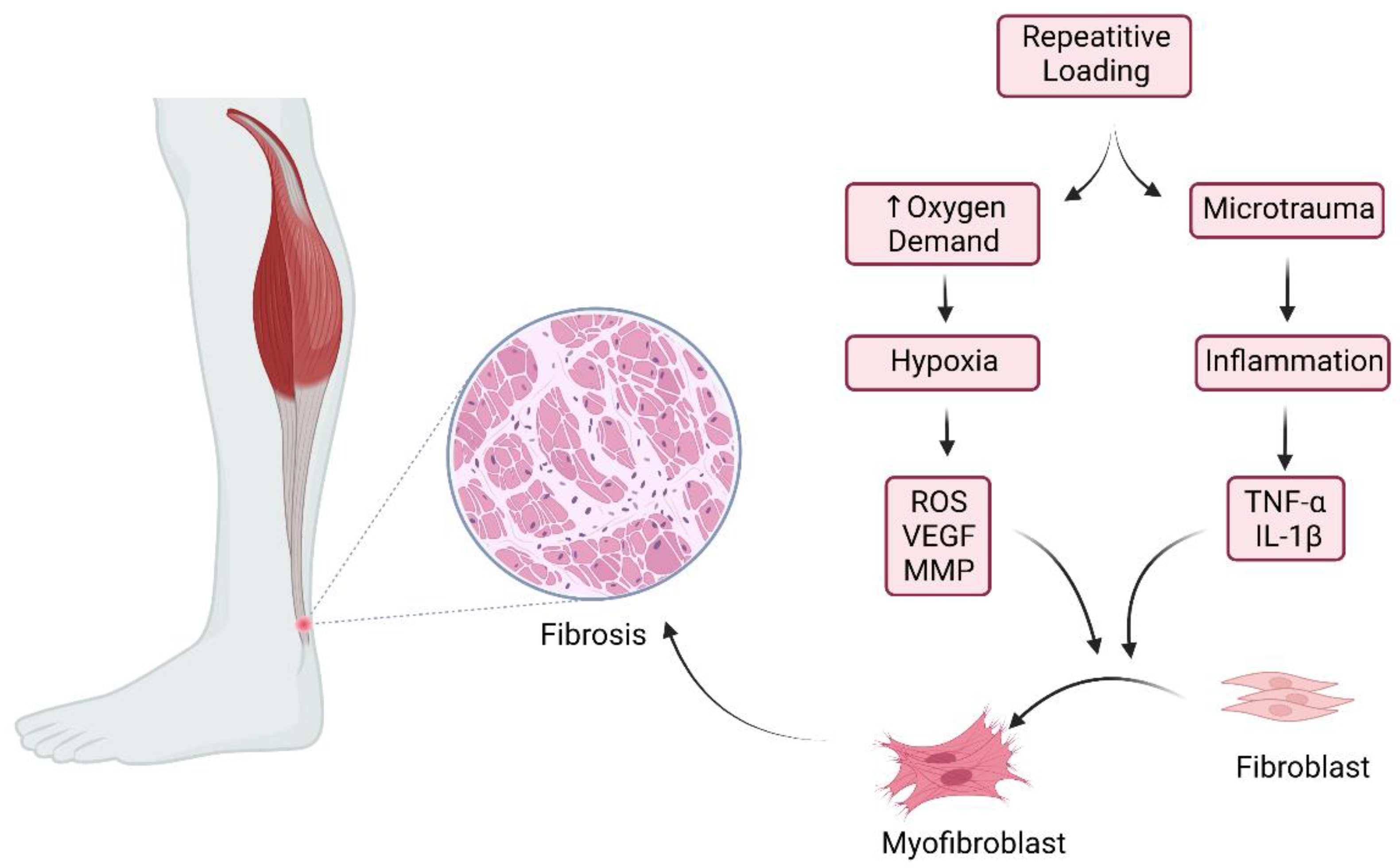
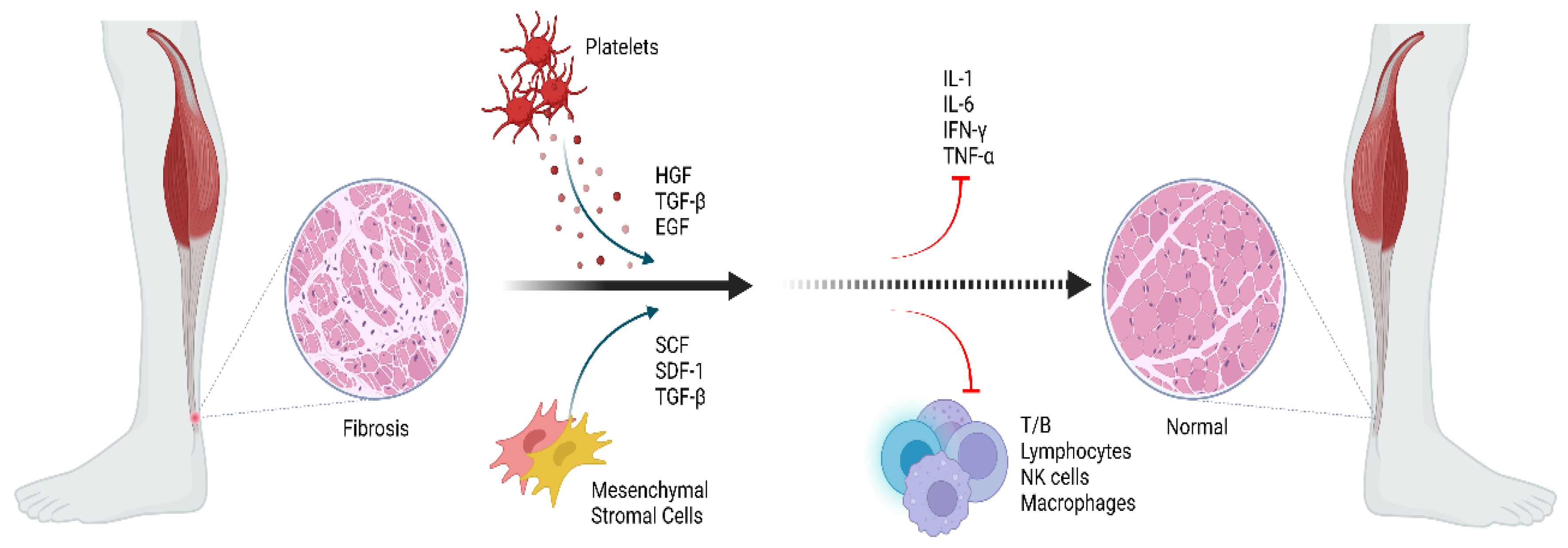
2.2. Inflammation
2.3. Neovascularization and Neoinnervation
2.4. Mechanical Loading and Unloading of the Tendon
3. Management of AT
4. Orthobiologics in AT
4.1. Cellular Therapy in AT
4.1.1. Embryonic Stem Cells (ESCs) and Induced Pluripotent Stem Cells (iPSCs)
4.1.2. Mesenchymal Stromal Cells (MSCs)
4.2. Acellular Therapy in Achilles Tendinopathy
4.2.1. Platelet-Rich Plasma (PRP)
4.2.2. Exosomes
5. Future Prospects in AT
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.-Y.; Hua, Y.-H. Achilles Tendinopathy: Current Concepts about the Basic Science and Clinical Treatments. BioMed Res. Int. 2016, 2016, 6492597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldecker, U.; Hofmann, G.; Drewitz, S. Epidemiologic investigation of 1394 feet: Coincidence of hindfoot malalignment and Achilles tendon disorders. Foot Ankle Surg. 2012, 18, 119–123. [Google Scholar] [CrossRef] [PubMed]
- A Fenwick, S.; Hazleman, B.L.; Riley, G.P. The vasculature and its role in the damaged and healing tendon. Arthritis Res. Ther. 2002, 4, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 203–222. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, M.S.; Behera, P.; Patel, S.; Shetty, V. Orthobiologics and platelet rich plasma. Indian J. Orthop. 2014, 48, 1–9. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, C.-Q.; Wang, J.H.-C. Augmenting tendon and ligament repair with platelet-rich plasma (PRP). Muscle Ligaments Tendons J. 2013, 3, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Alfredson, H.; Cook, J. A treatment algorithm for managing Achilles tendinopathy: New treatment options. Br. J. Sports Med. 2007, 41, 211–216. [Google Scholar] [CrossRef]
- Van der Vlist, A.C.; Winters, M.; Weir, A.; Ardern, C.L.; Welton, N.; Caldwell, D.M.; Verhaar, J.; de Vos, R.-J. Which treatment is most effective for patients with Achilles tendinopathy? A living systematic review with network meta-analysis of 29 randomised controlled trials. Br. J. Sports Med. 2021, 55, 249–256. [Google Scholar] [CrossRef]
- Van Der Plas, J.; de Jonge, S.; de Vos, R.J.; van der Heide, H.J.; Verhaar, J.; Weir, A.; Tol, J. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br. J. Sports Med. 2012, 46, 214–218. [Google Scholar] [CrossRef]
- O’Neill, S.; Watson, P.J.; Barry, S. A Delphi Study of Risk Factors for Achilles Tendinopathy—Opinions of World Tendon Experts. Int. J. Sports Phys. Ther. 2016, 11, 684–697. [Google Scholar]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Alfredson, H.; Ohberg, L.; Forsgren, S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg. Sports Traumatol. Arthrosc. 2003, 11, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, P.W. Neuronal regulation of tendon homoeostasis. Int. J. Exp. Pathol. 2013, 94, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Killian, M.L.; Cavinatto, L.; Galatz, L.M.; Thomopoulos, S. The role of mechanobiology in tendon healing. J. Shoulder Elb. Surg. 2012, 21, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Guzzoni, V.; Selistre-De-Araújo, H.S.; de Cássia Marqueti, R. Tendon Remodeling in Response to Resistance Training, Anabolic Androgenic Steroids and Aging. Cells 2018, 7, 251. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, S.P.; Kjaer, M. The impact of loading, unloading, ageing and injury on the human tendon. J. Physiol. 2019, 597, 1283–1298. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, S.P.; Langberg, H.; Kjaer, M. The pathogenesis of tendinopathy: Balancing the response to loading. Nat. Rev. Rheumatol. 2010, 6, 262–268. [Google Scholar] [CrossRef]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair—A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef]
- Yang, G.; Im, H.-J.; Wang, J.H.-C. Repetitive mechanical stretching modulates IL-1β induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 2005, 363, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.H.; Li, Z.; Wang, J.H.-C. Repeated Exposure of Tendon to Prostaglandin-E2 Leads to Localized Tendon Degeneration. Clin. J. Sport Med. 2005, 15, 27–33. [Google Scholar] [CrossRef]
- Sullo, A.; Maffulli, N.; Capasso, G.; Testa, V. The effects of prolonged peritendinous administration of PGE1 to the rat Achilles tendon: A possible animal model of chronic Achilles tendinopathy. J. Orthop. Sci. 2001, 6, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Morita, W.; Dakin, S.; Snelling, S.J.B.; Carr, A.J. Cytokines in tendon disease. Bone Jt. Res. 2017, 6, 656–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Chen, Y.; Huang, J.; Zhao, K.; Chen, X.; Yin, Z.; Heng, B.C.; Chen, W.; Shen, W. The roles of inflammatory mediators and immunocytes in tendinopathy. J. Orthop. Transl. 2018, 14, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Schubert, T.E.O.; Weidler, C.; Lerch, K.; Hofstädter, F.; Straub, R.H. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann. Rheum. Dis. 2005, 64, 1083–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lana, J.F.; Huber, S.C.; Purita, J.; Tambeli, C.H.; Santos, G.S.; Paulus, C.; Annichino-Bizzacchi, J.M. Leukocyte-rich PRP versus leukocyte-poor PRP—The role of monocyte/macrophage function in the healing cascade. J. Clin. Orthop. Trauma 2019, 10, S7–S12. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Orekhova, V.A.; Nikiforov, N.G.; Myasoedova, V.A.; Grechko, A.V.; Romanenko, E.B.; Zhang, N.; Chistiakov, D.A. Monocyte differentiation and macrophage polarization. Vessel Plus 2019, 3, 10. [Google Scholar] [CrossRef]
- Arvind, V.; Huang, A.H. Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Kaji, D.A.; Howell, K.L.; Balic, Z.; Hubmacher, D.; Huang, A.H. Tgfβ signaling is required for tenocyte recruitment and functional neonatal tendon regeneration. eLife 2020, 9, 1–19. [Google Scholar] [CrossRef]
- Ackermann, P.W.; Renström, P. Tendinopathy in Sport. Sports Health 2012, 4, 193–201. [Google Scholar] [CrossRef]
- Kohara, H.; Tajima, S.; Yamamoto, M.; Tabata, Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials 2010, 31, 8617–8625. [Google Scholar] [CrossRef] [PubMed]
- Emanueli, C.; Salis, M.B.; Pinna, A.; Graiani, G.; Manni, L.; Madeddu, P. Nerve Growth Factor Promotes Angiogenesis and Arteriogenesis in Ischemic Hindlimbs. Circulation 2002, 106, 2257–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, J.D.; Stride, M.; Scott, A. Tendons—Time to revisit inflammation. Br. J. Sports Med. 2014, 48, 1553–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesen, J.L.; Heinemeier, K.M.; Gemmer, C.; Kjaer, M.; Flyvbjerg, A.; Langberg, H. Exercise-dependent IGF-I, IGFBPs, and type I collagen changes in human peritendinous connective tissue determined by microdialysis. J. Appl. Physiol. 2007, 102, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Olesen, J.L.; Heinemeier, K.M.; Haddad, F.; Langberg, H.; Flyvbjerg, A.; Kjær, M.; Baldwin, K.M. Expression of insulin-like growth factor I, insulin-like growth factor binding proteins, and collagen mRNA in mechanically loaded plantaris tendon. J. Appl. Physiol. 2006, 101, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Bayer, M.L.; Schjerling, P.; Herchenhan, A.; Zeltz, C.; Heinemeier, K.M.; Christensen, L.; Krogsgaard, M.; Gullberg, D.; Kjaer, M. Release of Tensile Strain on Engineered Human Tendon Tissue Disturbs Cell Adhesions, Changes Matrix Architecture, and Induces an Inflammatory Phenotype. PLoS ONE 2014, 9, e86078. [Google Scholar] [CrossRef] [Green Version]
- Dideriksen, K.; Sindby, A.K.R.; Krogsgaard, M.; Schjerling, P.; Holm, L.; Langberg, H. Effect of acute exercise on patella tendon protein synthesis and gene expression. SpringerPlus 2013, 2, 109. [Google Scholar] [CrossRef] [Green Version]
- Boesen, A.P.; Dideriksen, K.; Couppé, C.; Magnusson, S.P.; Schjerling, P.; Boesen, M.; Kjaer, M.; Langberg, H. Tendon and skeletal muscle matrix gene expression and functional responses to immobilisation and rehabilitation in young males: Effect of growth hormone administration. J. Physiol. 2013, 591, 6039–6052. [Google Scholar] [CrossRef]
- Scott, A.; Huisman, E.; Khan, K. Conservative treatment of chronic Achilles tendinopathy. CMAJ 2011, 183, 1159–1165. [Google Scholar] [CrossRef] [Green Version]
- Hafsi, K.; McKay, J.; Li, J.; Lana, J.F.; Macedo, A.; Santos, G.S.; Murrell, W.D. Nutritional, metabolic and genetic considerations to optimise regenerative medicine outcome for knee osteoarthritis. J. Clin. Orthop. Trauma 2019, 10, 2–8. [Google Scholar] [CrossRef]
- Paavola, M.; Kannus, P.; Paakkala, T.; Pasanen, M.; Järvinen, M. Long-Term Prognosis of Patients with Achilles Tendinopathy. Am. J. Sports Med. 2000, 28, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.Y.; Khachfe, H.H.; Salhab, H.; Zbib, J.; Fares, Y.; Fares, J. Achilles tendinopathy: Exploring injury characteristics and current treatment modalities. Foot 2021, 46, 101715. [Google Scholar] [CrossRef] [PubMed]
- Lohrer, H.; David, S.; Nauck, T. Surgical treatment for achilles tendinopathy—A systematic review. BMC Musculoskelet. Disord. 2016, 17, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indino, C.; D’Ambrosi, R.; Usuelli, F.G. Biologics in the Treatment of Achilles Tendon Pathologies. Foot Ankle Clin. 2019, 24, 471–493. [Google Scholar] [CrossRef]
- Santos Duarte Lana, J.F.; Furtado da Fonseca, L.; Mosaner, T.; Tieppo, C.E.; Azzini, G.O.M.; Ribeiro, L.L.; Setti, T.; Purita, J. Bone marrow aspirate clot: A feasible orthobiologic. J. Clin. Orthop. Trauma 2020, 11, S789–S794. [Google Scholar] [CrossRef]
- Fogli, M.; Giordan, N.; Mazzoni, G. Efficacy and safety of hyaluronic acid (500–730 kDa) Ultrasound-guided injections on painful tendinopathies: A prospective, open label, clinical study. Muscles Ligaments Tendons J. 2017, 7, 388–395. [Google Scholar] [CrossRef]
- Huddleston, H.P.; Maheshwer, B.; Wong, S.E.; Chahla, J.; Cole, B.J.; Yanke, A.B. An Update on the Use of Orthobiologics: Use of Biologics for Osteoarthritis. Oper. Tech. Sports Med. 2020, 28, 150759. [Google Scholar] [CrossRef]
- Huang, G.; Ye, S.; Zhou, X.; Liu, D.; Ying, Q.-L. Molecular basis of embryonic stem cell self-renewal: From signaling pathways to pluripotency network. Cell. Mol. Life Sci. 2015, 72, 1741–1757. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef] [Green Version]
- Gordeeva, O.; Khaydukov, S. Tumorigenic and Differentiation Potentials of Embryonic Stem Cells Depend on TGFβFamily Signaling: Lessons from Teratocarcinoma Cells Stimulated to Differentiate with Retinoic Acid. Stem Cells Int. 2017, 2017, 7284872. [Google Scholar] [CrossRef] [Green Version]
- Blum, B.; Benvenisty, N. The Tumorigenicity of Human Embryonic Stem Cells. Adv. Cancer Res. 2008, 100, 133–158. [Google Scholar] [CrossRef]
- Watts, A.E.; Yeager, A.E.; Kopyov, O.V.; Nixon, A.J. Fetal derived embryonic-like stem cells improve healing in a large animal flexor tendonitis model. Stem Cell Res. Ther. 2011, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillot, P.V.; Cui, W.; Fisk, N.M.; Polak, D.J. Stem cell differentiation and expansion for clinical applications of tissue engineering. J. Cell. Mol. Med. 2007, 11, 935–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Song, X.-H.; Yin, Z.; Zou, X.-H.; Wang, L.-L.; Hu, H.; Cao, T.; Zheng, M.; Ouyang, H.W. Stepwise Differentiation of Human Embryonic Stem Cells Promotes Tendon Regeneration by Secreting Fetal Tendon Matrix and Differentiation Factors. Stem Cells 2009, 27, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Yin, Z.; Shen, W.L.; Chen, X.; Heng, B.C.; Zou, X.H.; Ouyang, H.W. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials 2010, 31, 9438–9451. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Parham, L. Ethical Issues in Stem Cell Research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef]
- Denker, H.-W. Potentiality of embryonic stem cells: An ethical problem even with alternative stem cell sources. J. Med. Ethic. 2006, 32, 665–671. [Google Scholar] [CrossRef]
- Denker, H.-W. Human Embryonic Stem Cells: The Real Challenge for Research as well as for Bioethics Is Still ahead of Us. CTO 2008, 187, 250–256. [Google Scholar] [CrossRef]
- De Wert, G. Human embryonic stem cells: Research, ethics and policy. Hum. Reprod. 2003, 18, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Wang, Y.; Liu, E.; Sun, Y.; Luo, Z.; Xu, Z.; Liu, W.; Zhong, L.; Lv, Y.; Wang, A.; et al. Human iPSC-Derived Neural Crest Stem Cells Promote Tendon Repair in a Rat Patellar Tendon Window Defect Model. Tissue Eng. Part A 2013, 19, 2439–2451. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, T.; Nakahata, A.; Yamada, N.; Yoshizawa, K.; Kato, T.M.; Iwasaki, M.; Zhao, C.; Kuroki, H.; Ikeya, M. Grafting of iPS cell-derived tenocytes promotes motor function recovery after Achilles tendon rupture. Nat. Commun. 2021, 12, 5012. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Hutchins, R. Embryonic Stem Cells. Stem Cells Dev. 2007, 16, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebolj, K.; Veber, M.; Drobnič, M.; Maličev, E. Hematopoietic stem cell and mesenchymal stem cell population size in bone marrow samples depends on patient’s age and harvesting technique. Cytotechnology 2018, 70, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.M. Mesenchymal stem cells: Will they have a role in the clinic? J. Cell. Biochem. 2002, 85, 73–79. [Google Scholar] [CrossRef]
- Papathanasopoulos, A.; Giannoudis, P.V. Biological considerations of mesenchymal stem cells and endothelial progenitor cells. Injury 2008, 39, S21–S32. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef] [Green Version]
- Sordi, V. Mesenchymal Stem Cell Homing Capacity. Transplantion 2009, 87, S42–S45. [Google Scholar] [CrossRef]
- De Witte, S.F.; Luk, F.; Sierra Parraga, J.M.; Gargesha, M.; Merino, A.; Korevaar, S.S.; Shankar, A.S.; O’Flynn, L.; Elliman, S.J.; Roy, D.; et al. Immunomodulation by Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells 2018, 36, 602–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondělková, K.; Vokurková, D.; Krejsek, J.; Borska, L.; Fiala, Z.; Andrys, C. Regulatory T cells (Treg) and Their Roles in Immune System with Respect to Immunopathological Disorders. Acta Med. Hradec Kralove 2010, 53, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Lana, J.; da Fonseca, L.; Azzini, G.; Santos, G.; Braga, M.; Junior, A.C.; Murrell, W.; Gobbi, A.; Purita, J.; de Andrade, M.P. Bone Marrow Aspirate Matrix: A Convenient Ally in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 2762. [Google Scholar] [CrossRef]
- Cassano, J.M.; Kennedy, J.G.; Ross, K.; Fraser, E.J.; Goodale, M.B.; Fortier, L.A. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 333–342. [Google Scholar] [CrossRef]
- Thampatty, B.P.; Li, H.; Im, H.-J.; Wang, J.H.-C. EP4 receptor regulates collagen type-I, MMP-1, and MMP-3 gene expression in human tendon fibroblasts in response to IL-1β treatment. Gene 2007, 386, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, A.K.; Ang, A.D.; Goh, J.C.; Hui, J.H.; Lim, A.Y.; Lee, E.H.; Lim, B.H. Bone Marrow-Derived Mesenchymal Stem Cells Influence Early Tendon-Healing in a Rabbit Achilles Tendon Model. J. Bone Jt. Surg. 2007, 89, 74–81. [Google Scholar] [CrossRef]
- Yuksel, S.; Guleç, M.A.; Gultekin, M.Z.; Adanır, O.; Caglar, A.; Beytemur, O.; Küçükyıldırım, B.O.; Avci, A.; Subaşı, C.; Inci, Ç.; et al. Comparison of the early period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on the Achilles tendon ruptures in rats. Connect. Tissue Res. 2016, 57, 360–373. [Google Scholar] [CrossRef]
- Alani, M.K.A.; Xu, K.; Sun, Y.; Pan, L.; Xu, Z.; Yang, L. Study of Bone Marrow Mesenchymal and Tendon-Derived Stem Cells Transplantation on the Regenerating Effect of Achilles Tendon Ruptures in Rats. Stem Cells Int. 2015, 2015, e984146. [Google Scholar] [CrossRef]
- McKenna, R.W.; Riordan, N.H. Minimally invasive autologous bone marrow concentrate stem cells in the treatment of the chronically injured Achilles tendon: A case report. CellR4 2014, 2, e1100. [Google Scholar]
- Stein, B.E.; Stroh, D.A.; Schon, L.C. Outcomes of acute Achilles tendon rupture repair with bone marrow aspirate concentrate augmentation. Int. Orthop. 2015, 39, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Thueakthong, W.; Netto, C.D.C.; Garnjanagoonchorn, A.; Day, J.; Friedman, G.; Auster, H.; Tan, E.; Schon, L.C. Outcomes of iliac crest bone marrow aspirate injection for the treatment of recalcitrant Achilles tendinopathy. Int. Orthop. 2021, 45, 2423–2428. [Google Scholar] [CrossRef] [PubMed]
- Farina, K.A.; Kandah, B.A.; Sowers, N.M.; Moore, G.A. Bone Marrow Aspirate Concentrate Injection of the Achilles Tendon in a Competitive Distance Runner. J. Musculoskelet. Res. 2021, 24, 214004. [Google Scholar] [CrossRef]
- Rodas, G.; Soler, R.; Balius, R.; Alomar, X.; Peirau, X.; Alberca, M.; Sánchez, A.; Sancho, J.G.; Rodellar, C.; Romero, A.; et al. Autologous bone marrow expanded mesenchymal stem cells in patellar tendinopathy: Protocol for a phase I/II, single-centre, randomized with active control PRP, double-blinded clinical trial. J. Orthop. Surg. Res. 2019, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rodas, G.; Soler-Rich, R.; Rius-Tarruella, J.; Alomar, X.; Balius, R.; Orozco, L.; Masci, L.; Maffulli, N. Effect of Autologous Expanded Bone Marrow Mesenchymal Stem Cells or Leukocyte-Poor Platelet-Rich Plasma in Chronic Patellar Tendinopathy (With Gap >3 mm): Preliminary Outcomes After 6 Months of a Double-Blind, Randomized, Prospective Study. Am. J. Sports Med. 2021, 49, 1492–1504. [Google Scholar] [CrossRef]
- Okamoto, N.; Kushida, T.; Oe, K.; Umeda, M.; Ikehara, S.; Iida, H. Treating Achilles Tendon Rupture in Rats with Bone-Marrow-Cell Transplantation Therapy. J. Bone Jt. Surg. 2010, 92, 2776–2784. [Google Scholar] [CrossRef]
- Sheikhani-Shahin, H.; Mehrabani, D.; Ashraf, M.J.; Rajabi, H.; Norouzian, M.; Rahmanifar, F.; Nazhvani, S.D.; Zare, S. The healing effect of bone marrow-derived stem cells and aquatic activity in Achilles tendon injury. J. Hell. Vet. Med. Soc. 2019, 70, 1373–1380. [Google Scholar] [CrossRef] [Green Version]
- Van den Boom, N.A.C.; Winters, M.; Haisma, H.J.; Moen, M.H. Efficacy of Stem Cell Therapy for Tendon Disorders: A Systematic Review. Orthop. J. Sports Med. 2020, 8. [Google Scholar] [CrossRef]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Sun, H.M.; Hwang, K.-C.; Kim, S.-W. Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 145–152. [Google Scholar] [CrossRef]
- Boquest, A.C.; Noer, A.; Collas, P. Epigenetic programming of mesenchymal stem cells from human adipose tissue. Stem Cell Rev. Rep. 2006, 2, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; He, D.; Kleiner, G.; Kuluz, J.T. Neuron-like Differentiation of Adipose-Derived Stem Cells from Infant Piglets In Vitro. J. Spinal Cord Med. 2007, 30, S35–S40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Nguyen, A.; Banyard, D.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Usuelli, F.G.; Grassi, M.; Maccario, C.; Viganò, M.; Lanfranchi, L.; Montrasio, U.A.; Girolamo, L. Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: Results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2000–2010. [Google Scholar] [CrossRef]
- Piccionello, A.P.; Riccio, V.; Senesi, L.; Volta, A.; Pennasilico, L.; Botto, R.; Rossi, G.; Tambella, A.M.; Galosi, L.; Marini, C.; et al. Adipose Micro-Grafts Enhance Tendinopathy Healing in Ovine Model: An in Vivo Experimental Perspective Study. STEM CELLS Transl. Med. 2021, 10, 1544–1560. [Google Scholar] [CrossRef]
- Khoury, M.; Tabben, M.; Rolón, A.U.; Levi, L.; Chamari, K.; D’Hooghe, P. Promising improvement of chronic lateral elbow tendinopathy by using adipose derived mesenchymal stromal cells: A pilot study. J. Exp. Orthop. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Norelli, J.B.; Plaza, D.P.; Stal, D.N.; Varghese, A.M.; Liang, H.; A Grande, D. Tenogenically differentiated adipose-derived stem cells are effective in Achilles tendon repair in vivo. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Kokubu, S.; Inaki, R.; Hoshi, K.; Hikita, A. Adipose-derived stem cells improve tendon repair and prevent ectopic ossification in tendinopathy by inhibiting inflammation and inducing neovascularization in the early stage of tendon healing. Regen. Ther. 2020, 14, 103–110. [Google Scholar] [CrossRef]
- Ferracini, R.; Artiaco, S.; Daghino, W.; Falco, M.; Gallo, A.; Garibaldi, R.; Tiraboschi, E.; Guidotti, C.; Bistolfi, A. Microfragmented Adipose Tissue (M-FATS) for Improved Healing of Surgically Repaired Achilles Tendon Tears: A Preliminary Study. Foot Ankle Spec. 2020, 2020, 1938640020974557. [Google Scholar] [CrossRef]
- Viganò, M.; Lugano, G.; Orfei, C.P.; Menon, A.; Ragni, E.; Colombini, A.; De Luca, P.; Randelli, P.; De Girolamo, L. Autologous Microfragmented Adipose Tissue Reduces the Catabolic and Fibrosis Response in an In Vitro Model of Tendon Cell Inflammation. Stem Cells Int. 2019, 2019, e5620286. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, L.; Grassi, M.; Viganò, M.; Orfei, C.P.; Montrasio, U.A.; Usuelli, F. Treatment of Achilles Tendinopathy with Autologous Adipose-derived Stromal Vascular Fraction. Orthop. J. Sports Med. 2016, 4 (Suppl. 4), 2325967116S00128. [Google Scholar] [CrossRef] [Green Version]
- Usuelli, F.G.; Grassi, M.; Montrasio, U.A.; De Girolamo, L.; Boga, M. Freshly Isolated Adipose-Derived Stem Cells for the Treatment of Achilles Tendinopathy. Foot Ankle Orthop. 2016, 1. [Google Scholar] [CrossRef] [Green Version]
- Boswell, S.G.; Cole, B.J.; Sundman, E.A.; Karas, V.; Fortier, L.A. Platelet-Rich Plasma: A Milieu of Bioactive Factors. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 429–439. [Google Scholar] [CrossRef]
- Galliera, E.; Corsi, M.M.; Banfi, G. Platelet rich plasma therapy: Inflammatory molecules involved in tissue healing. J. Biol. Regul. Homeost. agents 2013, 26 (Suppl. 1), 35S–42S. [Google Scholar]
- Bielecki, T.M.; Gazdzik, T.S.; Arendt, J.; Szczepanski, T.; Król, W.; Wielkoszynski, T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances. J. Bone Jt. Surgery. Ser. B. 2007, 89, 417–420. [Google Scholar] [CrossRef]
- Yoshida, R.; Murray, M.M. Peripheral blood mononuclear cells enhance the anabolic effects of platelet-rich plasma on anterior cruciate ligament fibroblasts. J. Orthop. Res. 2012, 31, 29–34. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The regenerative mechanisms of platelet-rich plasma: A review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H.-C. Platelet-Rich Plasma Releasate Promotes Differentiation of Tendon Stem Cells into Active Tenocytes. Am. J. Sports Med. 2010, 38, 2477–2486. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G.; Denaro, V. Novel approaches for the management of tendinopathy. J. Bone Jt. Surg. Am. 2010, 92, 2604–2613. [Google Scholar] [CrossRef]
- Hammond, J.W.; Hinton, R.Y.; Curl, L.A.; Muriel, J.M.; Lovering, R.M. Use of Autologous Platelet-rich Plasma to Treat Muscle Strain Injuries. Am. J. Sports Med. 2009, 37, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boesen, A.P.; Hansen, R.; Boesen, M.I.; Malliaras, P.; Langberg, H. Effect of High-Volume Injection, Platelet-Rich Plasma, and Sham Treatment in Chronic Midportion Achilles Tendinopathy: A Randomized Double-Blinded Prospective Study. Am. J. Sports Med. 2017, 45, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.J.; Weir, A.; Van Schie, H.T.M.; Bierma-Zeinstra, S.M.A.; Verhaar, J.; Weinans, H.; Tol, J.L. Platelet-Rich Plasma Injection for Chronic Achilles Tendinopathy: A Randomized Controlled Trial. JAMA 2010, 303, 144–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jonge, S.; De Vos, R.J.; Weir, A.; Van Schie, H.T.M.; Bierma-Zeinstra, S.M.A.; Verhaar, J.; Weinans, H.; Tol, J.L. One-Year Follow-up of Platelet-Rich Plasma Treatment in Chronic Achilles Tendinopathy. Am. J. Sports Med. 2011, 39, 1623–1630. [Google Scholar] [CrossRef]
- Ferrero, G.; Fabbro, E.; Orlandi, D.; Martini, C.; Lacelli, F.; Serafini, G.; Silvestri, E.; Sconfienza, L. Ultrasound-guided injection of platelet-rich plasma in chronic Achilles and patellar tendinopathy. J. Ultrasound 2012, 15, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Krogh, T.P.; Ellingsen, T.; Christensen, R.; Jensen, P.; Fredberg, U. Ultrasound-Guided Injection Therapy of Achilles Tendinopathy with Platelet-Rich Plasma or Saline. Am. J. Sports Med. 2016, 44, 1990–1997. [Google Scholar] [CrossRef]
- Albano, D.; Messina, C.; Usuelli, F.G.; De Girolamo, L.; Grassi, M.; Maccario, C.; Bignotti, B.; Tagliafico, A.; Sconfienza, L.M. Magnetic resonance and ultrasound in achilles tendinopathy: Predictive role and response assessment to platelet-rich plasma and adipose-derived stromal vascular fraction injection. Eur. J. Radiol. 2017, 95, 130–135. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Di Mateo, B. Platelet-rich plasma injections for the treatment of refractory Achilles tendinopathy: Results at 4 years. Blood Transfus. 2014, 12, 533–540. [Google Scholar] [CrossRef]
- Gaweda, K.; Tarczynska, M.; Krzyzanowski, W. Treatment of Achilles Tendinopathy with Platelet-Rich Plasma. Int. J. Sports Med. 2010, 31, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Deans, V.M.; Miller, A.; Ramos, J. A Prospective Series of Patients with Chronic Achilles Tendinopathy Treated with Autologous-conditioned Plasma Injections Combined with Exercise and Therapeutic Ultrasonography. J. Foot Ankle Surg. 2012, 51, 706–710. [Google Scholar] [CrossRef] [Green Version]
- Monto, R.R. Platelet Rich Plasma Treatment for Chronic Achilles Tendinosis. Foot Ankle Int. 2012, 33, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wan, Y.; Jiang, H. The effect of platelet-rich plasma injection on chronic Achilles tendinopathy and acute Achilles tendon rupture. Platelets 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Yu, K.-L.; Bai, J.-B.; Tian, D.-H.; Liu, G.-L. Platelet-rich plasma injection for the treatment of chronic Achilles tendinopathy. Medicine USA 2019, 98, e15278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Xu, S.-Z.; Gu, P.-C.; Du, J.-Y.; Cai, Y.-Z.; Zhang, C.; Lin, X.-J. Is Platelet-rich Plasma Injection Effective for Chronic Achilles Tendinopathy? A Meta-analysis. Clin. Orthop. Relat. Res. 2018, 476, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Madhi, I.; Yausep, O.E.; Khamdan, K.; Trigkilidas, D. The use of PRP in treatment of Achilles Tendinopathy: A systematic review of literature. Study design: Systematic review of literature. Ann. Med. Surg. 2020, 55, 320–326. [Google Scholar] [CrossRef]
- Salini, V.; Evanni, D.; Epantalone, A.; Eabate, M. Platelet Rich Plasma Therapy in Non-insertional Achilles Tendinopathy: The Efficacy is Reduced in 60-years Old People Compared to Young and Middle-Age Individuals. Front. Aging Neurosci. 2015, 7, 228. [Google Scholar] [CrossRef] [Green Version]
- Townsend, C.; Von Rickenbach, K.J.; Bailowitz, Z.; Gellhorn, A.C. Post-Procedure Protocols Following Platelet-Rich Plasma Injections for Tendinopathy: A Systematic Review. PMR 2020, 12, 904–915. [Google Scholar] [CrossRef]
- Zou, J.; Mo, X.; Shi, Z.; Li, T.; Xue, J.; Mei, G.; Li, X. A Prospective Study of Platelet-Rich Plasma as Biological Augmentation for Acute Achilles Tendon Rupture Repair. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Owens, R.-F., Jr.; Ginnetti, J.; Conti, S.-F.; Latona, C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot Ankle Int. 2011, 32, 1032–1039. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Q.; Jiang, D. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J. Transl. Med. 2019, 17, 211. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Yoneda, S.; Abu-Amer, Y.; Guilak, F.; Gelberman, R.H. Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J. Orthop. Res. 2020, 38, 117–127. [Google Scholar] [CrossRef]
- Trouski, F.F.; Parham, A. Exosomes Derived from Mesenchymal Stem Cells in the Treatment of Animal Tendon Injuries: A Review on Their Isolation and Application. Iran. J. Vet. Med. 2021, 15, 259–274. [Google Scholar]
- Shi, Y.; Kang, X.; Wang, Y.; Bian, X.; He, G.; Zhou, M.; Tang, K. Exosomes Derived from Bone Marrow Stromal Cells (BMSCs) Enhance Tendon-Bone Healing by Regulating Macrophage Polarization. Med. Sci. Monit. 2020, 26, e923328-1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, G.; Guo, Y.; Tang, H.; Shi, Y.; Bian, X.; Zhu, M.; Kang, X.; Zhou, M.; Lyu, J.; et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J. Cell. Mol. Med. 2019, 23, 5475–5485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Jia, J.; Li, S.; Cui, B.; Huang, J.; Guo, Z.; Ma, K.; Wang, L.; Cui, C. Exosomes derived from tendon stem cells promote cell proliferation and migration through the TGF β signal pathway. Biochem. Biophys. Res. Commun. 2021, 536, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xu, M.; Bai, J.; Lin, J.; Yu, B.; Liu, Y.; Guo, X.; Shen, J.; Sun, H.; Hao, Y.; et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology 2019, 71, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Di Matteo, B.; Kon, E.; Merli, G.; Marcacci, M. Platelet-rich plasma in tendon-related disorders: Results and indications. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 1984–1999. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Shi, M.; Zhang, T.; Lu, W.; Yang, S.; Cui, Q.; Li, Z. Adipose-derived mesenchymal stromal cell-derived exosomes promote tendon healing by activating both SMAD1/5/9 and SMAD2/3. Stem Cell Res. Ther. 2021, 12, 338. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Cui, Q.; Han, P.; Yang, S.; Shi, M.; Zhang, T.; Zhang, Z.; Li, Z. Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon. Stem Cell Res. Ther. 2020, 11, 402. [Google Scholar] [CrossRef]
- Clancy, R.; Gomez, P.; Abramson, S. Nitric oxide sustains nuclear factor kappaB activation in cytokine-stimulated chondrocytes. Osteoarthr. Cartil. 2004, 12, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Sun, B.; Wang, Z.; Yang, M.; Cui, Z.; Lin, S.; Jin, M.; Yi, C. Exosomes from M2 Macrophage Promote Peritendinous Fibrosis Posterior Tendon Injury via the MiR-15b-5p/FGF-1/7/9 Pathway by Delivery of circRNA-Ep400. Front. Cell Dev. Biol. 2021, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; He, Y.; Chen, S.; Zhang, D.; Yu, Y.; Fan, C. Macrophage-Derived miRNA-Containing Exosomes Induce Peritendinous Fibrosis after Tendon Injury through the miR-21-5p/Smad7 Pathway. Mol. Ther. Nucleic Acids 2019, 14, 114–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellings, E.P.; Huang, T.C.-T.; Li, J.; Peterson, T.E.; Hooke, A.W.; Rosenbaum, A.; Zhao, C.D.; Behfar, A.; Moran, S.L.; Houdek, M.T. Intrinsic Tendon Regeneration After Application of Purified Exosome Product: An In Vivo Study. Orthop. J. Sports Med. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cheng, J.; Shi, W.; Ren, B.; Zhao, F.; Shi, Y.; Yang, P.; Duan, X.; Zhang, J.; Fu, X.; et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020, 106, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.H.; Agrawal, D.K.; Thankam, F.G. “Smart Exosomes”: A Smart Approach for Tendon Regeneration. Tissue Eng. Part B Rev. 2021, 2. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, H.; Gao, S.; Yang, M.; Lyu, J.; Tang, K. Effect of Tendon Stem Cells in Chitosan/β-Glycerophosphate/Collagen Hydrogel on Achilles Tendon Healing in a Rat Model. Med. Sci. Monit. 2017, 23, 4633–4643. [Google Scholar] [CrossRef]
- Chailakhyan, R.K.; Kon, E.; Shekhter, A.B.; Ivannikov, S.V.; Telpukhov, V.I.; Grosheva, A.G.; Suslin, D.S.; Vorobieva, N.N.; Gerasimov, Y.V.; Churbanov, S.N.; et al. Autologous bone marrow-derived mesenchymal stem cells provide complete regeneration in a rabbit model of the Achilles tendon bundle rupture. Int. Orthop. 2021, 45, 3263–3276. [Google Scholar] [CrossRef]
- Guo, X.; Lv, H.; Fan, Z.; Duan, K.; Liang, J.; Zou, L.; Xue, H.; Huang, D.; Wang, Y.; Tan, M. Effects of hypoxia on Achilles tendon repair using adipose tissue-derived mesenchymal stem cells seeded small intestinal submucosa. J. Orthop. Surg. Res. 2021, 16, 570. [Google Scholar] [CrossRef]
- Shen, W.; Chen, J.; Yin, Z.; Chen, X.; Liu, H.; Heng, B.C.; Chen, W.; Ouyang, H.-W. Allogenous Tendon Stem/Progenitor Cells in Silk Scaffold for Functional Shoulder Repair. Cell Transplant. 2012, 21, 943–958. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, B.; Wang, J.H.-C. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials 2011, 32, 6972–6981. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Lui, P.P.Y.; Lee, W.Y.; Wong, Y.M. Scx-Transduced Tendon-Derived Stem Cells (TDSCs) Promoted Better Tendon Repair Compared to Mock-Transduced Cells in a Rat Patellar Tendon Window Injury Model. PLoS ONE 2014, 9, e97453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Z.; Li, J.; Xiong, H.; Cui, H.; Ning, J.; Wang, S.; Ouyang, X.; Qian, Y.; Fan, C. MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J. Nanobiotechnol. 2021, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Zhang, T.; Shi, M.; Song, X.; Yang, S.; Liu, H.; Zhang, M.; Cui, Q.; Li, Z. Hepatocyte Growth Factor-Induced Tendon Stem Cell Conditioned Medium Promotes Healing of Injured Achilles Tendon. Front. Cell Dev. Biol. 2021, 9, 654084. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-F.; Alberton, P.; Loffredo-Verde, E.; Volkmer, E.; Pietschmann, M.; Müller, P.; Schieker, M.; Docheva, D. Scaffold-free Scleraxis-programmed tendon progenitors aid in significantly enhanced repair of full-size Achilles tendon rupture. Nanomedicine 2016, 11, 1153–1167. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, C.S.; Clements, A.E.B.; Kink, J.A.; Choi, U.; Baer, G.S.; Halanski, M.A.; Hematti, P.; Vanderby, R. Extracellular Vesicle-Educated Macrophages Promote Early Achilles Tendon Healing. Stem Cells 2019, 37, 652–662. [Google Scholar] [CrossRef] [Green Version]
- Aktas, E.; Chamberlain, C.S.; Saether, E.E.; Duenwald-Kuehl, S.E.; Kondratko-Mittnacht, J.; Stitgen, M.; Lee, J.S.; Clements, A.E.; Murphy, W.L.; Vanderby, R. Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an Achilles tendon segmental defect. J. Orthop. Res. 2016, 35, 269–280. [Google Scholar] [CrossRef]
- Heidari, B.S.; Ruan, R.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Biofabrication and Signaling Strategies for Tendon/Ligament Interfacial Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 383–399. [Google Scholar] [CrossRef]
- Lui, P.P.Y. Mesenchymal Stem Cell-Derived Extracellular Vesicles for the Promotion of Tendon Repair—An Update of Literature. Stem Cell Rev. Rep. 2021, 17, 379–389. [Google Scholar] [CrossRef]
- Shearn, J.T.; Kinneberg, K.R.; A Dyment, N.; Galloway, M.T.; Kenter, K.; Wylie, C.; Butler, D.L. Tendon tissue engineering: Progress, challenges, and translation to the clinic. J. Musculoskelet. Neuronal Interact. 2011, 11, 163–173. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramires, L.C.; Jeyaraman, M.; Muthu, S.; Shankar A, N.; Santos, G.S.; da Fonseca, L.F.; Lana, J.F.; Rajendran, R.L.; Gangadaran, P.; Jogalekar, M.P.; et al. Application of Orthobiologics in Achilles Tendinopathy: A Review. Life 2022, 12, 399. https://doi.org/10.3390/life12030399
Ramires LC, Jeyaraman M, Muthu S, Shankar A N, Santos GS, da Fonseca LF, Lana JF, Rajendran RL, Gangadaran P, Jogalekar MP, et al. Application of Orthobiologics in Achilles Tendinopathy: A Review. Life. 2022; 12(3):399. https://doi.org/10.3390/life12030399
Chicago/Turabian StyleRamires, Luciano C., Madhan Jeyaraman, Sathish Muthu, Navaladi Shankar A, Gabriel Silva Santos, Lucas Furtado da Fonseca, José Fábio Lana, Ramya Lakshmi Rajendran, Prakash Gangadaran, Manasi P. Jogalekar, and et al. 2022. "Application of Orthobiologics in Achilles Tendinopathy: A Review" Life 12, no. 3: 399. https://doi.org/10.3390/life12030399
APA StyleRamires, L. C., Jeyaraman, M., Muthu, S., Shankar A, N., Santos, G. S., da Fonseca, L. F., Lana, J. F., Rajendran, R. L., Gangadaran, P., Jogalekar, M. P., Cardoso, A. A., & Eickhoff, A. (2022). Application of Orthobiologics in Achilles Tendinopathy: A Review. Life, 12(3), 399. https://doi.org/10.3390/life12030399










