The Role of Lifestyle Intervention, in Addition to Drugs, for Diabetic Kidney Disease with Sarcopenic Obesity
Abstract
1. Introduction
2. Early Detection the Sarcopenic Obesity in Diabetes Kidney Disease
3. Diagnosis of Sarcopenia Obesity
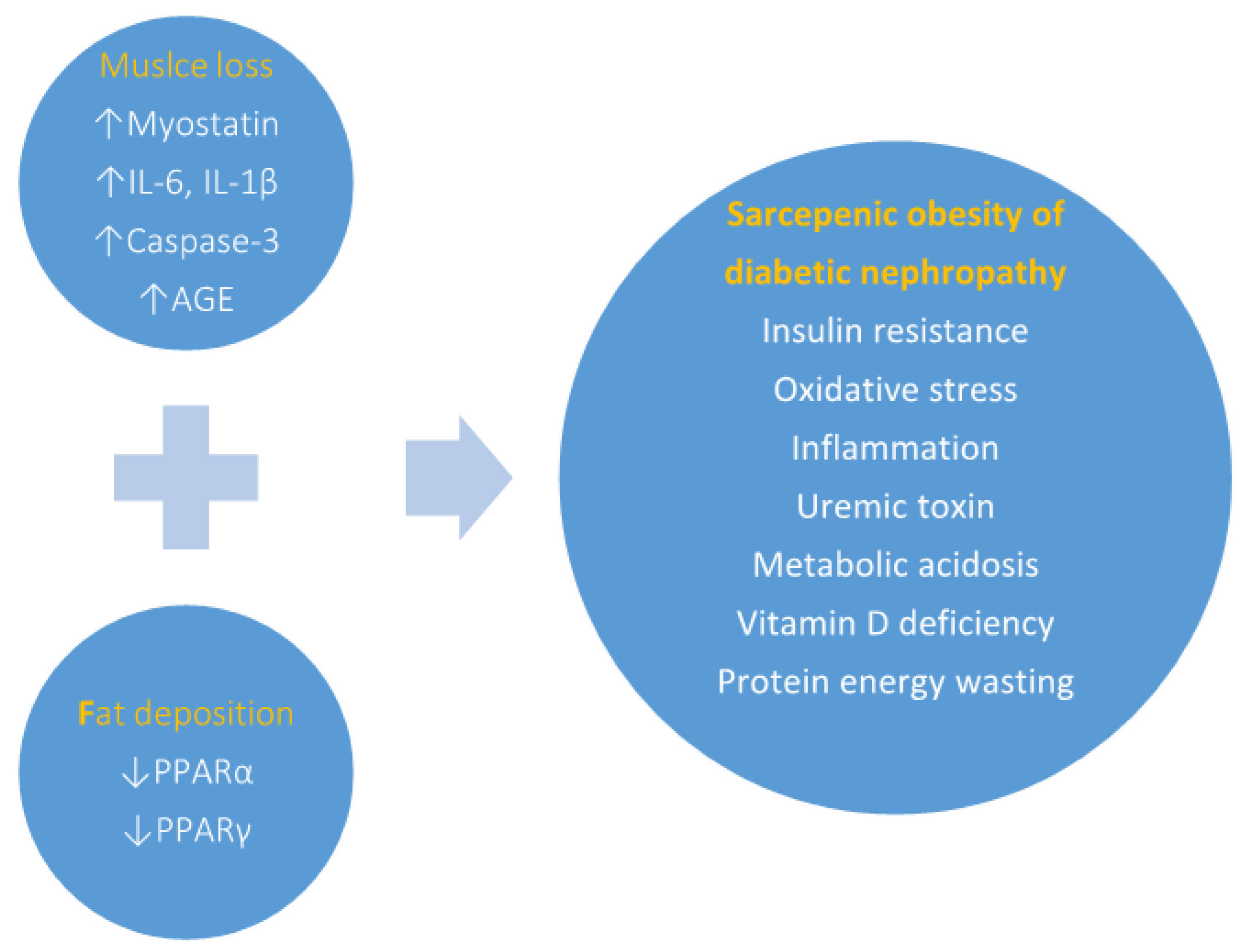
4. Relationship between Sarcopenic Obesity and Kidney Disease
5. Intervention of Sarcopenic Obesity in DM Nephropathy
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The United States Renal Data System (2020). Data source: National Health and Nutrition Examination Survey (NHANES). 2003–2006, 2007–2010, 2011–2014 and 2015–2018. Available online: http://adr.usrds.org/2020/chronic-kidney-disease/1-ckd-in-the-general-population (accessed on 2 March 2022).
- Philips, A.O. Diabetic nephropathy. Medicine 2011, 39, 470–474. [Google Scholar] [CrossRef]
- O’Brien, F. Merck Manual Professional Version. Washington University in St. Louis, 2021. Available online: https://www.msdmanuals.com/professional/genitourinary-disorders/glomerular-disorders/diabetic-nephropathy (accessed on 2 March 2022).
- The United States Renal Data System (2020) ESRD Database: International Comparisons. Available online: https://adr.usrds.org/2020/end-stage-renal-disease/11-international-comparisons (accessed on 2 March 2022).
- Matoba, K.; Takeda, Y.; Nagai, Y.; Kawanami, D.; Utsunomiya, K.; Nishimura, R. Unraveling the Role of Inflammation in the Pathogenesis of Diabetic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3393. [Google Scholar] [CrossRef]
- Ferrannini, E.; Simonson, D.C.; Katz, L.D.; Reichard, G., Jr.; Bevilacqua, S.; Barrett, E.J.; Olsson, M.; DeFronzo, R.A. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 1988, 37, 79–85. [Google Scholar] [CrossRef]
- Ma, R.C.W.; Chan, J.C.N. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann. N. Y. Acad. Sci. 2013, 1281, 64–91. [Google Scholar] [CrossRef]
- Ohse, T.; Inagi, R.; Tanaka, T.; Ota, T.; Miyata, T.; Kojima, I.; Ingelfinger, J.R.; Ogawa, S.; Fujita, T.; Nangaku, M. Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int. 2006, 70, 1447–1455. [Google Scholar] [CrossRef]
- Fang, L.; Xie, D.; Wu, X.; Cao, H.; Su, W.; Yang, J. Involvement of endoplasmic reticulum stress in albuminuria induced inflammasome activation in renal proximal tubular cells. PLoS ONE 2013, 8, e72344. [Google Scholar] [CrossRef]
- Cao, Y.; Fei, D.; Chen, M.; Sun, M.; Xu, J.; Kang, K.; Jiang, L.; Zhao, M. Role of the nucleotide-binding domain-like receptor protein 3 inflammasome in acute kidney injury. FEBS J. 2015, 282, 3799–3807. [Google Scholar] [CrossRef]
- Kawakami, T.; Inagi, R.; Wada, T.; Tanaka, T.; Fujita, T.; Nangaku, M. Indoxyl sulfate inhibits proliferation of human proximal tubular cells via endoplasmic reticulum stress. Am. J. Physiol. Renal. Physiol. 2010, 299, 568–576. [Google Scholar] [CrossRef]
- Hao, Z.; Konta, T.; Takasaki, S.; Abiko, H.; Ishikawa, M.; Takahashi, T.; Ikeda, A.; Ichikawa, K.; Kawata, S.; Kato, T.; et al. The association between microalbuminuria and metabolic syndrome in the general population in Japan: The Takahata study. Intern. Med. 2007, 46, 341–346. [Google Scholar] [CrossRef][Green Version]
- Gerstein, H.C.; Mann, J.F.; Yi, Q.; Zinman, B.; Dinneen, S.F.; Hoogwerf, B.; Hallé, J.P.; Young, J.; Rashkow, A.; Joyce, C.; et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001, 286, 421–426. [Google Scholar] [CrossRef]
- Fukuda, T.; Bouchi, R.; Asakawa, M.; Takeuchi, T.; Shiba, K.; Tsujimoto, K.; Komiya, C.; Yoshimoto, T.; Ogawa, Y.; Yamada, T. Sarcopenic obesity and faster renal function decline in type 2 diabetes. Diabet. Med. 2020, 37, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Hawkins, M.; Matthew, K. Abramowitz. Association of Sarcopenia with eGFR and Misclassification of Obesity in Adults with CKD in the United States. Clin. J. Am. Soc. Nephrol. 2014, 9, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Higashihara, T.; Inagi, R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients 2019, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-Y.; Yang, R.S.; Sheu, M.-L.; Chan, D.-C.; Yang, T.-H.; Tsai, K.-S.; Chiang, C.-K.; Liu, S.-H. Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. J. Pathol. 2016, 238, 470–482. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526. [Google Scholar] [CrossRef]
- Ye, M.; Qian, X.; Guo, X.; Wang, H.; Ni, Q.; Zhao, Y.; Xue, G.; Deng, H.; Zhang, L. Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio Predict Severity and Prognosis of Lower Limb Arteriosclerosis Obliterans. Ann. Vasc. Surg. 2020, 64, 221–227. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Xu, M.; Yang, L.; Deng, X.; Li, B. Study on the Clinical Implications of NLR and PLR for Diagnosing Frailty in Maintenance Hemodialysis Patients and Their Correlations with Patient Prognosis. J. Healthc. Eng. 2022, 2022, 1267200. [Google Scholar] [CrossRef]
- Foley, R.N.; Wang, C.; Ishani, A.; Collins, A.J.; Murray, A.M. Kidney Function and Sarcopenia in the United States General Population: NHANES III. Am. J. Nephrol. 2007, 27, 279–286. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Khadra, D.; Itani, L.; Tannir, H.; Kreidieh, D.; EIMasri, D.; EIGhoch, M. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: A systematic review and meta-analysis. World J. Diabetes 2019, 10, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Workeneh, B.T.; Mitch, W.E. Review of muscle wasting associated with chronic kidney disease. Am. J. Clin. Nutr. 2010, 91, 1128S–1132S. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.Z.; Aktas, G.; Atak, B.; Bilgin, S.; Kurtkulagi, O.; Duman, T.T.; Ozcil, I.E. The association between Vitamin D levels and handgrip strength in elderly men. Acta Endocrinol. 2020, 16, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Galvez, S.; Tiwari, S.; Rezk, B.M.; Semprun-Prieto, L.; Higashi, Y.; Sukhanov, S.; Yablonka-Reuveni, Z.; Delafontaine, P. Angiotensin II Inhibits Satellite Cell Proliferation and Prevents Skeletal Muscle Regeneration. J. Biol. Chem. 2013, 288, 23823–23832. [Google Scholar] [CrossRef]
- Dos Santos, L.R.; Lima, A.G.A.; Braz, A.F.; de Sousa Melo, S.R.; Morais, J.B.S.; Severo, J.S.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. Role of vitamin D in insulin resistance in obese individuals. Nutrire 2017, 42, 17. [Google Scholar] [CrossRef]
- Ceglia, L.; Harris, S.S. Vitamin D and its role in skeletal muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef]
- Rodrigues, G.G.C.; Dellê, H.; Brito, R.B.O.; Cardoso, V.; Fernandes, K.; Mesquita-Ferrari, R.; Cunha, R.S.; Stinghen, A.; Dalboni, M.; Barreto, F. Indoxyl sulfate contributes to uremic sarcopenia by inducing apoptosis in myoblasts. Arch. Med. Res. 2020, 51, 21–29. [Google Scholar] [CrossRef]
- Caldiroli, L.; Armelloni, S.; Eskander, A.; Messa, P.; Rizzo, V.; Margiotta, E. Association between the uremic toxins indoxyl-sulfate and p-cresyl-sulfate with sarcopenia and malnutrition in elderly patients with advanced chronic kidney disease. Exp. Gerontol. 2021, 147, 111266. [Google Scholar] [CrossRef]
- Mohamed, W.S. Obesity related Glomerulopathy. Eur. J. Gen. Med. 2017, 14, 67–72. [Google Scholar] [CrossRef]
- Ouali, F.; Djouadi, F.; Merlet-Bénichou, C.; Bastin, J. Dietary lipids regulate β-oxidation enzyme gene expression in the developing rat kidney. Am. J. Physiol. 1998, 275, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Febbraio, M.A.; Whitham, M. From cytokine to myokine: The emerging role of interleukin-6 in metabolic regulation. Immunol. Cell Biol. 2014, 92, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kristóf, E.; Klusóczki, Á.; Veress, R.; Shaw, A.; Combi, Z.S.; Varga, K.; Győry, F.; Balajthy, Z.; Bai, P.; Bacso, Z.; et al. Interleukin-6 released from differentiating human beige adipocytes improves browning. Exp. Cell Res. 2019, 377, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Koya, D.; Isono, M.; Sugimoto, T.; Kashiwagi, A.; Haneda, M. Peroxisome proliferator-activated receptor-gamma ligands inhibit TGF-beta 1-induced fibronectin expression in glomerular mesangial cells. Diabetes 2004, 53, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Sivarajah, A.; Chatterjee, P.K.; Patel, N.S.A.; Todorovic, Z.; Hattori, Y.; Brown, P.A.J.; Stewart, K.N.; Mota-Filipe, H.; Cuzzocrea, S.; Thiemermann, C. Agonists of peroxisome-proliferator activated receptor-gamma reduce renal ischemia/reperfusion injury. Am. J. Nephrol. 2003, 23, 267–276. [Google Scholar] [CrossRef]
- Barnouin, Y.; Armamento-Villareal, R.; Celli, A.; Jiang, B.; Paudyal, A.; Nambi, V.; Bryant, M.S.; Marcelli, M.; Garcia, J.M.; Qualls, C.; et al. Testosterone replacement therapy added to intensive lifestyle intervention in older men with obesity and hypogonadism. J. Clin. Endocrinol. Metab. 2021, 106, 1096–1110. [Google Scholar] [CrossRef]
- Kenny, A.M.; Kleppinger, A.; Annis, K.; Rathier, M.; Browner, B.; Judge, J.O.; McGee, D. Effects of Transdermal Testosterone on Bone and Muscle in Older Men with Low Bioavailable Testosterone Levels, Low Bone Mass, and Physical Frailty. J. Am. Geriatr. Soc. 2010, 58, 1134–1143. [Google Scholar] [CrossRef]
- Suh, J.; Lee, Y.S. Myostatin Inhibitors: Panacea or Predicament for Musculoskeletal Disorders? J. Bone Metab. 2020, 27, 151–165. [Google Scholar] [CrossRef]
- Fukami, K.; Yamagishi, S.-I.; Sakai, K.; Kaida, Y.; Yokoro, M.; Ueda, S.; Wada, Y.; Takeuchi, M.; Shimizu, M.; Yamazaki, H.; et al. Oral L-carnitine supplementation increases trimethylamine-N-oxide but reduces markers of vascular injury in hemodialysis patients. J. Cardiovasc. Pharmacol. 2015, 65, 289–295. [Google Scholar] [CrossRef]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; de Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; de Nicola, L.; Conte, G. Dietary Salt Restriction in Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef]
- Sulaiman, M.K. Diabetic nephropathy: Recent advances in pathophysiology and challenges in dietary management. Diabetol. Metab. Syndr. 2019, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naderpoor, N.; Johnson, J.; Sourris, K.; de Courten, M.P.J.; Wilson, K.; Scragg, R.; Plebanski, M.; de Courten, B. Effect of vitamin D supplementation on inflammation and nuclear factor kappa-B activity in overweight/obese adults: A randomized placebo-controlled trial. Sci. Rep. 2017, 7, 15154. [Google Scholar] [CrossRef] [PubMed]
- Taskapan, H.; Baysal, O.; Karahan, D.; Durmus, B.; Altay, Z.; Ulutas, O. Vitamin D and muscle strength, functional ability and balance in peritoneal dialysis patients with vitamin D deficiency. Clin. Nephrol. 2011, 76, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Cha, R.-H.; Kang, S.H.; Han, M.Y.; An, W.S.; Kim, S.-H.; Kim, J.C. Effects of AST-120 on muscle health and quality of life in chronic kidney disease patients: Results of RECOVERY study. J. Cachexia Sarcopenia Muscle 2022, 13, 397–408. [Google Scholar] [CrossRef]
- Watson, E.L.; Greening, N.J.; Viana, J.L.; Aulakh, J.; Bodicoat, D.H.; Barratt, J.; Feehally, J.; Smith, A.C. Progressive Resistance Exercise Training in CKD: A Feasibility Study. Am. J. Kidney Dis. 2015, 66, 249–257. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Kim, Y.; Jung, J.; Lee, J.; Chang, Y.; Lee, S.P.; Park, B.-C.; Wolfe, R.R.; Choi, C.S.; et al. Myostatin Inhibition-Induced Increase in Muscle Mass and Strength Was Amplified by Resistance Exercise Training, and Dietary Essential Amino Acids Improved Muscle Quality in Mice. Nutrients 2021, 13, 1508. [Google Scholar] [CrossRef]
- Enoki, Y.; Watanabe, H.; Arake, R.; Fujimura, R.; Ishiodori, K.; Imafuku, T.; Nishida, K.; Sugimoto, R.; Nagao, S.; Miyamura, S.; et al. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J. Cachexia Sarcopenia Muscle 2017, 8, 735–747. [Google Scholar] [CrossRef]
- Dierkes, J.; Dahl, H.; Welland, N.L.; Sandnes, K.; Sæle, K.; Sekse, I.; Marti, H.-P. High rates of central obesity and sarcopenia in CKD irrespective of renal replacement therapy—An observational cross-sectional study. BMC Nephrol. 2018, 19, 259. [Google Scholar]
- Howden, E.J.; Coombes, J.S.; Strand, H.; Douglas, B.; Campbell, K.L.; Isbel, N.M. Exercise Training in CKD: Efficacy, Adherence, and Safety. Am. J. Kidney Dis. 2015, 65, 583–591. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-H.; Liang, Y.-J. The Role of Lifestyle Intervention, in Addition to Drugs, for Diabetic Kidney Disease with Sarcopenic Obesity. Life 2022, 12, 380. https://doi.org/10.3390/life12030380
Chen S-H, Liang Y-J. The Role of Lifestyle Intervention, in Addition to Drugs, for Diabetic Kidney Disease with Sarcopenic Obesity. Life. 2022; 12(3):380. https://doi.org/10.3390/life12030380
Chicago/Turabian StyleChen, Shu-Hua, and Yao-Jen Liang. 2022. "The Role of Lifestyle Intervention, in Addition to Drugs, for Diabetic Kidney Disease with Sarcopenic Obesity" Life 12, no. 3: 380. https://doi.org/10.3390/life12030380
APA StyleChen, S.-H., & Liang, Y.-J. (2022). The Role of Lifestyle Intervention, in Addition to Drugs, for Diabetic Kidney Disease with Sarcopenic Obesity. Life, 12(3), 380. https://doi.org/10.3390/life12030380





