Partial Purification and Characterization of Exo-Polygalacturonase Produced by Penicillium oxalicum AUMC 4153
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Culturing Media
2.3. Sugar Beet Pulp Powder
2.4. Chemicals
2.5. Cultivation of Fungi and Exo-PG Production by Submerged Fermentation
2.6. Assay for Exo-Polygalacturonase Activity (Exo-PG)
2.7. Estimation of Protein Content
2.8. Purification of Exo-Polygalacturonase
2.8.1. Ammonium Sulfate Precipitation
2.8.2. Dialysis
2.8.3. Acetone Precipitation
2.8.4. Gel Filtration Chromatography
2.9. Characterization of Partially Purified Exo-Polygalacturonase
2.9.1. Effect of Temperature on the Exo-Polygalacturonase Activity
2.9.2. Effect of pH on the Exo-Polygalacturonase Activity
2.9.3. Thermal Stability of Exo-Polygalacturonase Activity
2.9.4. pH Stability of Exo-Polygalacturonase Activity
2.9.5. Kinetics Constants of Exo-Polygalacturonase
2.10. Statistical Analysis
3. Results
3.1. Purification of Exo-Polygalacturonase Enzyme
3.2. Characterization of Partially Purified Exo-Polygalacturonase
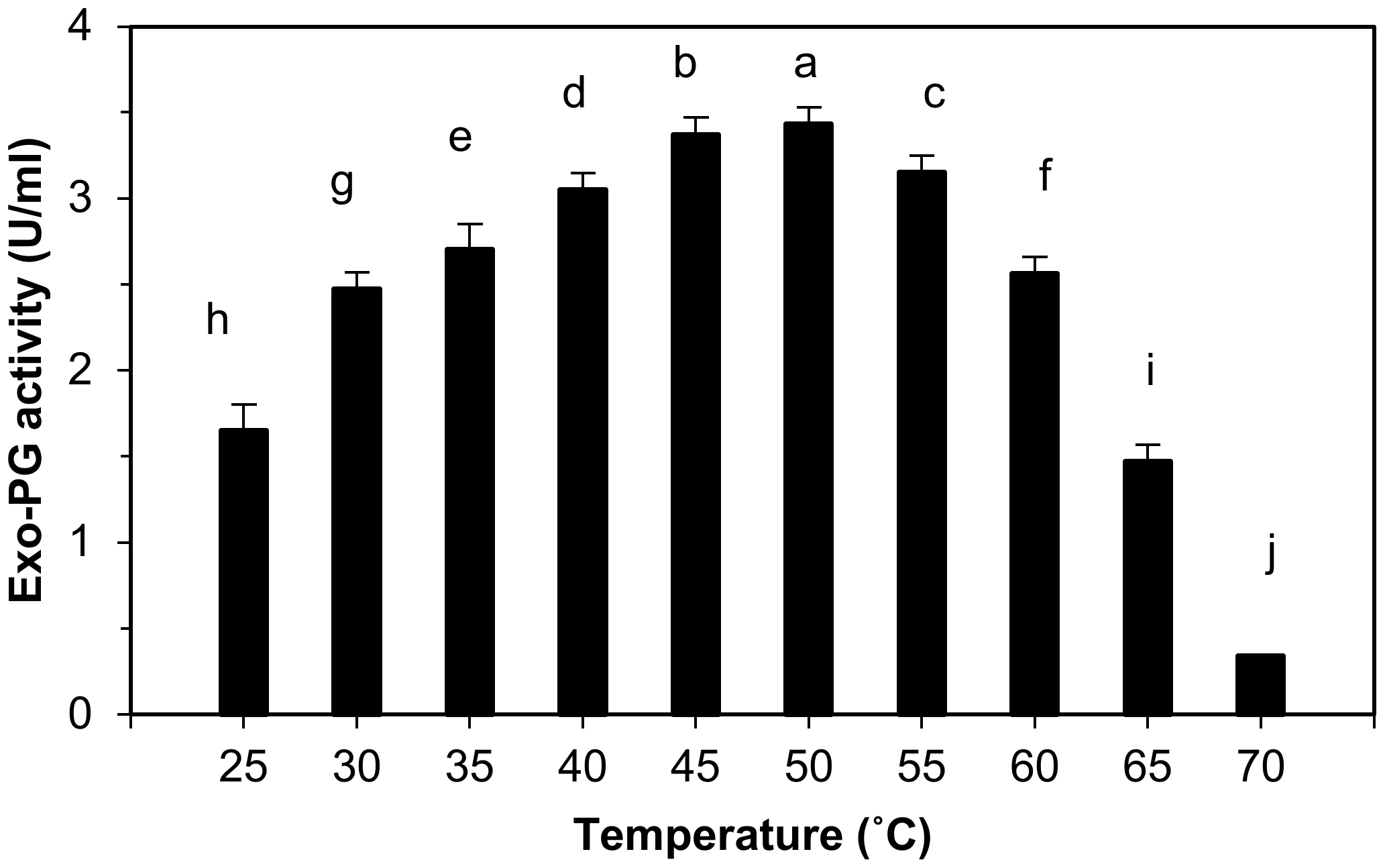
3.2.1. Effect of Temperature on the Exo-Polygalacturonase Activity
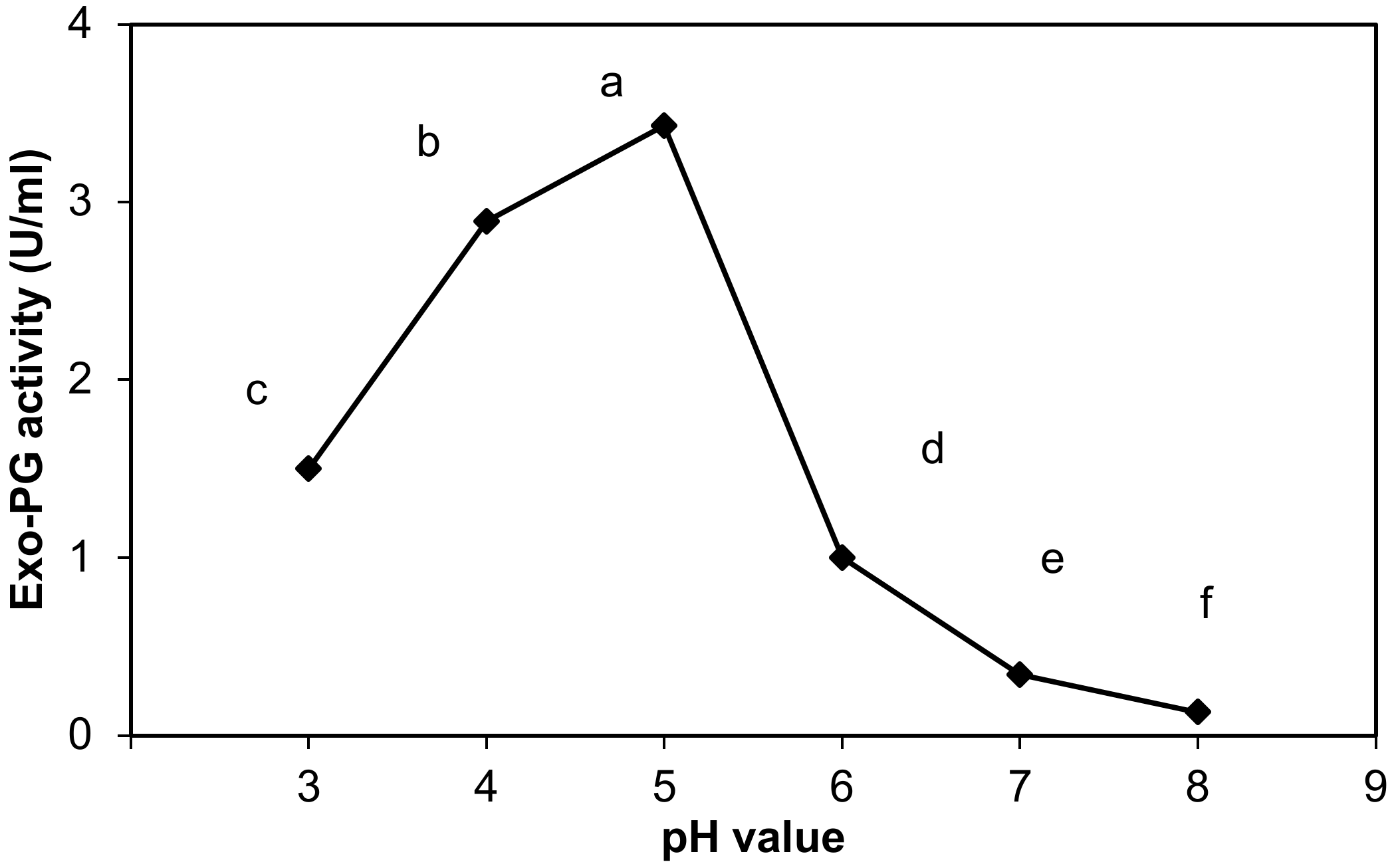
3.2.2. Effect of pH on the Exo-Polygalacturonase Activity
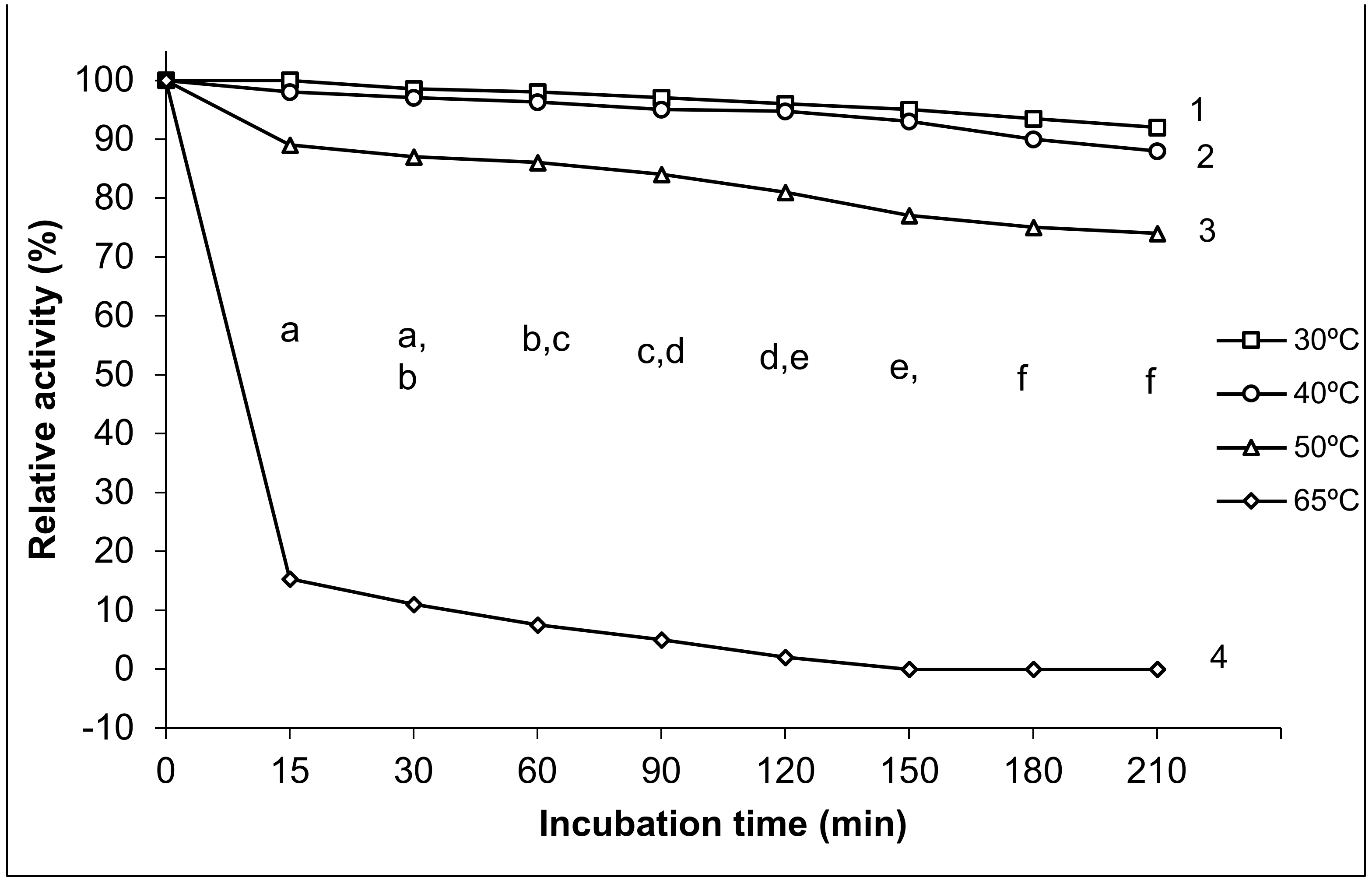
3.2.3. Thermal Stability of Exo-Polygalacturonase Activity
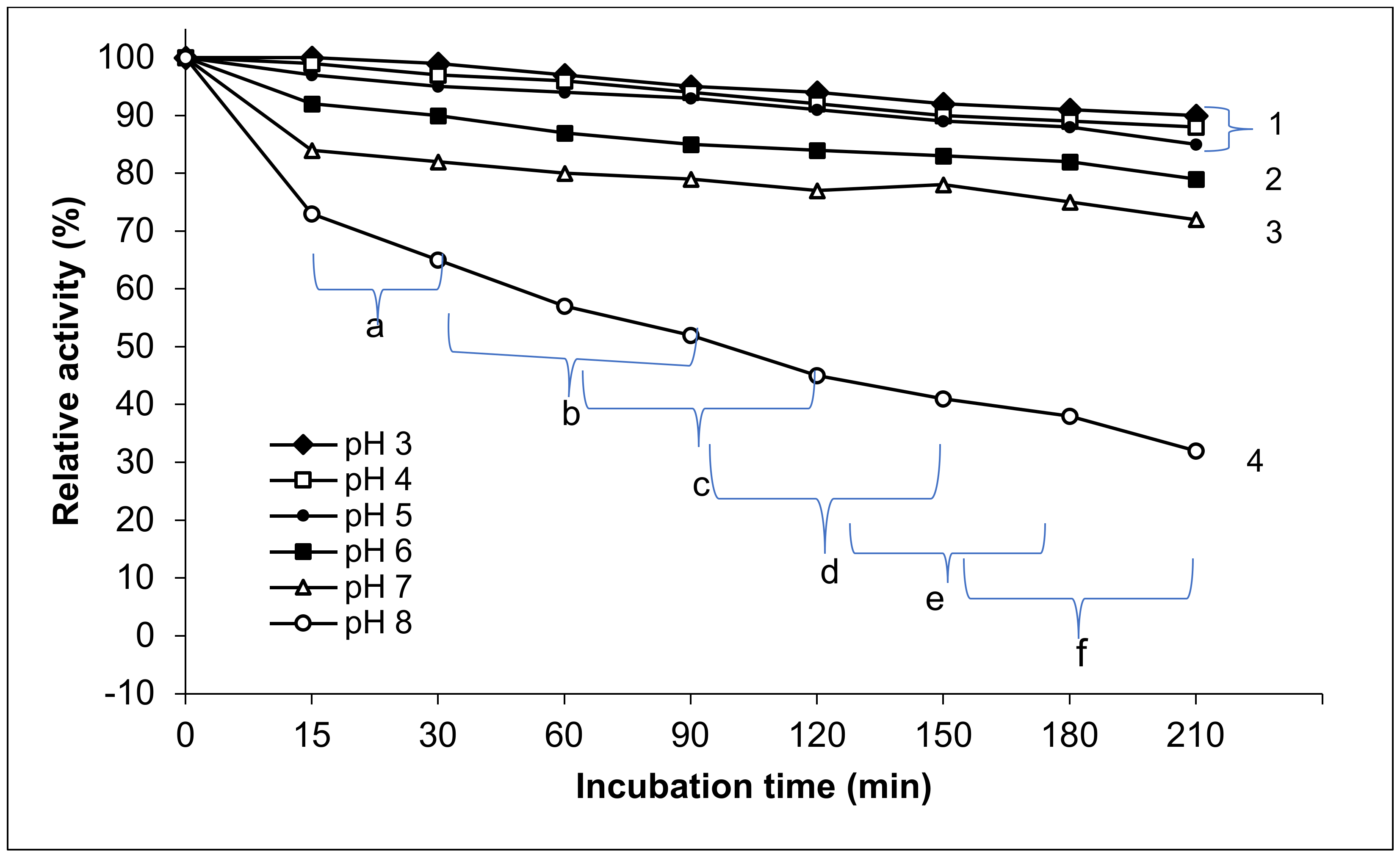
3.2.4. pH Stability of Exo-Polygalacturonase Activity
3.2.5. Kinetic Constants of Exo-Polygalacturonase Activity
4. Discussion
4.1. Purification of Exo-Polygalacturonase Enzyme
4.2. Characterization of Partially Purified Exo-Polygalacturonase
4.2.1. Effect of Temperature on Exo-Polygalacturonase Activity
4.2.2. Effect of pH on the Exo-Polygalacturonase Activity
4.2.3. Thermal Stability of Exo-Polygalacturonase Activity
4.2.4. pH Stability of Exo-Polygalacturonase Activity
4.2.5. Kinetic Constants of Exo-Polygalacturonase Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alkorta, I.; Garbisu, C.; Liama, M.J.; Serra, J.S. Industrial applications of pectic enzymes: A review. Process. Biochem. 1998, 33, 21–28. [Google Scholar] [CrossRef]
- Kashyab, D.R.; Vohra, P.K.; Chopra, S.; Tewari, R. Application of pectinases in the commercial sector: A review. Bioresour. Technol. 2001, 77, 215–227. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Kapoor, M.; Rustagi, R. Enhanced production of an alkaline pectinase from Streptomyces sp. RCK-SC by whole-cell immobilization and solid-state cultivation. World J. Microbiol. Biotechnol. 2004, 20, 257–263. [Google Scholar] [CrossRef]
- Li, Z.M.; Jin, B.; Zhang, H.X. purification and characterization of three alkaline endo- polygalacturonase from a newly isolated Bacillus gibsonii. Chin. J. Process. Eng. 2008, 8, 690–695. [Google Scholar]
- Palagiri, S.; Mayukha, M.; Sagar, G.; Chourasiya, R.; Sibi, G. Production of Pectinases and Pectinolytic Enzymes: Microorganisms, Cultural Conditions and Substrates. Adv. Biotechnol. Microbiol. 2019, 14, 53–59. [Google Scholar] [CrossRef]
- Singh, S.A.; Ramakrishna, M.; Rao, A.G.A. Optimization of down-stream processing parameters for the recovery of pectinase from the fermented broth of Aspergillus carbonarious. Process. Biochem. 1999, 35, 411–417. [Google Scholar] [CrossRef]
- Prathyusha, K.; Suneetha, V. Bacterial pectinases and their potent biotechnological application in fruit processing/juice production industry: A review. J. Phytol. Res. 2011, 3, 6–19. [Google Scholar]
- Solis, S.; Flores, M.E.; Huitron, C. Isolation of endo- polygalacturonases hyperproducing mutant of Aspergillus sp. CH-Y-1043. Biotechnol. Lett. 1990, 12, 751–756. [Google Scholar] [CrossRef]
- Blanco, P.; Sieiro, C.; Villa, T.G. Production of pectic enzymes in yeast. FEMS Microbiol. Lett. 1999, 175, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Favela-Torres, E.; Volke-Sepulveda, T.; Viniegra-Gonzalez, G. Production of hydrolytic depolymerising pectinases: A review. Food Technol. Biotechnol. 2006, 44, 221–227. [Google Scholar]
- Jing, L.; Wang, B.; Long, F.; Yue, B.; Zhang, M.; Zng, Q. Study on the composition of substrate and ferment condition of Penicillium oxalicum Curri & Thom production pectinase. J. Shenyang Agric. Univ. 2008, 39, 38–43. [Google Scholar]
- Reid, I.; Ricard, M. Pectinase in paper making Solving retention problems in mechanical pulps bleached with hydrogen peroxide. Enzym. Microb. Technol. 2000, 26, 115–123. [Google Scholar]
- Kapoor, M.; Beg, Q.K.; Bushan, B.; Sing, K.; Dadhich, K.S.; Hoondal, G.S. Application of an alkaline and thermostable polygalacturonase from Bacillus sp. MG-CP-2 in degumming of ramie (Boehmeria nivea) and sunn hemp (Crotaloria juneea) bast fibers. Process. Biochem. 2001, 36, 803–807. [Google Scholar] [CrossRef]
- Hoondal, G.S.; Tiwari, R.P.; Tewari, R.; Dahiya, N.; Beg, O.K. Microbial alkaline pectinases and their industrial applications: A review. Appl. Microbiol. Biotechnol. 2002, 59, 409–418. [Google Scholar]
- Voragen, A.; Wolters, H.; Verdonschot-Kroef, T.; Rombouts, F.M.; Pilnik, W. Effect of Juice-Releasing Enzymes on Juice Quality. In Proceedings of the International Fruit Juice Symposium, Zurich, Switzerland, 15–18 October 1986; Juris Durk Verlag: Zurich, Switzerland, 1986; pp. 453–462. [Google Scholar]
- Li, J.; Zhou, P.; Liu, H.; Lin, J.; Gong, Y.; Xiao, W.; Liu, Z. Monosaccharides and ethanol production from superfine ground sugarcane bagasse using enzyme cocktail. Bioresources 2014, 9, 2529–2540. [Google Scholar] [CrossRef] [Green Version]
- Said, S.; Fonseca, M.G.V.; Siessere, V. Pectinase production by Penicillium frequentans. World J. Microbiol. Biotechnol. 1991, 7, 607–608. [Google Scholar] [CrossRef]
- Ismail, A.S. Utilization of orange peels for the production of multienzyme complexes by some fungal strains. Process. Biochem. 1996, 31, 645–650. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Ismail, A.S.; El-Abd, M.A.; Hegazy, E.M.; El-Diwany, A.I. Effective technological pectinases by Aspergillus carneus NRC1 utilizing Egyptian orange juice industry scarps. Int. Biodeterior. Biodegrad. 2009, 63, 12–18. [Google Scholar] [CrossRef]
- Heerd, D.; Diercks, S.; Fernandes-Lahore, M. Efficient polygalacturonase production from solid-state culture of Aspergillus sojae under optimized conditions. Springer Plus 2014, 3, 742–755. [Google Scholar] [CrossRef] [Green Version]
- Viikari, L.; Tenkanen, M.; Suuranakki, M. Biotechnology Set; Wiley: Hoboken, NJ, USA, 2001; pp. 523–546. [Google Scholar] [CrossRef]
- FAO. 2011. Available online: http://www.fao.org (accessed on 11 January 2022).
- OECD/FAO, 2021. OECD-FAO Agricultural Outlook (Edition 2021). Available online: https://www.oecd-ilibrary.org/content/data/4bde2d83-en (accessed on 11 August 2021).
- Foster, B.L.; Dale, B.E.; Doran-Peterson, J.B. Enzymatic hydrolysis of ammonia-treated sugar beet pulp. Appl. Biochem. Biotechnol. 2001, 91–93, 269–282. [Google Scholar] [CrossRef]
- Jacob, N. Pectinolytic enzymes. In Biotechnology for Agro-Industrial Residues Utilization; Springer: Amsterdam, The Netherlands, 2009; pp. 383–396. [Google Scholar] [CrossRef]
- Zhang, C.H.; Li, Z.M.; Peng, X.W.; Jia, Y.; Zhang, H.X.; Bai, Z.H. Separation, purification and characterization of three endo- polygalacturonase from newly isolated Penicillum oxalicum. Chin. J. Process. Eng. 2009, 9, 242–249. [Google Scholar]
- Lekha, P.K.; Lonsane, B.K. Comparative titres, location and properties of tannin acyl hydrolase produced by Aspergillus niger PKL104 in solid-state, liquid surface and submerged fermentations. Process. Biochem. 1991, 29, 497–503. [Google Scholar] [CrossRef]
- Gummadi, S.N.; Panda, T. Purification and biochemical properties of microbial pectinases: A review. Process. Biochem. 2003, 38, 987–996. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Pande, V.; Arif, M. Production, Purification, and Characterization of Polygalacturonase from Rhizomucor pusillus Isolated from Decomposting Orange Peels. Enzym. Res. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Meena, K.K.; Jaipal, M.K.; Singh, U. Production kinetics and characterization of pectinase enzyme from Aspergillus niger. South Asian J. Food Technol. Environ. 2015, 1, 131–135. [Google Scholar] [CrossRef]
- Almowallad, S.A. Studies on Fungal Pectinase Enzymes. Master’s Thesis, Assiut University, Assiut, Egypt, May 2008. [Google Scholar]
- Almowallad, S.A.; Aljobair, M.O.; Alkuraieef, A.N.; Aljahani, A.H.; Alsuhaibani, A.M.; Alsayadi, M.M. Utilization of agro- industrial orange peel and sugar beet pulp waste for fungal endo-polygalacturonase production. Saudi J. Biol. Sci. 2022, 29, 963–969. [Google Scholar] [CrossRef]
- Almowallad, S.A. Studies on Partial Purification and Characterization of Fungal Pectinases Enzymes and Their Application in Food Processing. Ph.D. Thesis, Assiut University, Assiut, Egypt, October 2012. [Google Scholar]
- Paterson, R.R.; Bridge, P.D.; Crosswaite, M.J.; Howksorth, D.L. A reapprasial of terverticillate pencillia using biochemical, physiological and morphological features III. An evaluation of pectinase and amylase isenozymes for species characterization. J. Gen Microbiol. 1989, 135, 2979–2991. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for the determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Baracat, M.C.; Valentim, C.; Muchovej, J.J.; Silvia, D.O. Selection of pectinolytic fungi for degumming natural fibers. Biotechnol. Lett. 1989, 11, 809–902. [Google Scholar] [CrossRef]
- Moyo, S.; Gashe, B.A.; Collison, E.K.; Mpuchane, S. Optimizing growth conditions for the pectinolytic activity of Kluyveromyces wickerhamii by using response surface methodology. Int. J. Food Microbiol. 2003, 85, 87–100. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ramachandran, S. Isolation, Purification and Characterization of Pectinase from Penicillium Citrinum of Pectinase: Characteristics and Applications. Ph.D. Thesis, Cairo University, Cairo, Egypt, October 2005. [Google Scholar]
- Hara, T.; Lim, J.Y.; Fujio, Y.; Ueda, S. Purification and some properties of exo- polygalacturonase from Aspergillus niger cultured in the medium containing Satsuma mandarin peel. Nipp. Shok. Kog. Gakk. 1984, 31, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Hebert, G.A.; Pelham, P.L.; Pittman, B. Determination of the optimal ammonium sulfate concentration for the fractionation of Rabbit, Sheep, Horse and Goat Antisera. Appl. Microbiol. 1973, 25, 26–36. [Google Scholar] [CrossRef]
- Mansour, S.M. Studies on Pectinolytic Enzymes of Fungi. Ph.D. Thesis, Ain Shams University, Ain Shams, Egypt, November 1996. [Google Scholar]
- Arbaisah, S.M.; Asbi, B.A.; Junainah, A.H.; Jamilah, B. Purification and properties of pectinesterase from soursop (Anona muricata) pulp. Food Chem. 1997, 59, 33–40. [Google Scholar] [CrossRef]
- Farinas, C.S.; Scarpelini, L.M.; Miranda, E.A.; Bertucci Neto, V. Evaluation of operational parameters on the precipitation of endoglucanase and xylanase produced by solid state fermentation of Aspergillus niger. Braz. J. Chem. Eng. 2011, 28, 17–26. [Google Scholar] [CrossRef]
- Kumar, A.; Galaev, I.Y.; Mattiasson, B. Precipitation of proteins: Nonspecific and specific. In Isolation and Purification of Proteins; Hutti-Kaul, R., Mattiasson, B., Eds.; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Jalil, M.T.M.; Ibrahim, D. Partial purification and characterization of pectinase produced by Aspergillus niger LFP-1 grown on pomelo peels as a substrate. Top. Life Sci. Res. 2021, 32, 1–22. [Google Scholar] [CrossRef]
- Coelho, M.A.Z.; Medronho, R.C.; Leite, S.G.F.; Couri, S. Partial purification of a polygalacturonase produced by solid state cultures of Aspergillus niger 3T5P. Braz. J. Microbiol. 1995, 26, 318–322. [Google Scholar]
- Doukani, K. Microbial Production of Pectinase: Characteristics and Applications. Ph.D. Thesis, Cairo University, Cairo, Egypt, September 2009. [Google Scholar]
- Lee, T.H.; Kim, B.Y.; Chung, Y.R.; Lee, S.Y.; Lee, C.W.; Kim, J.W. Purification and characterization of an exo- polygalacturonase from Botrytis cinerea. J. Microbiol. 1997, 35, 134–140. [Google Scholar]
- Kaur, G.; Kumar, S.; Satyanarayana, T. Production, characterization and application of a thermostable exo- polygalacturonase of thermophilic mould Sporotrichum thermophile Apinis. Bioresour. Technol. 2004, 94, 239–243. [Google Scholar] [CrossRef]
- Khatri, B.P.; Bhattarai, T.; Shrestha., S.; Maharjan, J. lkaline thermostable pectinase enzyme from Aspergillus niger strain MCAS2 isolated from Manaslu Conservation Area, Gorkha, Nepal. Springer Plus 2015, 4, 488. [Google Scholar] [CrossRef] [Green Version]
- Ire, F.S.; Vinking, E.G. Production, purification and characterization of polygalacturonase from Aspergillus niger in solid state and submerged fermentation using banana peels. J. Adv. Biol. Biotechnol. 2016, 10, 1–15. [Google Scholar] [CrossRef]
- Raak, N.; Abbate, R.A.; Lederer, A.; Rohm, H.; Jaros, D. Size separation techniques for the characterization of cross-linked casein: A review of methods and their applications. Separations 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Barense, R.I.; Chellegatti, M.A.S.; Fonseca, M.J.V.; Said, S. Partial Purification and characterization of exo- polygalacturonase II and III from Penicillium frequentans. Braz. J. Microbiol. 2001, 32, 327–330. [Google Scholar] [CrossRef]
- Chellegatti, M.A.S.; Fonseca, M.J.V.; Said, S. Purification and partial characterization of exo- polygalacturonase from Penicillium frequentans. Microbiol. Res. 2002, 157, 19–24. [Google Scholar] [CrossRef]
- Ahmed, I.; Zia, M.A.; Hussain, M.A.; Akram, Z.; Naveed, M.T.; Nowrouzi, A. Bioprocessing of citrus waster peel for induced pectinase production by Aspergillus niger; its purification and characterization. J. Radiat. Res. Appl. Sci. 2016, 9, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Dinu, D.; Nechifor, M.T.; Stoian, G.; Costache, M.; Dinischiotu, A. Enzyme with new biochemical properties in the pectinolytic complex produced by Aspergillus niger MIUG 16. J. Biotechnol. 2007, 131, 128–137. [Google Scholar] [CrossRef]
- Sharma, D.C.; Satyanarayan, T. Production and application of pectinolytic enzymes of Sporotrichum thermopohile and Bacillus pumilus. In Biotechnolgical Approaches for Sustainable Development; Reddy, M.S., Khanna, S., Eds.; Allied Publishers: New Delhi, India, 2004; pp. 164–169. [Google Scholar]
- Kulkarni, N.S.; Jaiswal, J.V.; Bodhankar, M.G. Influence of agro-waste amendment on soil microbial population in relation to plant growth response. J. Environ. Biol. 2007, 28, 623–626. [Google Scholar]
- Gewali, M.; Maharjan, J.; Thapa, S.; Shrestha, J.K. Studies on polygalacturonase from Aspergillus flavus. Sci. World 2007, 5, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Sohail, M. Characterization of pectinase from Geotrichum candidum AA15 and its potential application in orange juice clarification. J. King Saud Univ. Sci. 2020, 32, 955–961. [Google Scholar] [CrossRef]
- Maciel, M.D.C.; Herculano, P.N.; Porto, T.S.; Teixeira, M.F.S.; Moreira, K.A.; Souza-Motta, C.M. Production and partial characterization of pectinases from forage palm by Aspergillus niger URM4645. Afr. J. Biotechnol. 2011, 10, 2469–2475. [Google Scholar]
- Kumar, S.S.; Palanivelu, P. Purification and characterization of an exo- polygalacturonase from the thermophilic fungus, Thermomyces lanuginosus. World J. Microbiol. Biotechnol. 1999, 15, 643–646. [Google Scholar] [CrossRef]
- Galiotou-Panayotou, M.P.R.; Kapantai, M.; Kalantzi, O. Growth conditions of Aspergillus sp. ATHUM-3428 for polygalacturonase production. Appl. Microbiol. Biotechnol. 1997, 47, 425–429. [Google Scholar] [CrossRef] [PubMed]
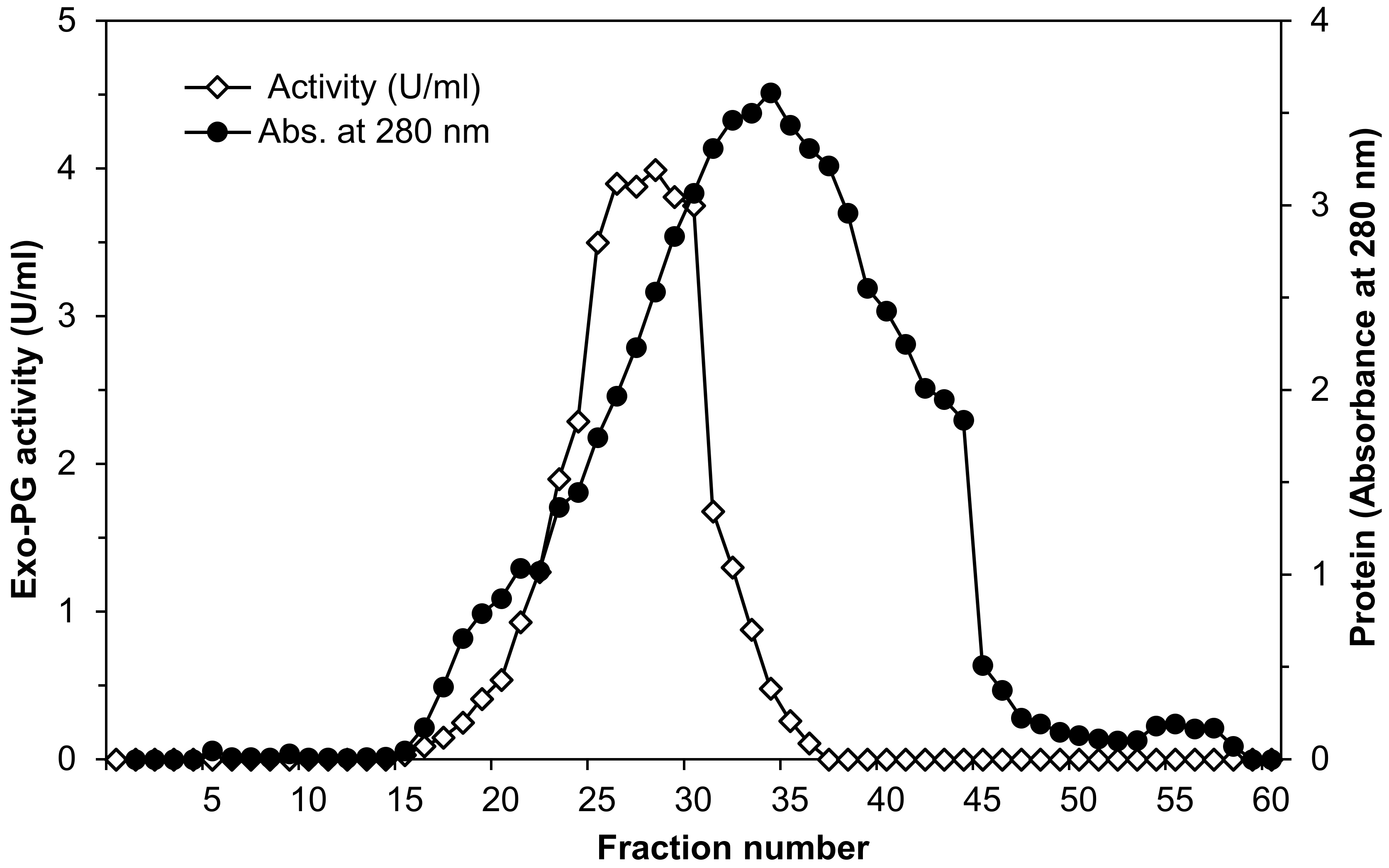

| Purification Steps | Total Activity (Units) | Total Protein (mg) | Specific Activity (U/mg) | Yield (%) | Purification (Fold) |
|---|---|---|---|---|---|
| Crude enzyme | 141 | 699 | 0.2 | 100 | 1 |
| Ammonium sulphate precipitation 20–80% | 109 | 78 | 1.4 | 77 | 7 |
| Acetone precipitation 66% | 89 | 58.5 | 1.52 | 63 | 7.6 |
| Gel filtration chromatography | 81 | 14.4 | 5.63 | 57 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almowallad, S.A.; Alshammari, G.M.; Alsayadi, M.M.; Aljafer, N.; Al-Sanea, E.A.; Yahya, M.A.; Al-Harbi, L.N. Partial Purification and Characterization of Exo-Polygalacturonase Produced by Penicillium oxalicum AUMC 4153. Life 2022, 12, 284. https://doi.org/10.3390/life12020284
Almowallad SA, Alshammari GM, Alsayadi MM, Aljafer N, Al-Sanea EA, Yahya MA, Al-Harbi LN. Partial Purification and Characterization of Exo-Polygalacturonase Produced by Penicillium oxalicum AUMC 4153. Life. 2022; 12(2):284. https://doi.org/10.3390/life12020284
Chicago/Turabian StyleAlmowallad, Shamsan A., Ghedeir M. Alshammari, Muneer M. Alsayadi, Naofel Aljafer, Ekram A. Al-Sanea, Mohammed Abdo Yahya, and Laila Naif Al-Harbi. 2022. "Partial Purification and Characterization of Exo-Polygalacturonase Produced by Penicillium oxalicum AUMC 4153" Life 12, no. 2: 284. https://doi.org/10.3390/life12020284
APA StyleAlmowallad, S. A., Alshammari, G. M., Alsayadi, M. M., Aljafer, N., Al-Sanea, E. A., Yahya, M. A., & Al-Harbi, L. N. (2022). Partial Purification and Characterization of Exo-Polygalacturonase Produced by Penicillium oxalicum AUMC 4153. Life, 12(2), 284. https://doi.org/10.3390/life12020284






