Proteomic Alterations in Follicular Fluid of Human Small Antral Follicles Collected from Polycystic Ovaries—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Follicular Fluid Sample Acquisition from Small Antral Follicles
2.3. Proteomics Analysis
2.3.1. Reagents and Solutions
2.3.2. Sample Preparation
2.3.3. Mass Spectrometry Data Acquisition
2.3.4. Mass Spectrometry Data Analysis
2.4. Bioinformatics and Statistical Analyses
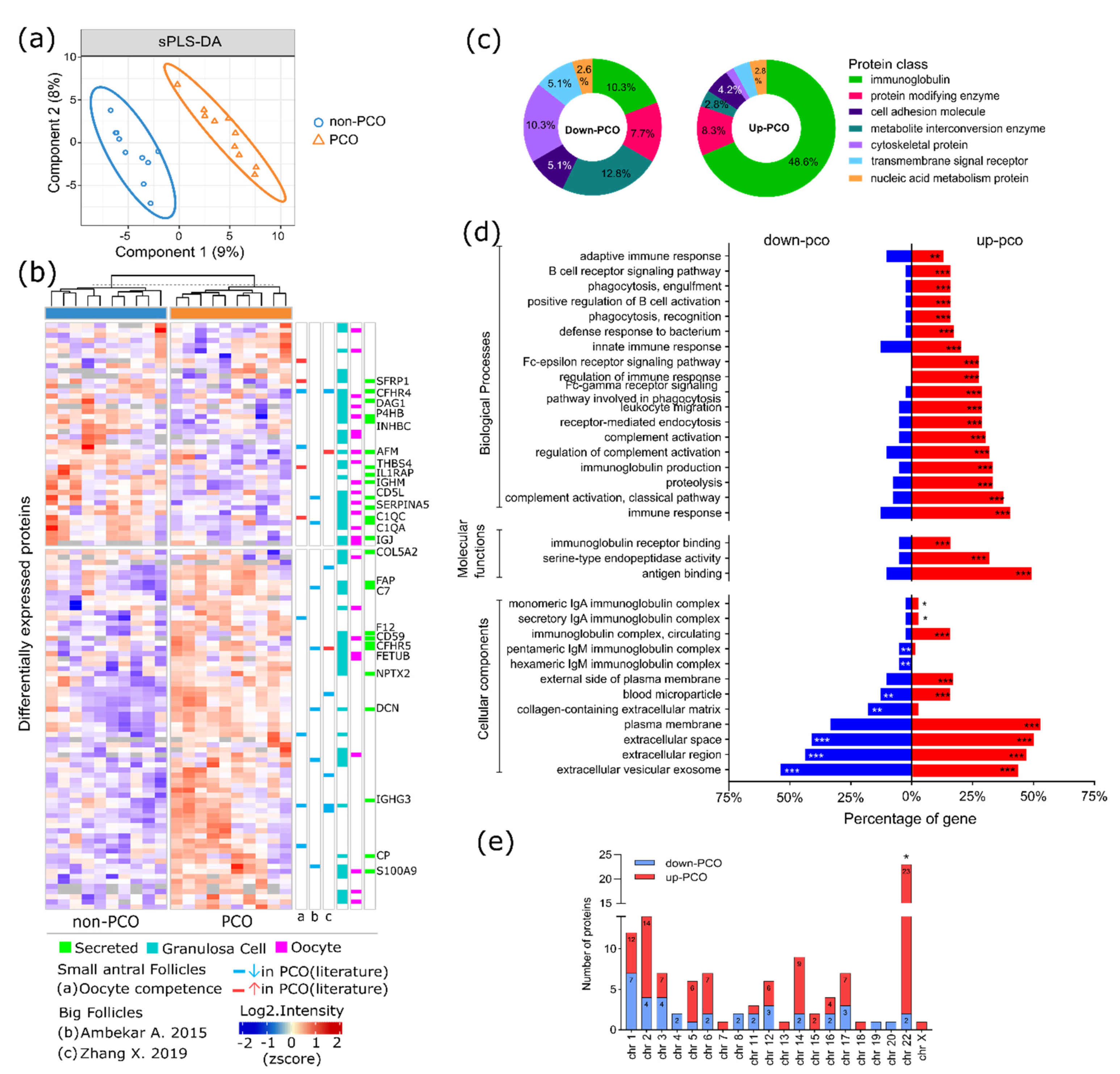
3. Results
3.1. Protein Identification and Quantification
3.2. Dysregulated Protein Profile in FF of hSAF from PCO
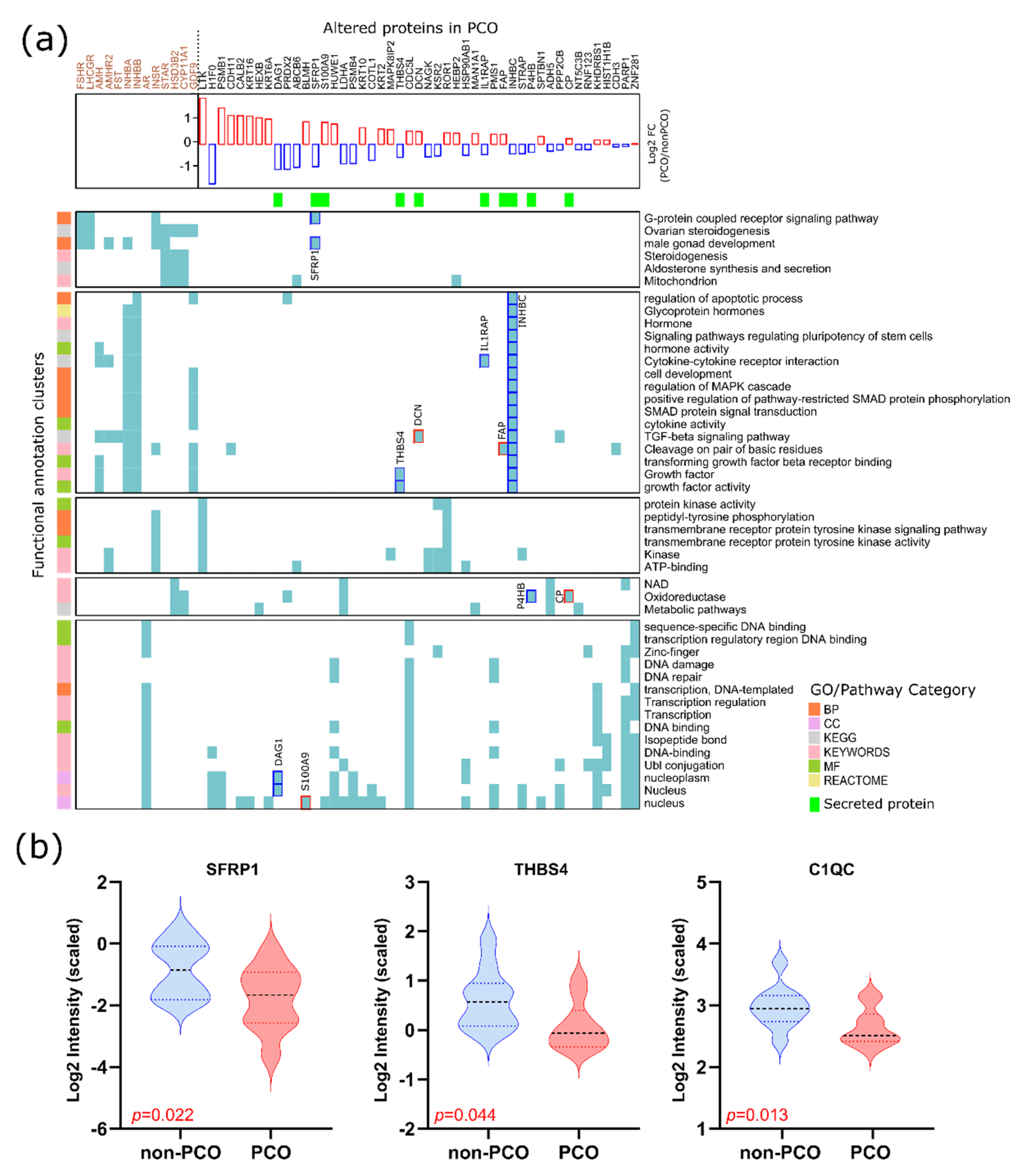
3.3. Altered Pathways FF of hSAF from PCO
3.4. The Functionality of Dysregulated Secreted Proteins
4. Discussion
4.1. Diminished Cell Signaling and Communication in FF hSAF from PCO
4.2. Increased Immune System and Inflammatory Processes and Their Link to Oxidative Stress
4.3. Altered Secreted Proteins and Their Role in Oocyte Competence
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, N.; Khan, S.Z.; Shaikh, R. Polycystic ovary syndrome (PCOS) and genetic predisposition: A review article. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3, 100060. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E. Polycystic ovarian syndrome: Pathophysiology, molecular aspects and clinical implications. Expert Rev. Mol. Med. 2008, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yding Andersen, C. Biological activity of hormones in preovulatory follicular fluid and serum of women undergoing ovarian stimulation. Dan. Med. Bull. 1997, 44, 257–273. [Google Scholar]
- Rodgers, R.J.; Irving-Rodgers, H.F. Formation of the ovarian follicular antrum and follicular fluid. Biol. Reprod. 2010, 82, 1021–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, M.K.Y.; Cheng, C.Y. The Blood-Follicle Barrier (BFB) in disease and in ovarian function. Adv. Exp. Med. Biol. 2012, 763, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Huang, X.; Chang, X.; Yao, J.; He, Q.; Shen, Z.; Ji, Y.; Wang, K. S100-A9 protein in exosomes derived from follicular fluid promotes inflammation via activation of NF-κB pathway in polycystic ovary syndrome. J. Cell. Mol. Med. 2019, 24, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xu, X.; Li, P.; Zhou, F.; Kong, L.; Qiu, J.; Yuan, Z.; Tan, J. TMT Based Proteomic Analysis of Human Follicular Fluid From Overweight/Obese and Normal-Weight Patients With Polycystic Ovary Syndrome. Front. Endocrinol. 2019, 10, 821. [Google Scholar] [CrossRef]
- Ambekar, A.S.; Kelkar, D.S.; Pinto, S.M.; Sharma, R.; Hinduja, I.; Zaveri, K.; Pandey, A.; Prasad, T.S.K.; Gowda, H.; Mukherjee, S. Proteomics of Follicular Fluid From Women With Polycystic Ovary Syndrome Suggests Molecular Defects in Follicular Development. J. Clin. Endocrinol. Metab. 2015, 100, 744–753. [Google Scholar] [CrossRef]
- Domingues, T.S.; Bonetti, T.C.S.; Pimenta, D.C.; Mariano, D.O.C.; Barros, B.; Aquino, A.P.; Motta, E.L.A. Proteomic profile of follicular fluid from patients with polycystic ovary syndrome (PCOS) submitted to in vitro fertilization (IVF) compared to oocyte donors. J. Bras. Reprod. Assist. 2019, 23, 367–391. [Google Scholar] [CrossRef]
- Poulsen, L.l.C.; Pla, I.; Sanchez, A.; Grøndahl, M.L.; Marko-Varga, G.; Yding Andersen, C.; Englund, A.L.M.; Malm, J. Progressive changes in human follicular fluid composition over the course of ovulation: Quantitative proteomic analyses. Mol. Cell. Endocrinol. 2019, 495, 110522. [Google Scholar] [CrossRef] [PubMed]
- Pla, I.; Sanchez, A.; Pors, S.E.; Pawlowski, K.; Appelqvist, R.; Sahlin, K.B.; Poulsen, L.L.C.; Marko-Varga, G.; Andersen, C.Y.; Malm, J. Proteome of fluid from human ovarian small antral follicles reveals insights in folliculogenesis and oocyte maturation. Hum. Reprod. 2020, 36, 2020. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C.; et al. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio: Boston, MA, USA, 2020. [Google Scholar]
- Chung, D.; Keles, S. Sparse partial least squares classification for high dimensional data. Stat. Appl. Genet. Mol. Biol. 2010, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A.A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [Green Version]
- Lottering, R.T.; Govender, M.; Peerbhay, K.; Lottering, S. Comparing partial least squares (PLS) discriminant analysis and sparse PLS discriminant analysis in detecting and mapping Solanum mauritianum in commercial forest plantations using image texture. ISPRS J. Photogramm. Remote Sens. 2020, 159, 271–280. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.J.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Mi, H.; Ebert, D.; Muruganujan, A.; Mills, C.; Albou, L.P.; Mushayamaha, T.; Thomas, P.D. PANTHER version 16: A revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 2021, 49, D394–D403. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. 1D and 2D annotation enrichment: A statistical method integrating quantitative proteomics with complementary high-throughput data. BMC Bioinformatics 2012, 13 (Suppl. 1), S12. [Google Scholar] [CrossRef] [Green Version]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kõks, S.; Velthut, A.; Sarapik, A.; Altmäe, S.; Reinmaa, E.; Schalkwyk, L.C.; Fernandes, C.; Lad, H.V.; Soomets, U.; Jaakma, Ü.; et al. The differential transcriptome and ontology profiles of floating and cumulus granulosa cells in stimulated human antral follicles. MHR Basic Sci. Reprod. Med. 2010, 16, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virant-Klun, I.; Leicht, S.; Hughes, C.; Krijgsveld, J. Identification of Maturation-Specific Proteins by Single-Cell Proteomics of Human Oocytes. Mol. Cell. Proteomics 2016, 15, 2616–2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, L.A.; Kristensen, S.G.; Lerner, A.; Christopoulos, G.; Lavery, S.; Hanyaloglu, A.C.; Hardy, K.; Yding Andersen, C.; Franks, S. Gene Expression in Granulosa Cells From Small Antral Follicles From Women With or Without Polycystic Ovaries. J. Clin. Endocrinol. Metab. 2019, 104, 6182–6192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, S.G.; Kumar, A.; Kalra, B.; Pors, S.E.; Bøtkjær, J.A.; Mamsen, L.S.; Colmorn, L.B.; Fedder, J.; Ernst, E.; Owens, L.A.; et al. Quantitative Differences in TGF-β Family Members Measured in Small Antral Follicle Fluids From Women With or Without PCO. J. Clin. Endocrinol. Metab. 2019, 104, 6371–6384. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.L.; Anderson, N.G. The Human Plasma Proteome: History, Character, and Diagnostic Prospects. Mol. Cell. Proteomics 2002, 1, 845–867. [Google Scholar] [CrossRef] [Green Version]
- Zamah, A.M.; Hassis, M.E.; Albertolle, M.E.; Williams, K.E. Proteomic analysis of human follicular fluid from fertile women. Clin. Proteomics 2015, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bizzarri, M.; Logoteta, P.; Monastra, G.; Laganà, A.S. An innovative approach to polycystic ovary syndrome: Vittorio unfer and his pioneering research on inositols. J. Obstet. Gynaecol. 2021. [Google Scholar] [CrossRef]
- Ambekar, A.S.; Nirujogi, R.S.; Srikanth, S.M.; Chavan, S.; Kelkar, D.S.; Hinduja, I.; Zaveri, K.; Prasad, T.S.K.S.K.; Harsha, H.C.C.; Pandey, A.; et al. Proteomic analysis of human follicular fluid: A new perspective towards understanding folliculogenesis. J. Proteomics 2013, 87, 68–77. [Google Scholar] [CrossRef]
- Irving-Rodgers, H.F.; Rodgers, R.J. Extracellular matrix of the developing ovarian follicle. Semin. Reprod. Med. 2006, 24, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Irving-Rodgers, H.F.; Van Wezel, I.L. Extracellular matrix in ovarian follicles. Mol. Cell. Endocrinol. 2000, 163, 73–79. [Google Scholar] [CrossRef]
- Hu, C.; Pang, B.; Ma, Z.; Yi, H. Immunophenotypic profiles in polycystic ovary syndrome. Mediators Inflamm. 2020, 2020, 5894768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, H.; Li, Z.; Fan, H.; Yan, X.; Liu, X.; Xuan, J.; Feng, D.; Wei, X. The Release of Peripheral Immune Inflammatory Cytokines Promote an Inflammatory Cascade in PCOS Patients via Altering the Follicular Microenvironment. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Esfandyari, S.; Elkafas, H.; Chugh, R.M.; Park, H.S.; Navarro, A.; Al-Hendy, A. Exosomes as Biomarkers for Female Reproductive Diseases Diagnosis and Therapy. Int. J. Mol. Sci. 2021, 22, 2165. [Google Scholar] [CrossRef]
- González, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Increased activation of nuclear factor kappaB triggers inflammation and insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 1508–1512. [Google Scholar] [CrossRef]
- González, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Esparvarinha, M.; Nickho, H.; Mohammadi, H.; Aghebati-Maleki, L.; Abdolalizadeh, J.; Majidi, J. The role of free kappa and lambda light chains in the pathogenesis and treatment of inflammatory diseases. Biomed. Pharmacother. 2017, 91, 632–644. [Google Scholar] [CrossRef]
- Hossein, G.; Khanmohammadi, M.; Fard, P.S.; Heidarian, Y.; Kazemnejad, S.; Akhondi, M.M. Exogenous secreted frizzled-related protein-4 modulates steroidogenesis of rat granulosa cells through Wnt/β-catenin and PI3K/AKT signaling pathways. Avicenna J. Med. Biotechnol. 2016, 8, 159–168. [Google Scholar]
- Häusler, K.D.; Horwood, N.J.; Chuman, Y.; Fisher, J.L.; Ellis, J.; Martin, T.J.; Rubin, J.S.; Gillespie, M.T. Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J. Bone Miner. Res. 2004, 19, 1873–1881. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Rutkowski, H.; Vrezas, I. Cytokines and steroidogenesis. Mol. Cell. Endocrinol. 2004, 215, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Saller, S.; Ströbl, S.; Hennebold, J.D.; Dissen, G.A.; Ojeda, S.R.; Stouffer, R.L.; Berg, D.; Berg, U.; Mayerhofer, A. Decorin is a part of the ovarian extracellular matrix in primates and may act as a signaling molecule. Hum. Reprod. 2012, 27, 3249–3258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543. [Google Scholar] [CrossRef] [PubMed]


| Non-PCO | PCO | |
|---|---|---|
| Women (N) | 5 | 5 |
| Age in years (mean ± SD) | 27.4 ± 5.5 | 25.1 ± 4.4 |
| Ovarian volume in mL (mean ± SD) | 8.5 ± 1.1 | 16.5 ± 3.5 |
| Median number of FF/woman (range) | 5.6 (5–6) | 9.8 (7–14) |
| Follicular diameter in mm (mean ± SD) | 7.1 ± 1.1 | 6.8 ± 1.0 |
| AMH in pmol/L (mean ± SEM) | 16.8 ± 3.5 | 39.1 ± 16.8 |
| FSH in IU/L (mean ± SEM) | 5.0 ± 1.9 | 5.6 ± 1.6 |
| LH in IU/L (mean ± SEM) | 5.0 ± 2.5 | 10.3 ± 6.4 |
| LH/FSH ratio (mean ± SEM) | 1.0 ± 0.3 | 1.7 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pla, I.; Sanchez, A.; Pors, S.E.; Kristensen, S.G.; Appelqvist, R.; Sahlin, K.B.; Marko-Varga, G.; Andersen, C.Y.; Malm, J. Proteomic Alterations in Follicular Fluid of Human Small Antral Follicles Collected from Polycystic Ovaries—A Pilot Study. Life 2022, 12, 391. https://doi.org/10.3390/life12030391
Pla I, Sanchez A, Pors SE, Kristensen SG, Appelqvist R, Sahlin KB, Marko-Varga G, Andersen CY, Malm J. Proteomic Alterations in Follicular Fluid of Human Small Antral Follicles Collected from Polycystic Ovaries—A Pilot Study. Life. 2022; 12(3):391. https://doi.org/10.3390/life12030391
Chicago/Turabian StylePla, Indira, Aniel Sanchez, Susanne Elisabeth Pors, Stine Gry Kristensen, Roger Appelqvist, K. Barbara Sahlin, György Marko-Varga, Claus Yding Andersen, and Johan Malm. 2022. "Proteomic Alterations in Follicular Fluid of Human Small Antral Follicles Collected from Polycystic Ovaries—A Pilot Study" Life 12, no. 3: 391. https://doi.org/10.3390/life12030391
APA StylePla, I., Sanchez, A., Pors, S. E., Kristensen, S. G., Appelqvist, R., Sahlin, K. B., Marko-Varga, G., Andersen, C. Y., & Malm, J. (2022). Proteomic Alterations in Follicular Fluid of Human Small Antral Follicles Collected from Polycystic Ovaries—A Pilot Study. Life, 12(3), 391. https://doi.org/10.3390/life12030391







