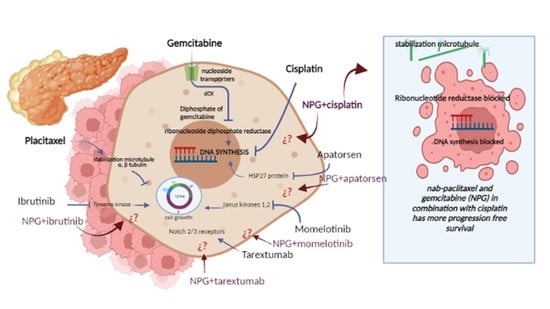
A Review on the Efficacy and Safety of Nab-Paclitaxel with Gemcitabine in Combination with Other Therapeutic Agents as New Treatment Strategies in Pancreatic Cancer
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment of Included Studies
3. Results
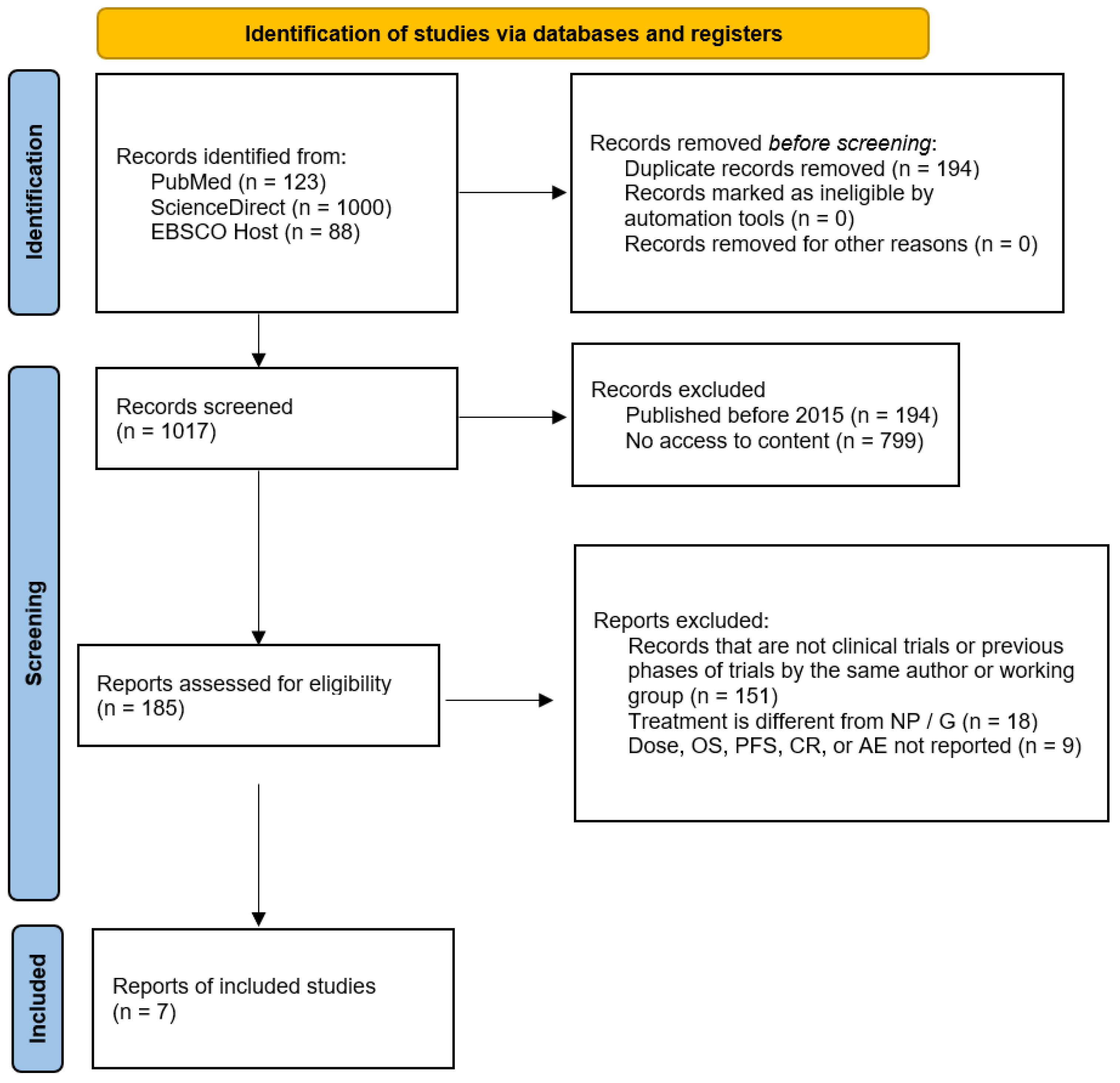
3.1. Selection of Sources of Evidence
3.2. Characteristics of Sources Evidence
3.3. Critical Appraisal within Sources of Evidence
3.4. Results of Individual Sources of Evidence
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| 5-FU | Fluorouracil |
| AE | Adverse events |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| CNTs | concentrative nucleoside transporters |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CR | Complete response |
| ENTs | Equilibrative nucleoside transporters |
| FOLFIRINOX | Chemotherapy regimen containing fluorouracil, folinic acid, irinotecan, and oxaliplatin |
| MMRI | Molecular magnetic resonance imaging |
| NP/G | Nab-paclitaxel plus gemcitabine |
| ORR | Objective response rate |
| OS | Overall survival |
| PDAC | Pancreatic ductal adenocarcinoma |
| PFS | Progression-free survival |
| PR | Partial response |
| PRISMA | Preferred Reporting Items for Systematic Reviews |
| TNBC | Triple-negative breast cancer |
| TNM | Tumor/Node/Metastasis staging system from the American Joint Committee on Cancer |
| UTI | Urinary tract infection |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Hand, F.; Conlon, K.C. Pancreatic Cancer. Surgery 2019, 37, 319–326. [Google Scholar] [CrossRef]
- Tuveson, D.A.; Neoptolemos, J.P. Understanding Metastasis in Pancreatic Cancer: A Call for New Clinical Approaches. Cell 2012, 148, 21–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippi, G.; Mattiuzzi, C. The Global Burden of Pancreatic Cancer. Arch. Med. Sci. 2020, 16, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Soweid, A.M. The Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma: Definition. Endosc. Ultrasound 2017, 6, 76. [Google Scholar] [CrossRef]
- Roalsø, M.; Aunan, J.R.; Søreide, K. Refined TNM-Staging for Pancreatic Adenocarcinoma—Real Progress or Much Ado about Nothing? Eur. J. Surg. Oncol. 2020, 46, 1554–1557. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Cheng, H.; Jin, K.; Guo, M.; Lu, Y.; Wang, Z.; Yang, C.; Long, J.; Ni, Q.; Yu, X.; et al. Application of the Eighth Edition of the American Joint Committee on Cancer Staging for Pancreatic Adenocarcinoma. Pancreas 2018, 47, 742–747. [Google Scholar] [CrossRef]
- Allen, P.J.; Kuk, D.; del Castillo, C.F.; Basturk, O.; Wolfgang, C.L.; Cameron, J.L.; Lillemoe, K.D.; Ferrone, C.R.; Morales-Oyarvide, V.; He, J.; et al. Multi-Institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients with Pancreatic Adenocarcinoma. Ann. Surg. 2017, 265, 185. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, M.E.; Erbarut-Seven, I.; Pehlivanoglu, B.; Adsay, V. Pathologic Classification of “Pancreatic Cancers”: Current Concepts and Challenges. Chin. Clin. Oncol. 2017, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Puckett, Y.; Garfield, K. Pancreatic Cancer; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic Cancer: A Review of Clinical Diagnosis, Epidemiology, Treatment and Outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.L.; Gargesha, M.; Liu, Y.; Laney, V.E.A.; Hall, R.C.; Vaidya, A.M.; Gilmore, H.; Gawelek, K.; Scott, B.B.; Roy, D.; et al. Magnetic Resonance Molecular Imaging of Extradomain B Fibronectin Enables Detection of Pancreatic Ductal Adenocarcinoma Metastasis. Magn. Reson. Imaging 2022, 86, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.-B.; Huang, J.-Q.; Huang, S.-Y.; Ahir, B.K.; Li, L.-M.; Mo, Z.-N.; Zhong, J.-H. Advances in the Detection of Pancreatic Cancer Through Liquid Biopsy. Front. Oncol. 2021, 11, 801173. [Google Scholar] [CrossRef]
- Goulart, M.R.; Watt, J.; Siddiqui, I.; Lawlor, R.T.; Imrali, A.; Hughes, C.; Saad, A.; ChinAleong, J.; Hurt, C.; Cox, C.; et al. Pentraxin 3 Is a Stromally-Derived Biomarker for Detection of Pancreatic Ductal Adenocarcinoma. NPJ Precis. Oncol. 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Hipperson, L.; Hadden, W.J.; Nahm, C.B.; Gill, A.J.; Samra, J.S.; Dona, A.; Mittal, A.; Sahni, S. Urinary Metabolite Prognostic Biomarker Panel for Pancreatic Ductal Adenocarcinomas. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129966. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Direct Endoplasmic Reticulum Targeting by the Selective Alkylphospholipid Analog and Antitumor Ether Lipid Edelfosine as a Therapeutic Approach in Pancreatic Cancer. Cancers 2021, 13, 4173. [Google Scholar] [CrossRef]
- Qi, D.; Song, X.; Xue, C.; Yao, W.; Shen, P.; Yu, H.; Zhang, Z. AKT1/FOXP3 Axis-Mediated Expression of CerS6 Promotes P53 Mutant Pancreatic Tumorigenesis. Cancer Lett. 2021, 522, 105–118. [Google Scholar] [CrossRef]
- Yuan, L.; Zhao, J.; Zhao, S.; Dong, T.; Dong, R.; Liu, D.; Ma, E.; Li, Y. ASPER-29 Suppresses the Metastasis of Pancreatic Cancer Cells by Dual Inhibition of Cathepsin-L and Cathepsin-S. Chem.-Biol. Interact. 2022, 353, 109811. [Google Scholar] [CrossRef]
- Roacho-Pérez, J.A.; Garza-Treviño, E.N.; Delgado-Gonzalez, P.; G-Buentello, Z.; Delgado-Gallegos, J.L.; Chapa-Gonzalez, C.; Sánchez-Domínguez, M.; Sánchez-Domínguez, C.N.; Islas, J.F. Target Nanoparticles against Pancreatic Cancer: Fewer Side Effects in Therapy. Life 2021, 11, 1187. [Google Scholar] [CrossRef]
- Demirtürk, N.; Bilensoy, E. Nanocarriers Targeting the Diseases of the Pancreas. Eur. J. Pharm. Biopharm. 2022, 170, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Brachi, G.; Bussolino, F.; Ciardelli, G.; Mattu, C. Nanomedicine for Imaging and Therapy of Pancreatic Adenocarcinoma. Front. Bioeng. Biotechnol. 2019, 7, 307. [Google Scholar] [CrossRef] [PubMed]
- Mousa, D.S.; El-Far, A.H.; Saddiq, A.A.; Sudha, T.; Mousa, S.A. Nanoformulated Bioactive Compounds Derived from Different Natural Products Combat Pancreatic Cancer Cell Proliferation. Int. J. Nanomed. 2020, 15, 2259–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redruello, P.; Perazzoli, G.; Cepero, A.; Quiñonero, F.; Mesas, C.; Doello, K.; Láinez-Ramos-Bossini, A.; Rivera-Izquierdo, M.; Melguizo, C.; Prados, J. Nanomedicine in Pancreatic Cancer: A New Hope for Treatment. Curr. Drug Targets 2020, 21, 1580–1592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jiang, Z.; Chen, L.; Pan, C.; Sun, S.; Liu, C.; Li, Z.; Ren, W.; Wu, A.; Huang, P. PCN-Fe(III)-PTX Nanoparticles for MRI Guided High Efficiency Chemo-Photodynamic Therapy in Pancreatic Cancer through Alleviating Tumor Hypoxia. Nano Res. 2020, 13, 273–281. [Google Scholar] [CrossRef]
- Ren, S.; Song, L.; Tian, Y.; Zhu, L.; Guo, K.; Zhang, H.; Wang, Z. Emodin-Conjugated PEGylation of Fe3O4 Nanoparticles for FI/MRI Dual-Modal Imaging and Therapy in Pancreatic Cancer. Int. J. Nanomed. 2021, 16, 7463–7478. [Google Scholar] [CrossRef]
- Lei, F.; Xi, X.; Rachagani, S.; Seshacharyulu, P.; Talmon, G.A.; Ponnusamy, M.P.; Batra, S.K.; Bronich, T.K. Nanoscale Platform for Delivery of Active IRINOX to Combat Pancreatic Cancer. J. Control. Release 2021, 330, 1229–1243. [Google Scholar] [CrossRef]
- Ou, A.; Zhao, X.; Lu, Z. The Potential Roles of P53 Signaling Reactivation in Pancreatic Cancer Therapy. Biochim. Biophys. Acta (BBA) Rev. Cancer 2022, 1877, 188662. [Google Scholar] [CrossRef]
- Polireddy, K.; Chen, Q. Cancer of the Pancreas: Molecular Pathways and Current Advancement in Treatment. J. Cancer 2016, 7, 1497–1514. [Google Scholar] [CrossRef] [Green Version]
- Digiacomo, G.; Volta, F.; Garajova, I.; Balsano, R.; Cavazzoni, A. Biological Hallmarks and New Therapeutic Approaches for the Treatment of PDAC. Life 2021, 11, 843. [Google Scholar] [CrossRef]
- Koga, F.; Kawaguchi, Y.; Shimokawa, M.; Murayama, K.; Nakashita, S.; Oza, N.; Ureshino, N.; Takahashi, H.; Ueda, Y.; Nakazawa, J.; et al. Gemcitabine plus Nab-Paclitaxel in Older Patients with Metastatic Pancreatic Cancer: A Post-Hoc Analysis of the Real-World Data of a Multicenter Study (the NAPOLEON Study). J. Geriatr. Oncol. 2022, 13, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Vogl, U.M.; Andalibi, H.; Klaus, A.; Vormittag, L.; Schima, W.; Heinrich, B.; Kafka, A.; Winkler, T.; Öhler, L. Nab-Paclitaxel and Gemcitabine or FOLFIRINOX as First-Line Treatment in Patients with Unresectable Adenocarcinoma of the Pancreas: Does Sequence Matter? 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. BMC Cancer 2019, 19, 28. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Ishido, K.; Kudo, D.; Kimura, N.; Wakiya, T.; Nakayama, Y.; Hakamada, K. Combination Therapy with Gemcitabine and Nab-Paclitaxel for Locally Advanced Unresectable Pancreatic Cancer. Mol. Clin. Oncol. 2017, 6, 963–967. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, N.; Abdalla, K.; Ibnshamsa, F.; Alselwi, W.; Al-Shakir, S.; Alqahtani, M. Exceptional Response to Second-Line Gemcitabine/Nab-Paclitaxel Chemotherapy in Patients with Metastatic Pancreatic Adenocarcinoma. Cureus 2021, 13, e18756. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Song, Q.; Li, J.; Jiang, Y.; Wang, Z.; Lu, S. Opportunities and Challenges in Targeted Therapy and Immunotherapy for Pancreatic Cancer. Expert Rev. Mol. Med. 2021, 23, e21. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.; Gavoille, C.; Conroy, T. Current Status on the Place of FOLFIRINOX in Metastatic Pancreatic Cancer and Future Directions. Ther. Adv. Gastroenterol. 2017, 10, 631–645. [Google Scholar] [CrossRef] [Green Version]
- Moris, D.; Karachaliou, G.-S.; Lazarou, V.; Giannis, D.; Astras, G.; Petrou, A. Initial Experience with Neoadjuvant FOLFIRINOX as First Line Therapy for Locally Advanced Pancreatic Cancer. J. Balk. Union Oncol. 2020, 25, 2525–2527. [Google Scholar]
- Loveday, B.P.T.; Lipton, L.; Thomson, B.N. Pancreatic Cancer: An Update on Diagnosis and Management. Aust. J. Gen. Pract. 2019, 48, 826–831. [Google Scholar] [CrossRef] [Green Version]
- Chiorean, E.G.; Coveler, A.L. Pancreatic Cancer: Optimizing Treatment Options, New, and Emerging Targeted Therapies. Drug Des. Dev. Ther. 2015, 9, 3529–3545. [Google Scholar] [CrossRef] [Green Version]
- Muranaka, T.; Kuwatani, M.; Komatsu, Y.; Sawada, K.; Nakatsumi, H.; Kawamoto, Y.; Yuki, S.; Kubota, Y.; Kubo, K.; Kawahata, S.; et al. Comparison of Efficacy and Toxicity of FOLFIRINOX and Gemcitabine with Nab-Paclitaxel in Unresectable Pancreatic Cancer. J. Gastrointest. Oncol. 2017, 8, 566–571. [Google Scholar] [CrossRef] [Green Version]
- Blomstrand, H.; Scheibling, U.; Bratthäll, C.; Green, H.; Elander, N.O. Real World Evidence on Gemcitabine and Nab-Paclitaxel Combination Chemotherapy in Advanced Pancreatic Cancer. BMC Cancer 2019, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Khot, V.M.; Salunkhe, A.B.; Pricl, S.; Bauer, J.; Thorat, N.D.; Townley, H. Nanomedicine-Driven Molecular Targeting, Drug Delivery, and Therapeutic Approaches to Cancer Chemoresistance. Drug Discov. Today 2021, 26, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Pancione, M.; Olivieri, N.; Parcesepe, P.; Velocci, M.; di Raimo, T.; Coppola, L.; Toffoli, G.; D’Andrea, M.R. Nano Albumin Bound-Paclitaxel in Pancreatic Cancer: Current Evidences and Future Directions. World J. Gastroenterol. 2017, 23, 5875–5886. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.H.; Horwitz, S.B. Taxol®: The First Microtubule Stabilizing Agent. Int. J. Mol. Sci. 2017, 18, 1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cretella, D.; Fumarola, C.; Bonelli, M.; Alfieri, R.; la Monica, S.; Digiacomo, G.; Cavazzoni, A.; Galetti, M.; Generali, D.; Petronini, P.G. Pre-Treatment with the CDK4/6 Inhibitor Palbociclib Improves the Efficacy of Paclitaxel in TNBC Cells. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- López Juárez, K. Review Research Protocol. Efficacy and Safety of Nab-Paclitaxel with Gemcitabine in Combination with Other Drugs in the Treatment of Pancreatic Cancer; Zenodo: Meyrin, Switzerland, 2021. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Halpern, S.H.; Douglas, M.J. (Eds.) Appendix: Jadad Scale for Reporting Randomized Controlled Trials. Evidence-based Obstetric Anesthesia; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; pp. 237–238. [Google Scholar] [CrossRef]
- Olivo, S.A.; Macedo, L.G.; Gadotti, I.C.; Fuentes, J.; Stanton, T.; Magee, D.J. Scales to Assess the Quality of Randomized Controlled Trials: A Systematic Review. Phys. Ther. 2008, 88, 156–175. [Google Scholar] [CrossRef] [Green Version]
- Khalil, H.; Peters, M.; Godfrey, C.M.; Mcinerney, P.; Soares, C.B.; Parker, D. An Evidence-Based Approach to Scoping Reviews. Worldviews Evid.-Based Nurs. 2016, 13, 118–123. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Tempero, M.; Oh, D.Y.; Tabernero, J.; Reni, M.; van Cutsem, E.; Hendifar, A.; Waldschmidt, D.T.; Starling, N.; Bachet, J.B.; Chang, H.M.; et al. Ibrutinib in Combination with Nab-Paclitaxel and Gemcitabine for First-Line Treatment of Patients with Metastatic Pancreatic Adenocarcinoma: Phase III RESOLVE Study. Ann. Oncol. 2021, 32, 600–608. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Barone, D.; Mahalingam, D.; Bekaii-Saab, T.; Shao, S.H.; Wolf, J.; Rosano, M.; Krause, S.; Richards, D.A.; Yu, K.H.; et al. Randomised Phase II Trial of Gemcitabine and Nab-Paclitaxel with Necuparanib or Placebo in Untreated Metastatic Pancreas Ductal Adenocarcinoma. Eur. J. Cancer 2020, 132, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.I.; Bendell, J.C.; Bullock, A.; LoConte, N.K.; Hatoum, H.; Ritch, P.; Hool, H.; Leach, J.W.; Sanchez, J.; Sohal, D.P.S.; et al. A Randomized Phase II Trial of Nab-Paclitaxel and Gemcitabine with Tarextumab or Placebo in Patients with Untreated Metastatic Pancreatic Cancer. Cancer Med. 2019, 8, 5148–5157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, A.H.; Murphy, P.B.; Peyton, J.D.; Shipley, D.L.; Al-Hazzouri, A.; Rodriguez, F.A.; Womack, M.S.; Xiong, H.Q.; Waterhouse, D.M.; Tempero, M.A.; et al. A Randomized, Double-Blinded, Phase II Trial of Gemcitabine and Nab-Paclitaxel Plus Apatorsen or Placebo in Patients with Metastatic Pancreatic Cancer: The RAINIER Trial. Oncologist 2017, 22, 1427-e129. [Google Scholar] [CrossRef] [Green Version]
- Jameson, G.S.; Borazanci, E.; Babiker, H.M.; Poplin, E.; Niewiarowska, A.A.; Gordon, M.S.; Barrett, M.T.; Rosenthal, A.; Stoll-D’Astice, A.; Crowley, J.; et al. Response Rate Following Albumin-Bound Paclitaxel Plus Gemcitabine Plus Cisplatin Treatment Among Patients with Advanced Pancreatic Cancer: A Phase 1b/2 Pilot Clinical Trial. JAMA Oncol. 2020, 6, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Mahipal, A.; Tella, S.H.; Kommalapati, A.; Goyal, G.; Soares, H.; Neuger, A.; Copolla, D.; Kim, J.; Kim, R. Phase 1 Trial of Enzalutamide in Combination with Gemcitabine and Nab-Paclitaxel for the Treatment of Advanced Pancreatic Cancer. Investig. New Drugs 2019, 37, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Hendifar, A.; Starodub, A.; Chaves, J.; Yang, Y.; Koh, B.; Barbie, D.; Hahn, W.C.; Fuchs, C.S. Phase 1 Dose-Escalation Study of Momelotinib, a Janus Kinase 1/2 Inhibitor, Combined with Gemcitabine and Nab-Paclitaxel in Patients with Previously Untreated Metastatic Pancreatic Ductal Adenocarcinoma. Investig. New Drugs 2019, 37, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Yardley, D.A. Nab-Paclitaxel Mechanisms of Action and Delivery. J. Control. Release 2013, 170, 365–372. [Google Scholar] [CrossRef]
- Veltkamp, S.A.; Jansen, R.S.; Callies, S.; Pluim, D.; Visseren-Grul, C.M.; Rosing, H.; Kloeker-Rhoades, S.; Andre, V.A.M.; Beijnen, J.H.; Slapak, C.A.; et al. Oral Administration of Gemcitabine in Patients with Refractory Tumors: A Clinical and Pharmacologic Study. Clin. Cancer Res. 2008, 14, 3477–3486. [Google Scholar] [CrossRef] [Green Version]
- Espey, M.G.; Chen, P.; Chalmers, B.; Drisko, J.; Sun, A.Y.; Levine, M.; Chen, Q. Pharmacologic Ascorbate Synergizes with Gemcitabine in Preclinical Models of Pancreatic Cancer. Free Radic. Biol. Med. 2011, 50, 1610–1619. [Google Scholar] [CrossRef] [Green Version]
- Barton-Burke, M. Gemcitabine: A Pharmacologic and Clinical Overview. Cancer Nurs. 1999, 22, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Dilruba, S.; Kalayda, G.V. Platinum-Based Drugs: Past, Present and Future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Hato, S.V.; Khong, A.; de Vries, I.J.M.; Lesterhuis, W.J. Molecular Pathways: The Immunogenic Effects of Platinum-Based Chemotherapeutics. Clin. Cancer Res. 2014, 20, 2831–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Vitale, I.; Michels, J.; Brenner, C.; Szabadkai, G.; Harel-Bellan, A.; Castedo, M.; Kroemer, G. Systems Biology of Cisplatin Resistance: Past, Present and Future. Cell Death Dis. 2014, 5, e1257. [Google Scholar] [CrossRef] [Green Version]
- Reedijk, J.; Lohman, P.H.M. Cisplatin: Synthesis, Antitumour Activity and Mechanism of Action. Pharm. Weekbl. Sci. Ed. 1985, 7, 7–173. [Google Scholar] [CrossRef]
- Ibrutinib: Uses, Interactions, Mechanism of Action. DrugBank Online. Available online: https://go.drugbank.com/drugs/DB09053 (accessed on 16 January 2022).
- Hughes, D.L. Patent Review of Manufacturing Routes to Recently Approved Oncology Drugs: Ibrutinib, Cobimetinib, and Alectinib. Org. Process Res. Dev. 2016, 20, 1855–1869. [Google Scholar] [CrossRef]
- Necuparanib: Uses, Interactions, Mechanism of Action. DrugBank Online. Available online: https://go.drugbank.com/drugs/DB16251 (accessed on 16 January 2022).
- MacDonald, A.; Priess, M.; Curran, J.; Guess, J.; Farutin, V.; Oosterom, I.; Chu, C.L.; Cochran, E.; Zhang, L.; Getchell, K.; et al. Necuparanib, A Multitargeting Heparan Sulfate Mimetic, Targets Tumor and Stromal Compartments in Pancreatic Cancer. Mol. Cancer Ther. 2019, 18, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Tarextumab: Uses, Interactions, Mechanism of Action. DrugBank Online. Available online: https://go.drugbank.com/drugs/DB12104 (accessed on 16 January 2022).
- Shah, J.; O’Young, G.; Wei, J.; Fischer, M.; Yen, W.-C.; Cancilla, B.; Kapoun, A.; Lewicki, J.; Cain, J.; Hoey, T. Tarextumab (Anti-NOTCH2/3) Reverses NOTCH2 and NOTCH3-Dependent Tumorigenicity and Metastases in Small Cell Lung Cancer. Cancer Res. 2015, 75, 2323. [Google Scholar] [CrossRef]
- Apatorsen: Uses, Interactions, Mechanism of Action. DrugBank Online. Available online: https://go.drugbank.com/drugs/DB06094 (accessed on 16 January 2022).
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X. Hai Antisense Technology: An Overview and Prospectus. Nat. Rev. Drug Discov. 2021, 20, 427–453. [Google Scholar] [CrossRef]
- Cisplatin: Uses, Interactions, Mechanism of Action. DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00515 (accessed on 16 January 2022).
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Enzalutamide: Uses, Interactions, Mechanism of Action. DrugBank Online. Available online: https://go.drugbank.com/drugs/DB08899 (accessed on 16 January 2022).
- Erdogan, B.; Kostek, O.; Bekirhacioglu, M. Enzalutamide in Prostate Cancer, A Review on Enzalutamide and Cancer. Eurasian J. Med. Oncol. 2018, 2, 121–129. [Google Scholar] [CrossRef]
- Momelotinib: Uses, Interactions, Mechanism of Action. DrugBank Online. Available online: https://go.drugbank.com/drugs/DB11763 (accessed on 16 January 2022).
- Azhar, M.; Kincaid, Z.; Kesarwani, M.; Ahmed, A.; Wunderlich, M.; Latif, T.; Starczynowski, D.; Azam, M. Momelotinib Is a Highly Potent Inhibitor of FLT3-Mutant AML. Blood Adv. 2021, 6, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. JNCI J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Litière, S.; Isaac, G.; de Vries, E.G.E.; Bogaerts, J.; Chen, A.; Dancey, J.; Ford, R.; Gwyther, S.; Hoekstra, O.; Huang, E.; et al. RECIST 1.1 for Response Evaluation Apply Not Only to Chemotherapy-Treated Patients but Also to Targeted Cancer Agents: A Pooled Database Analysis. J. Clin. Oncol. 2019, 37, 1102–1110. [Google Scholar] [CrossRef]
| Study | Clinical Phase | Sex | Age | |
|---|---|---|---|---|
| Male | Female | Median | ||
| M. Tempero et al. (2021) [53] | III | 189 | 235 | 64 |
| E. M. O’Reilly et al. (2020) [54] | II | 58 | 62 | 64 |
| Z. I. Hu et al. (2019) [55] | II | 73 | 104 | 66 |
| A. H. Ko et al. (2017) [56] | II | 57 | 75 | 66 |
| G. S. Jameson et al. (2020) [57] | 1b/2 | 11 | 14 | 65 |
| R. K. Mahipal et al. (2020) [58] | I | 4 | 16 | 68 |
| K. Ng et al. (2019) [59] | I | 8 | 17 | 61 |
| M. Tempero et al. (2021) [53] | E. M. O’Reilly et al. (2020) [54] | Z. I. Hu et al. (2019) [55] | A. H. Ko et al. (2017) [56] | |
|---|---|---|---|---|
| Randomized | Yes | Yes | Yes | Yes |
| Appropriately randomized | No | No | No | No |
| Described withdrawals | Yes | Yes | Yes | Yes |
| Double-blinded | Yes | No | No | No |
| Described blinding | Yes | Yes | Yes | No |
| Jadad score | 4 | 3 | 3 | 2 |
| Study | Question | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | |
| G. S. Jameson et al. (2020) [57] | Yes | Yes | Yes | Not applicable | Yes | Unclear | Yes | Unclear | Yes |
| R. K. Mahipal et al. (2020) [58] | Yes | Yes | Yes | Not applicable | Yes | No | Yes | Unclear | Yes |
| K. Ng et al. (2019) [59] | Yes | Yes | Yes | Not applicable | Yes | Yes | Yes | Unclear | Yes |
| Study | Patients n | Regimen * | OS, Months | PFS, Months | PR, n (%) | CR, n (%) | ORR |
|---|---|---|---|---|---|---|---|
| M. Tempero et al. (2021) [53] | 213 | NP/G + placebo | 10.8 | 6 | 90 (42%) | 3 (1%) | 43 |
| 211 | NP/G + ibrutinib (560 mg) | 9.7 | 5.3 | 62 (29%) | 0 | 29 | |
| E. M. O´Reilly et al. (2020) [54] | 58 | NP/G + placebo | 9.99 | 6.93 | 8 (14%) | 2 (3%) | 17 |
| 62 | NP/G + necuparanib (5 mg/kg) | 10.71 | 5.52 | 14 (23%) | 0 | 23 | |
| Z. I. Hu et al. (2019) [55] | 88 | NP/G + placebo | 7.9 | 5.5 | 28 (32%) | 0 | 32 |
| 89 | NP/G + tarextumab (15 mg/kg) | 6.4 | 3.7 | 18 (20%) | 0 | 20 | |
| A. H. Ko et al. (2017) [56] | 66 | NP/G + placebo | 6.9 | 3.8 | 12 (18%) | 0 | 18 |
| 66 | NP/G + apatorsen (600 mg) | 5.3 | 2.7 | 12 (18%) | 0 | 18 | |
| G. S. Jameson et al. (2020) [57] | 25 | NP/G + cisplatin (25 mg/m2) | 16.4 | 10.1 | 15 (62.5%) | 2 (8.33%) | 71 |
| A. Mahipal et al. (2020) [58] | 12 | NP/G + enzalutamide (80 and 160 mg) | 9.73 | 7.53 | 4 (33%) | 0 | 33 |
| 12 | NP/G + enzalutamide (160 mg) | 1 (8.33%) | 0 | 8.33 | |||
| K. Ng et al. (2019) [59] | 7 | NP/G + momelotinib (100 mg once daily) | 8.7 | 5.7 | 2 (28.6%) | 0 | 29 |
| 4 | NP/G + momelotinib (150 mg once daily) | 1 (25%) | 0 | 25 | |||
| 7 | NP/G + momelotinib (200 mg once daily) | 3 (42.9%) | 0 | 43 | |||
| 3 | NP/G + momelotinib (150 mg twice daily) | 1 (33.3%) | 0 | 33.33 |
| Disorders | M. Tempero et al. (2021) [53] | E. M. O´Reilly et al. (2020) [54] | Z. I. Hu et al. (2019) [55] | A. H Ko et al. (2017) [56] | |||
|---|---|---|---|---|---|---|---|
| Placebo, n = 212 | Ibrutinib, n = 208 | Placebo, n = 57 | Necuparanib, n = 60 | Placebo, n = 85 | Tarextumab, n = 87 | Apatorsen, n = 12 | |
| Abdominal pain | 34% | 32% | 26% | 25% | — | — | — |
| Alopecia | 41% | 43% | — | — | — | — | — |
| ALT increase | — | — | 12% | 35% | — | — | — |
| Anemia | 45% | 44% | — | — | 26% | 29% | 17% |
| AST increase | — | — | 16% | 27% | — | — | — |
| Constipation | 45% | 49% | — | — | — | — | 8% |
| Decreased appetite | 37% | 33% | — | — | 13% | 17% | 17% |
| Dehydration | 36% | 41% | — | — | 12% | 9% | 8% |
| Diarrhea | 52% | 71% | 21% | 50% | 40% | 72% | 58% |
| Dysgeusia | 20% | 13% | — | — | 9% | 13% | 8% |
| Dysphagia | — | — | — | — | — | — | 8% |
| Dyspnea | 31% | 38% | — | — | — | — | — |
| Epistaxis | 52% | 56% | — | — | 1% | 10% | — |
| Fall | — | — | — | — | — | — | 8% |
| Fatigue | 40% | 35% | 54% | 60% | 59% | 52% | 42% |
| Fever | 36% | 29% | — | — | 12% | 9% | — |
| Hyperbilirubinemia | — | — | 5% | 3% | — | — | — |
| Hyperglycemia | — | — | — | — | — | — | 8% |
| Hypersensitivity | — | — | — | — | — | — | 8% |
| Hypokalemia | — | — | 12% | 15% | — | — | 17% |
| Hypomagnesemia | — | — | — | — | — | — | 8% |
| Hyponatremia | — | — | 12% | 22% | — | — | — |
| Hypophosphatemia | — | — | 2% | 10% | — | — | — |
| Insomnia | — | — | — | — | — | — | 8% |
| Mucosal inflammation | — | — | — | — | — | — | 8% |
| Myalgia | — | — | — | — | — | — | 8% |
| Nausea | 30% | 33% | 33% | 53% | 31% | 41% | 67% |
| Neuropathy peripheral | — | — | 25% | 18% | — | — | — |
| Neutropenia | 6% | 7% | — | — | 18% | 9% | — |
| Peripheral edema | 41% | 44% | 21% | 27% | — | — | 8% |
| Peripheral embolism | — | — | — | — | — | — | 8% |
| Pericardial effusion | — | — | — | 3% | — | — | — |
| Peripheral sensory neuropathy | — | — | 9% | 8% | — | — | 8% |
| Pleural effusion | — | — | 2% | 7% | — | — | — |
| Pneumonia | — | — | 4% | 7% | — | — | — |
| Pruritus | — | — | — | — | — | — | 8% |
| Rash | — | — | — | — | — | — | 24% |
| Sinus tachycardia | — | — | — | — | — | — | 8% |
| Stomatitis | — | — | — | — | — | — | 25% |
| Temperature intolerance | — | — | — | — | — | — | 8% |
| Thrombocytopenia | 26% | 37% | — | — | 25% | 49% | 17% |
| Vomiting | 42% | 42% | — | — | 16% | 22% | 42% |
| Weight loss | — | — | — | — | — | — | 8% |
| Adverse Event | G. S. Jameson et al. (2020) [57] * | K. Ng et al. (2019) [59] | A. Mahipal et al. (2020) [58] |
|---|---|---|---|
| Abdominal pain | — | 44% | 41.67% |
| Acute cryptosporidiosis | yes | — | — |
| Alkaline phosphatase increase | — | — | 66.67% |
| Alopecia | — | 40% | 20.84 |
| ALT increase | — | — | 58.34% |
| Anemia | yes | 68% | 91.67% |
| Anorectal infection | yes | — | — |
| Arthralgia | — | — | 37.5% |
| AST increase | — | — | 50.01% |
| Bilirubin increase | — | — | 16.67% |
| Cachexia | — | 4.00% | — |
| Constipation | — | 52% | — |
| Cough | — | — | 12.5% |
| Death | yes | — | — |
| Decreased appetite | — | 40% | — |
| Decreased neutrophil count | yes | 8.00% | 58.34% |
| Decreased weight | — | 4.00% | — |
| Deep vein thrombosis | — | 4.00% | — |
| Dehydration | yes | 4.00% | 12.5% |
| Diarrhea | yes | 64% | 62.51% |
| Dizziness | — | — | 12.5% |
| Dysgeusia | — | 40% | — |
| Dyspnea | — | — | 37.5% |
| Edema limbs | — | — | 25% |
| Embolic stroke | — | 4.00% | — |
| Epistaxis | yes | — | 16.67% |
| Fall | — | — | 12.5% |
| Fatigue | yes | 80% | 62.5% |
| Febrile neutropenia | yes | 4.00% | — |
| Fever | yes | 4.00% | 41.67% |
| Generalized edema | — | — | — |
| Generalized muscle weakness | — | — | 20.84% |
| Headache | — | — | 20.84% |
| Hyperkalemia | — | — | 29.17% |
| Hypertension | — | 36% | 16.66% |
| Hypoalbuminemia | — | — | 45.84% |
| Hypokalemia | yes | — | 20.83% |
| Hyponatremia | — | — | 41.67% |
| Increased blood uric acid | — | 4.00% | — |
| Lung infection | — | — | 12.5% |
| Lymphocyte count decreased | yes | — | 37.5% |
| Lymphocyte count increased | yes | — | — |
| Maculopapular rash | — | — | 25% |
| Malaise | — | 4.00% | — |
| Mucositis | — | — | 25% |
| Myalgia | — | — | 16.67 |
| Nausea | yes | 76% | 70.84% |
| Nephrolithiasis | — | 4.00% | — |
| Neutropenia | — | 16% | — |
| Peripheral edema | — | 48% | — |
| Peripheral motor neuropathy | yes | — | — |
| Peripheral neuropathy | — | 36% | — |
| Peripheral sensory motor neuropathy | — | 8% | 54.17% |
| Peripheral sensory neuropathy | — | 36% | — |
| Platelet count decreased | yes | — | 70.84% |
| Pneumonia | — | 24% | — |
| Polyneuropathy | — | 4.00% | — |
| Pyrexia | — | 56% | — |
| Respiratory distress | — | 4.00% | — |
| Stroke | yes | — | — |
| Thrombocytopenia | — | 8% | — |
| Thromboembolic event | — | — | 20.83% |
| Tremor | — | 4.00% | — |
| Urinary tract infection (UTI) | — | — | 12.5% |
| Vomiting | yes | 52% | 50% |
| White blood cell decreased | yes | — | 66.67% |
| Wound infection | — | — | 8.33% |
| Ref | Therapeutic Agent | Structure | Description |
|---|---|---|---|
| [53] | Ibrutinib | 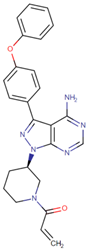 | Ibrutinib is a Bruton’s tyrosine kinase inhibitor that forms a covalent bond with a cysteine residue (Cys 481). Ibrutinib is used to treat chronic lymphocytic leukemia, mantle cell lymphoma, and Waldenstrom‘s macroglobulinemia, leading to inhibition of BTK activity [69,70]. ClinicalTrials.gov identifier: NCT024366. Phase III RESOLVE study. Ibrutinib plus nab-paclitaxel/gemcitabine did not improve OS or PFS for patients with metastatic PDAC. |
| [54] | Necuparanib | — | Necuparanib (a heparin mimetic) acts as a multitargeting therapeutic, altering multiple signaling pathways simultaneously by binding and sequestering different proteins [71,72]. ClinicalTrials.gov identifier: NCT01621243. A randomized phase II trial. Necuparanib plus nab-paclitaxel/gemcitabine did not improve OS. |
| [55] | Tarextumab | — | Monoclonal antibodies (mAb, anti-Notch2/3, OMP-59R5) are fully human monoclonal antibodies that target the Notch2 and Notch3 receptors. They have been used in trials studying the treatment of solid tumors, stage IV pancreatic cancer, and stage IV small cell lung cancer [73,74]. ClinicalTrials.gov identifier: NCT01647828. A randomized phase II trial. Tarextumab plus nab-paclitaxel/gemcitabine did not improve OS, PFS, or ORR in first-line metastatic PDAC |
| [56] | Apatorsen |  | Apatorsen is a second-generation antisense drug in preclinical experiments that inhibits the production of heat shock protein 27 (Hsp27), a cell survival protein found at elevated levels in many human cancers, including prostate, lung, breast, ovarian, bladder, renal, pancreatic, multiple myeloma, and liver cancer [75,76]. ClinicalTrials.gov identifier: NCT01844817. A randomized, double-blinded, phase II trial. The RAINIER trial. Addition of apatorsen to nab-paclitaxel/gemcitabine regimen did not improve survival or other clinically relevant endpoints in patients with metastatic pancreatic cancer. |
| [57] | Cisplatin | 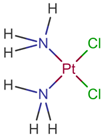 | Cisplatin is a platinum-based chemotherapy agent used to treat various sarcomas, carcinomas, lymphomas, and germ cell tumors. Cisplatin exerts its anticancer activities by generating DNA lesions through interactions with purine bases, leading to the activation of various signal transduction pathways leading to apoptosis [68,77,78]. ClinicalTrials.gov identifier: NCT01893801. A nonrandomized phase 1b/2 pilot clinical trial. The addition of cisplatin to nab-paclitaxel/gemcitabine resulted in a high response rate and evolving OS. |
| [58] | Enzalutamide | 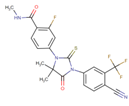 | Enzalutamide is a rationally designed, targeted androgen-receptor inhibitor used to treat castration-resistant prostate cancer. Enzalutamide acts both by inhibiting the translocation of the androgen receptor into the nucleus and by reducing the transcriptional activity of this receptor [79,80]. ClinicalTrials.gov identifier: NCT02138383. A phase I trial. Enzalutamide plus nab-paclitaxel/gemcitabine was safely administered with no unexpected toxicities and resulted in consistent reductions in CA 19–9 (biological marker) levels. |
| [59] | Momelotinib | 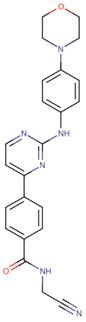 | Momelotinib is a benzamide that acts as an ATP-competitive JAK1/JAK2 inhibitor. Momelotinib has been used in trials studying the treatment of polycythemia vera, primary myelofibrosis, post-polycythemia vera, essential thrombocythemia, and primary myelofibrosis (PMF), among others [81,82]. ClinicalTrials.gov identifier: NCT02101021. Phase 1 dose-escalation study. Momelotinib plus nab-paclitaxel/gemcitabine was safe and well tolerated, with no OS or PFS benefits. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapa-González, C.; López, K.; Lomelí, K.M.; Roacho-Pérez, J.A.; Stevens, J.C. A Review on the Efficacy and Safety of Nab-Paclitaxel with Gemcitabine in Combination with Other Therapeutic Agents as New Treatment Strategies in Pancreatic Cancer. Life 2022, 12, 327. https://doi.org/10.3390/life12030327
Chapa-González C, López K, Lomelí KM, Roacho-Pérez JA, Stevens JC. A Review on the Efficacy and Safety of Nab-Paclitaxel with Gemcitabine in Combination with Other Therapeutic Agents as New Treatment Strategies in Pancreatic Cancer. Life. 2022; 12(3):327. https://doi.org/10.3390/life12030327
Chicago/Turabian StyleChapa-González, Christian, Karina López, Kimberly Michelle Lomelí, Jorge Alberto Roacho-Pérez, and Jazmín Cristina Stevens. 2022. "A Review on the Efficacy and Safety of Nab-Paclitaxel with Gemcitabine in Combination with Other Therapeutic Agents as New Treatment Strategies in Pancreatic Cancer" Life 12, no. 3: 327. https://doi.org/10.3390/life12030327
APA StyleChapa-González, C., López, K., Lomelí, K. M., Roacho-Pérez, J. A., & Stevens, J. C. (2022). A Review on the Efficacy and Safety of Nab-Paclitaxel with Gemcitabine in Combination with Other Therapeutic Agents as New Treatment Strategies in Pancreatic Cancer. Life, 12(3), 327. https://doi.org/10.3390/life12030327