Percutaneous Coronary Intervention versus Coronary Artery Bypass Grafting for Non-Protected Left Main Coronary Artery Disease: 1-Year Outcomes in a High Volume Single Center Study
Abstract
:1. Introduction
2. Materials and Methods
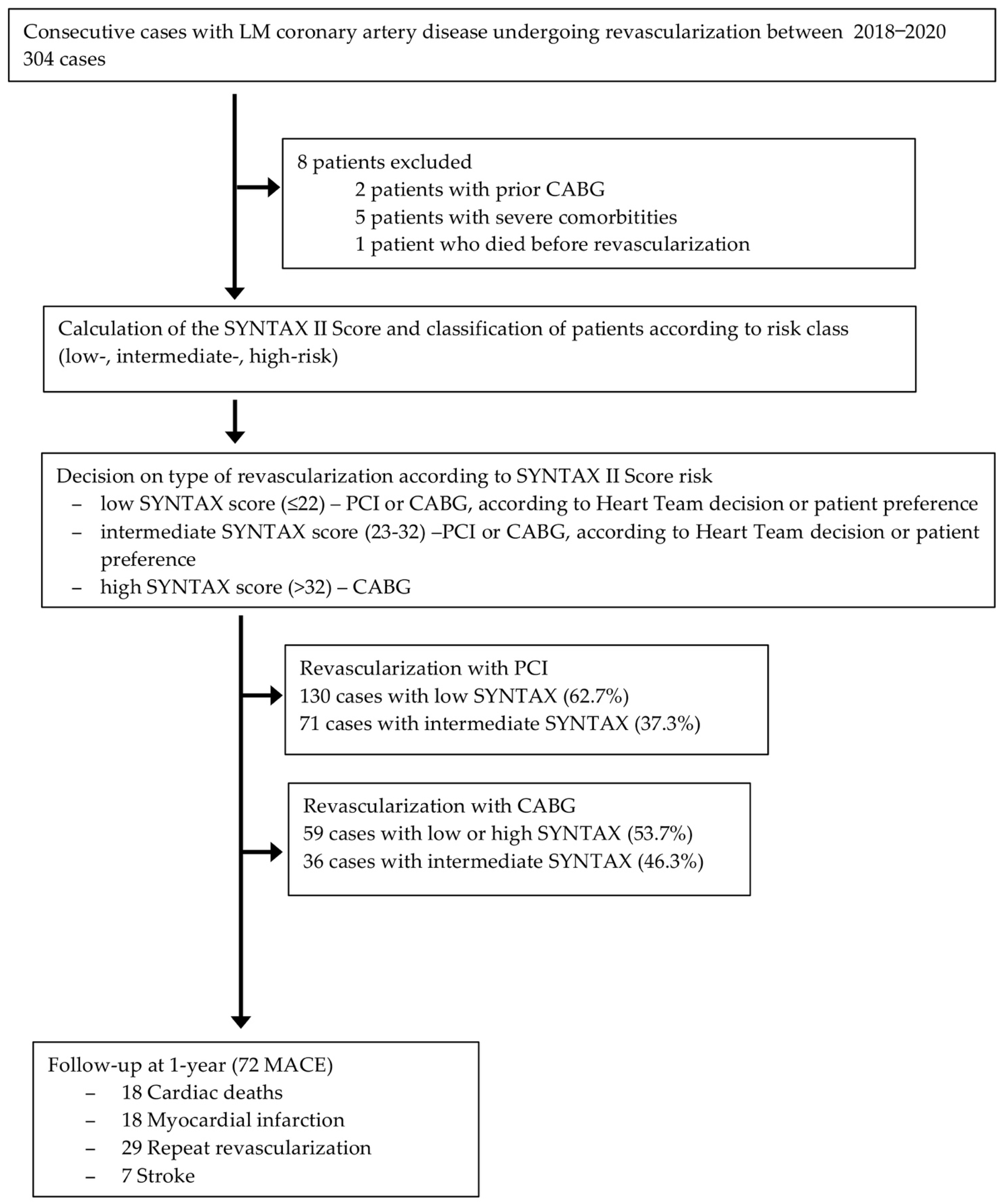
2.1. Study Population
2.2. Procedures
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
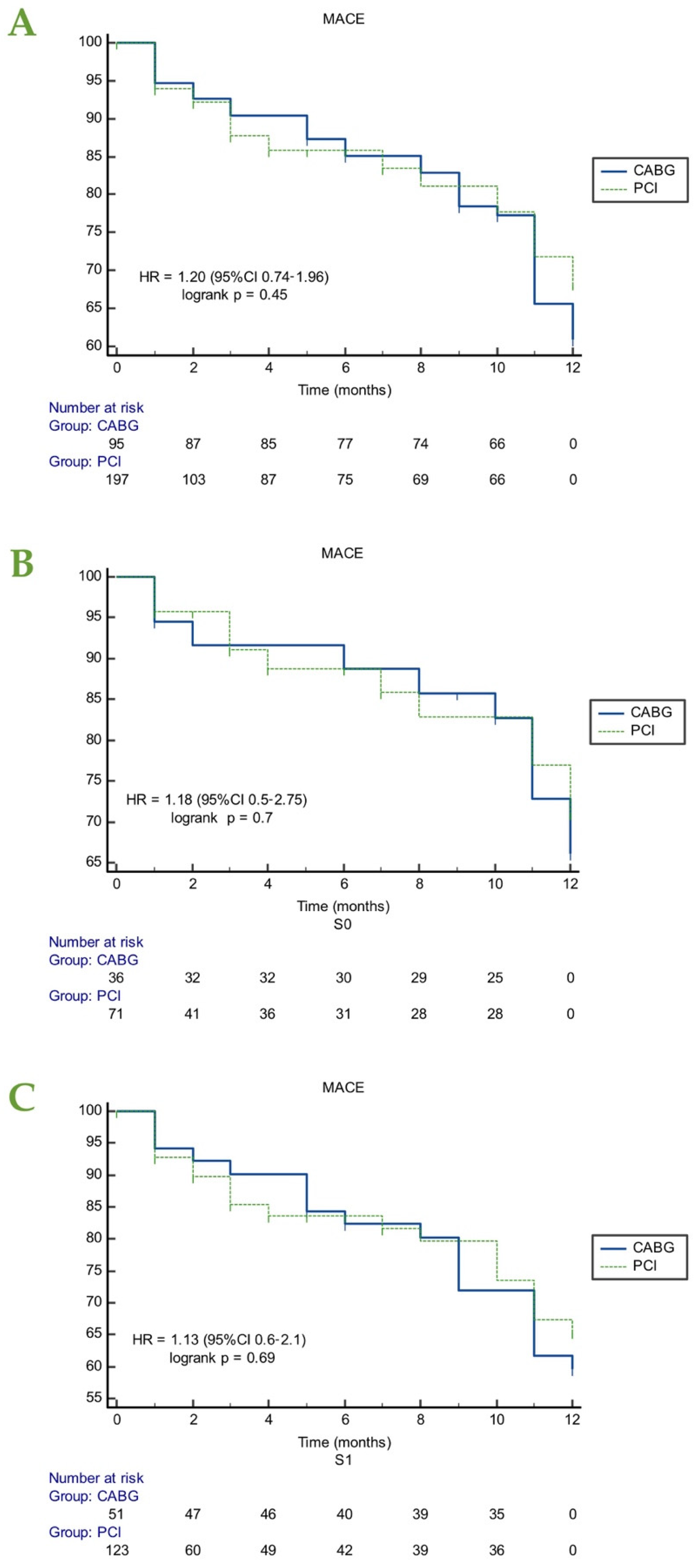
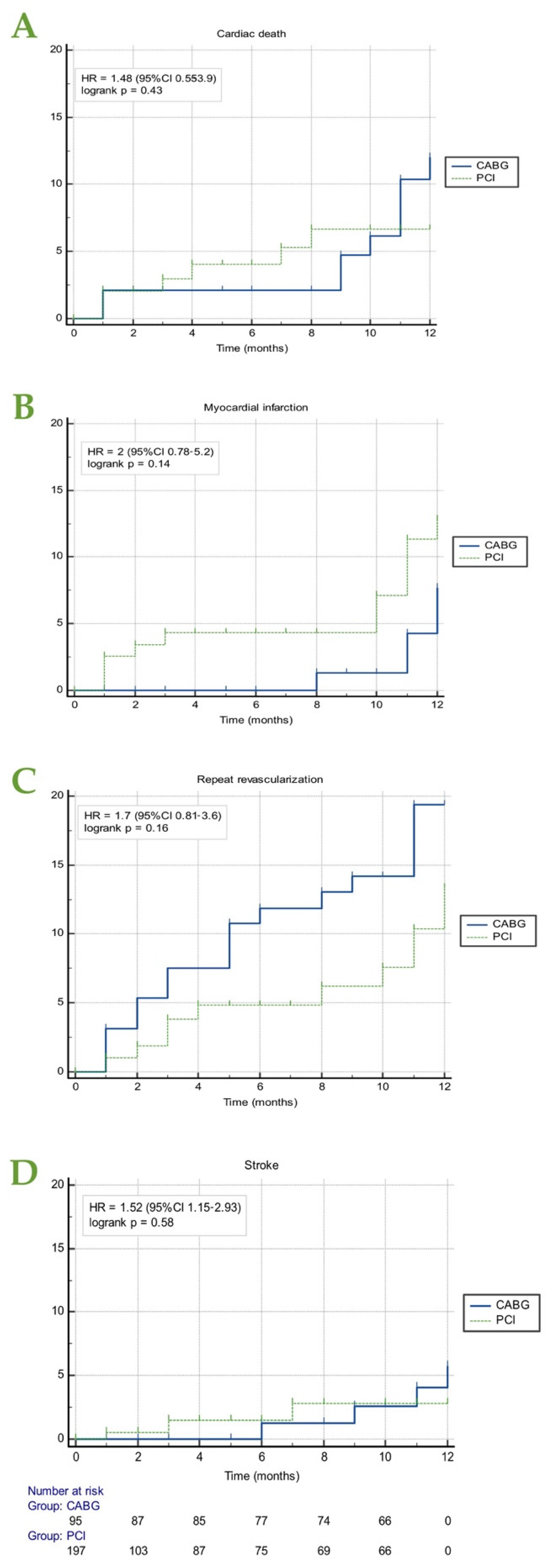
3.2. Major Cardiac Adverse Events (MACE)
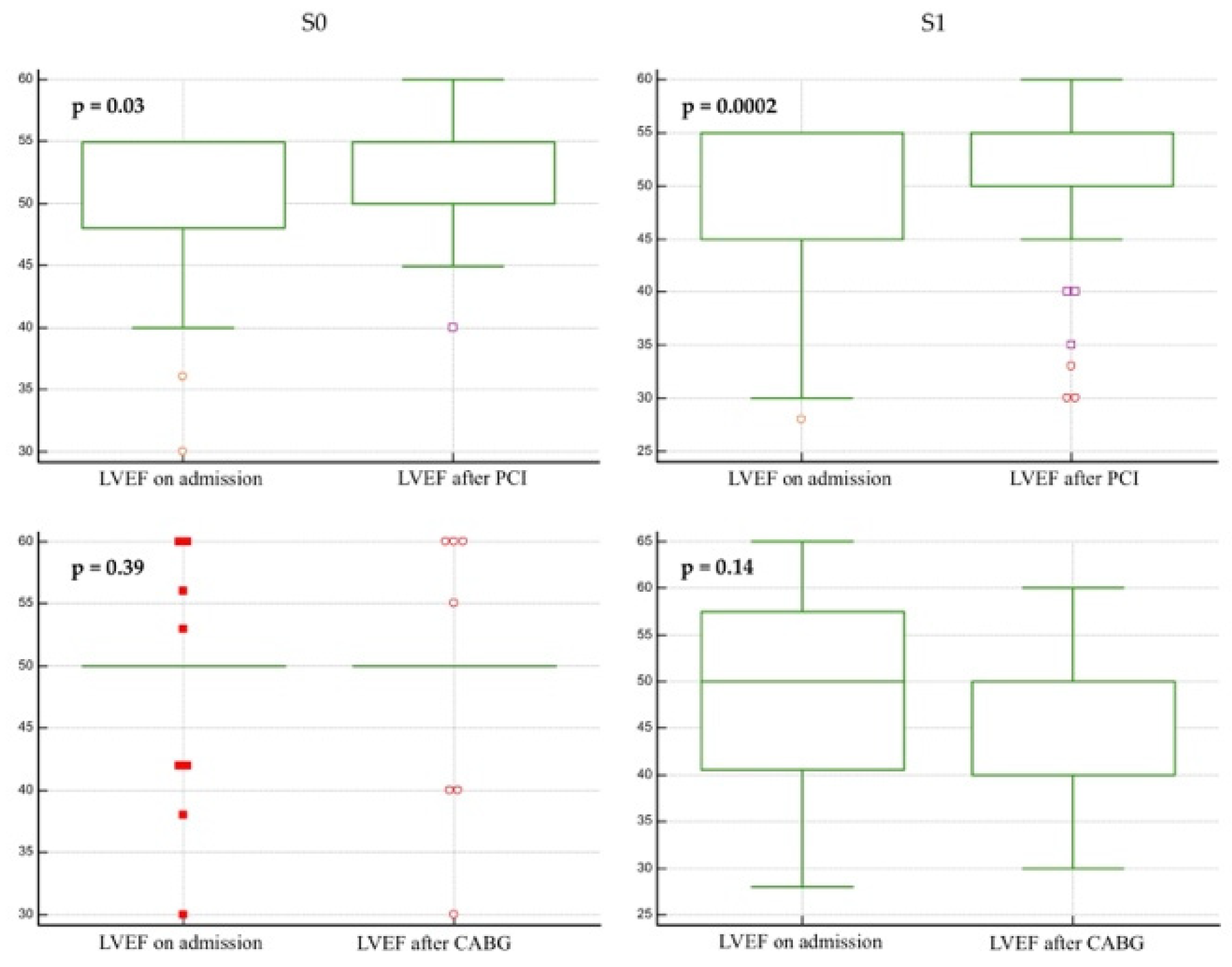
3.3. Left Ventricular Ejection Fraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeMots, H.; Rösch, J.; McAnulty, J.H.; Rahimtoola, S.H. Left main coronary artery disease. Cardiovasc. Clin. 1977, 8, 201–211. [Google Scholar] [CrossRef]
- Leaman, D.M.; Brower, R.W.; Meester, G.T.; Serruys, P.; van den Brand, M. Coronary artery atherosclerosis: Severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation 1981, 63, 285–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, H.A.; Deumite, N.J.; Chaitman, B.R.; Davis, K.B.; Killip, T.; Rogers, W.J. Asymptomatic left main coronary artery disease in the Coronary Artery Surgery Study (CASS) registry. Circulation 1989, 79, 1171–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serruys, P.W.; Morice, M.-C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; Van Den Brand, M.; Bass, E.J.; et al. SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef]
- Ahn, J.M.; Roh, J.H.; Kim, Y.H.; Park, D.W.; Yun, S.C.; Lee, P.H.; Chang, M.; Park, H.W.; Lee, S.W.; Lee, C.W.; et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease: 5-year outcomes of the PRECOMBAT study. J. Am. Coll. Cardiol. 2015, 65, 2198–2206. [Google Scholar] [CrossRef] [Green Version]
- Mohr, F.W.; Morice, M.-C.; Kappetein, A.P.; E Feldman, T.; Ståhle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R.; Morel, M.-A.; Van Dyck, N.; et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef]
- Morice, M.-C.; Serruys, P.W.; Kappetein, A.P.; Feldman, T.E.; Ståhle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R.; Choi, J.W.; Ruzyllo, W.; et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. Circulation 2014, 129, 2388–2394. [Google Scholar] [CrossRef] [Green Version]
- Thuijs, D.J.F.M.; Kappetein, A.P.; Serruys, P.W.; Mohr, F.-W.; Morice, M.-C.; Mack, M.J.; Holmes, D.R.; Curzen, N.; Davierwala, P.; Noack, T.; et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019, 394, 1325–1334. [Google Scholar] [CrossRef]
- Stone, G.W.; Sabik, J.F.; Serruys, P.W.; Simonton, C.A.; Généreux, P.; Puskas, J.; Kandzari, D.E.; Morice, M.-C.; Lembo, N.; Brown, W.M.; et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N. Engl. J. Med. 2016, 375, 2223–2235. [Google Scholar] [CrossRef]
- Mäkikallio, T.; Holm, N.R.; Lindsay, M.; Spence, M.S.; Erglis, A.; Menown, I.B.; Trovik, T.; Eskola, M.; Romppanen, H.; Kellerth, T.; et al. Percutaneous coronary angioplasty versus coronary artery bypass ing in treatment of unprotected left main stenosis (NOBLE): A prospective, randomised, open-label, non-inferiority trial. Lancet 2016, 388, 2743–2752. [Google Scholar] [CrossRef] [Green Version]
- Holm, N.R.; Mäkikallio, T.; Lindsay, M.M.; Spence, M.S.; Erglis, A.; A Menown, I.B.; Trovik, T.; Kellerth, T.; Kalinauskas, G.; Mogensen, L.J.H.; et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: Updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet 2020, 395, 191–199. [Google Scholar] [CrossRef]
- Ranucci, M.; Castelvecchio, S.; Menicanti, L.; Frigiola, A.; Pelissero, G. Risk of assessing mortality risk in elective cardiac operations: Age, creatinine, ejection fraction, and the law of parsimony. Circulation 2009, 119, 3053–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Conley, M.J.; Ely, R.L.; Kisslo, J.; Lee, K.L.; McNeer, J.F.; A Rosati, R. The prognostic spectrum of left main stenosis. Circulation 1978, 57, 947. [Google Scholar] [CrossRef] [Green Version]
- Nerlekar, N.; Ha, F.J.; Verma, K.P.; Bennett, M.R.; Cameron, J.D.; Meredith, I.T.; Brown, A.J. Percutaneous Coronary Intervention Using Drug-Eluting Stents Versus Coronary Artery Bypass Grafting for Unprotected Left Main Coronary Artery Stenosis: A Meta-Analysis of Randomized Trials. Circ. Cardiovasc. Interv. 2016, 9, e004729. [Google Scholar] [CrossRef]
- Sabatine, M.S.; A Bergmark, B.; A Murphy, S.; O’Gara, P.T.; Smith, P.K.; Serruys, P.W.; Kappetein, A.P.; Park, S.-J.; Park, D.-W.; Christiansen, E.H.; et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease: An individual patient data meta-analysis. Lancet 2021, 398, 2247–2257. [Google Scholar] [CrossRef]
- Testa, L.; Latib, A.; Bollati, M.; Montone, R.A.; Colombo, A.; Crea, F.; Bedogni, F. Unprotected left main revascularization: Percutaneous coronary intervention versus coronary artery bypass. An updated systematic review and meta-analysis of randomised controlled trials. PLoS ONE 2017, 12, e0179060. [Google Scholar] [CrossRef]
- Athappan, G.; Patvardhan, E.; Tuzcu, M.E.; Ellis, S.; Whitlow, P.; Kapadia, S.R. Left main coronary artery stenosis: A meta-analysis of drug-eluting stents versus coronary artery bypass grafting. JACC Cardiovasc. Interv. 2013, 6, 1219–1230. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.-C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown, W.M.; Lembo, N.J.; et al. Five-year outcomes after PCI or CABG for left main coronary disease. N. Engl. J. Med. 2019, 381, 1820–1830. [Google Scholar] [CrossRef]
- Giacoppo, D.; Colleran, R.; Cassese, S.; Frangieh, A.H.; Wiebe, J.; Joner, M.; Schunkert, H.; Kastrati, A.; Byrne, R.A. Percutaneous Coronary Intervention vs Coronary Artery Bypass Grafting in Patients With Left Main Coronary Artery Stenosis: A Systematic Review and Meta-analysis. JAMA Cardiol. 2017, 2, 1079–1088. [Google Scholar] [CrossRef]
- Lee, C.W.; Ahn, J.-M.; Cavalcante, R.; Sotomi, Y.; Onuma, Y.; Suwannasom, P.; Tenekecioglu, E.; Yun, S.-C.; Park, D.-W.; Kang, S.-J.; et al. Coronary Artery Bypass Surgery Versus Drug-Eluting Stent Implantation for Left Main or Multivessel Coronary Artery Disease: A Meta-Analysis of Individual Patient Data. JACC Cardiovasc. Interv. 2016, 9, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Hildick-Smith, D.; Egred, M.; Banning, A.; Brunel, P.; Ferenc, M.; Hovasse, T.; Wlodarczak, A.; Pan, M.; Schmitz, T.; Silvestri, M.; et al. The European bifurcation club Left Main Coronary Stent study: A randomized comparison of stepwise provisional vs. systematic dual stenting strategies (EBC MAIN). Eur. Heart J. 2021, 42, 3829–3839. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Ono, M.; Garg, S.; Hara, H.; Kawashima, H.; Pompilio, G.; Andreini, D.; Holmes, D.R.; Onuma, Y.; Iii, S.B.K. Percutaneous Coronary Revascularization: JACC Historical Breakthroughs in Perspective. J. Am. Coll. Cardiol. 2021, 78, 384–407. [Google Scholar] [CrossRef] [PubMed]
- Head, S.J.; Kaul, S.; Mack, M.J.; Serruys, P.W.; Taggart, D.P.; Holmes, J.D.R.; Leon, M.B.; Marco, J.; Bogers, A.J.J.C.; Kappetein, A.P. The rationale for Heart Team decision-making for patients with stable, complex coronary artery disease. Eur. Heart J. 2013, 34, 2510–2518. [Google Scholar] [CrossRef] [PubMed]


| Variable | All Patients (n = 296) | PCI Group (n = 201) 68% | CABG Group (n = 95) 32% | p Value |
|---|---|---|---|---|
| Age, years, mean ± SD | 66 ± 9.6 | 66.5 ± 9.9 | 65.4 ± 9.1 | 0.35 |
| Male sex, n (%) | 223 (75.6) | 145 (72.1) | 78 (82.1) | 0.044 |
| Hypertension, n (%) | 256 (87.4) | 179 (89) | 77 (81) | 0.108 |
| Diabetes, n (%) | 102 (34.8) | 75 (37.3) | 27 (28.4) | 0.157 |
| Dyslipidemia, n (%) | 236 (80.5) | 173 (86.1) | 63 (66.3) | 0.0002 |
| Smoking, n (%) | 37 (12.7) | 29 (14.4) | 8 (8.4) | 0.162 |
| Obesity, n (%) | 72 (24.7) | 58 (28.9) | 14 (14.7) | 0.008 |
| Chronic kidney disease, n (%) | 45 (15.4) | 38 (18.9) | 7 (7.4) | 0.011 |
| Prior ischemic stroke, n (%) | 25 (8.6) | 17 (8.45) | 8 (8.42) | 0.96 |
| Prior MI—treated conservative, n (%) | 50 (17.1) | 32 (16) | 18 (18.9) | 0.48 |
| Prior PCI | ||||
| Bare metal stent, n (%) | 11 (3.8) | 8 (4) | 3 (3.16) | 0.746 |
| Drug-eluting stent (%) | 27 (9.2) | 20 (10) | 7 (7.37) | 0.496 |
| Clinical presentation | ||||
| Stable angina, n (%) | 179 (60.5) | 95 (47.5) | 83 (87.4) | |
| Unstable angina, n (%) | 89 (30.1) | 85 (42.5) | 4 (4.2) | <0.0001 |
| NSTEMI, n (%) | 18 (6.1) | 13 (6.5) | 5 (5.3) | |
| STEMI, n (%) | 10 (3.4) | 7 (3.5) | 3 (3.1) | |
| LV dilatation, n (%) | 47 (16.3) | 32 (16) | 15 (15.8) | 0.856 |
| Hipokinesia, n (%) | 216 | 154 (76.6) | 62 (65.3) | 0.186 |
| LVEF on admission, %, mean ± SD | 49.3 ± 8.3 | 49.24 ± 7.87 | 49.5 ± 9.18 | 0.808 |
| SYNTAX II score | ||||
| 0, n (%) | 107 (36.1) | 71 (35.3) | 36 (37.9) | 0.392 |
| 1, n (%) | 189 (63.9) | 130 (64.7) | 59 (62.1) | |
| Other coronary artery stenosis, n (%) | 172 (50.7) | 105 (52.2) | 75 (79) | <0.0001 |
| No. of supplementary coronary stenoses | ||||
| 1, n (%) | 76 (26.4) | 48 (24) | 28 (29.5) | |
| 2, n (%) | 40 (13.9) | 20 (10) | 20 (21) | 0.09 |
| 3, n (%) | 52 (18.1) | 41 (20.4) | 11 (11.6) | |
| 4–5, n (%) | 7 (2.4) | 7 (3.5) | 7 (7.4) | |
| Complete revascularization, n (%) | 189 (63.9) | 138 (68.7) | 51(53.7) | 0.012 |
| LVEF on follow-up, %, mean ± SD | 49.7 ± 7.1 | 47.4 ± 8.4 | 52 ± 5.8 | 0.0001 |
| Follow-up event | ||||
| Cardiac death, n (%) | 18 (6.1) | 9 (4.5) | 9 (9.5) | |
| MI, n (%) | 18 (6.1) | 13 (6.5) | 5 (5.3) | 0.002 |
| Stroke, n (%) | 7 (2.4) | 3 (1.5) | 4 (4.2) | |
| Repeat revascularization, n (%) | 29 (9.8) | 12 (6) | 17 (17.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moț, Ș.D.C.; Șerban, A.M.; Beyer, R.Ș.; Cocoi, M.; Iuga, H.; Mureșan, I.D.; Cozma, S.; Dădârlat-Pop, A.; Tomoaia, R.; Pop, D. Percutaneous Coronary Intervention versus Coronary Artery Bypass Grafting for Non-Protected Left Main Coronary Artery Disease: 1-Year Outcomes in a High Volume Single Center Study. Life 2022, 12, 347. https://doi.org/10.3390/life12030347
Moț ȘDC, Șerban AM, Beyer RȘ, Cocoi M, Iuga H, Mureșan ID, Cozma S, Dădârlat-Pop A, Tomoaia R, Pop D. Percutaneous Coronary Intervention versus Coronary Artery Bypass Grafting for Non-Protected Left Main Coronary Artery Disease: 1-Year Outcomes in a High Volume Single Center Study. Life. 2022; 12(3):347. https://doi.org/10.3390/life12030347
Chicago/Turabian StyleMoț, Ștefan Dan Cezar, Adela Mihaela Șerban, Ruxandra Ștefana Beyer, Mihai Cocoi, Horia Iuga, Ioana Dănuța Mureșan, Simona Cozma, Alexandra Dădârlat-Pop, Raluca Tomoaia, and Dana Pop. 2022. "Percutaneous Coronary Intervention versus Coronary Artery Bypass Grafting for Non-Protected Left Main Coronary Artery Disease: 1-Year Outcomes in a High Volume Single Center Study" Life 12, no. 3: 347. https://doi.org/10.3390/life12030347
APA StyleMoț, Ș. D. C., Șerban, A. M., Beyer, R. Ș., Cocoi, M., Iuga, H., Mureșan, I. D., Cozma, S., Dădârlat-Pop, A., Tomoaia, R., & Pop, D. (2022). Percutaneous Coronary Intervention versus Coronary Artery Bypass Grafting for Non-Protected Left Main Coronary Artery Disease: 1-Year Outcomes in a High Volume Single Center Study. Life, 12(3), 347. https://doi.org/10.3390/life12030347






