Partial Purification and Characterization of Exo-Polygalacturonase Produced by Penicillium oxalicum AUMC 4153
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Culturing Media
2.3. Sugar Beet Pulp Powder
2.4. Chemicals
2.5. Cultivation of Fungi and Exo-PG Production by Submerged Fermentation
2.6. Assay for Exo-Polygalacturonase Activity (Exo-PG)
2.7. Estimation of Protein Content
2.8. Purification of Exo-Polygalacturonase
2.8.1. Ammonium Sulfate Precipitation
2.8.2. Dialysis
2.8.3. Acetone Precipitation
2.8.4. Gel Filtration Chromatography
2.9. Characterization of Partially Purified Exo-Polygalacturonase
2.9.1. Effect of Temperature on the Exo-Polygalacturonase Activity
2.9.2. Effect of pH on the Exo-Polygalacturonase Activity
2.9.3. Thermal Stability of Exo-Polygalacturonase Activity
2.9.4. pH Stability of Exo-Polygalacturonase Activity
2.9.5. Kinetics Constants of Exo-Polygalacturonase
2.10. Statistical Analysis
3. Results
3.1. Purification of Exo-Polygalacturonase Enzyme
3.2. Characterization of Partially Purified Exo-Polygalacturonase
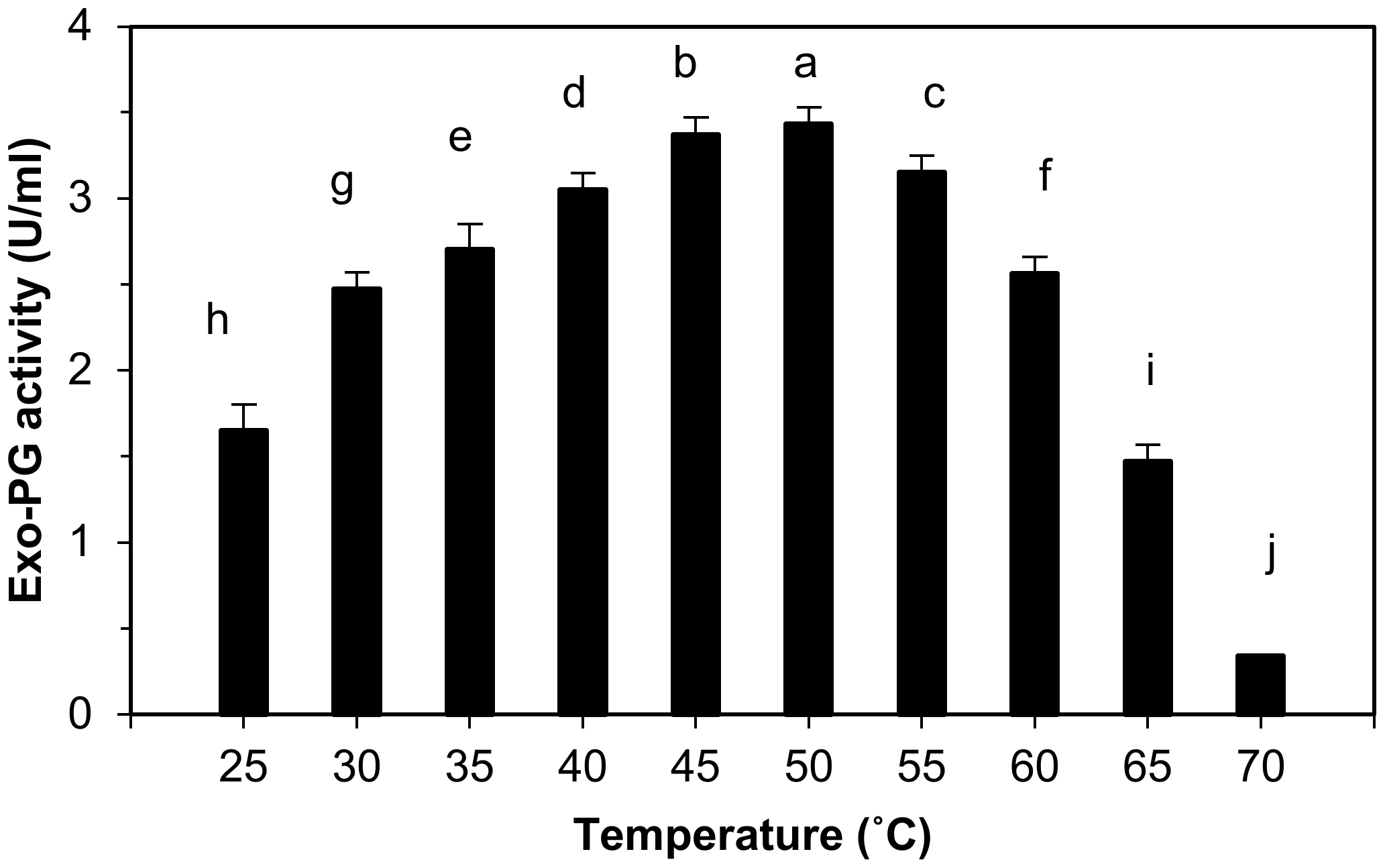
3.2.1. Effect of Temperature on the Exo-Polygalacturonase Activity
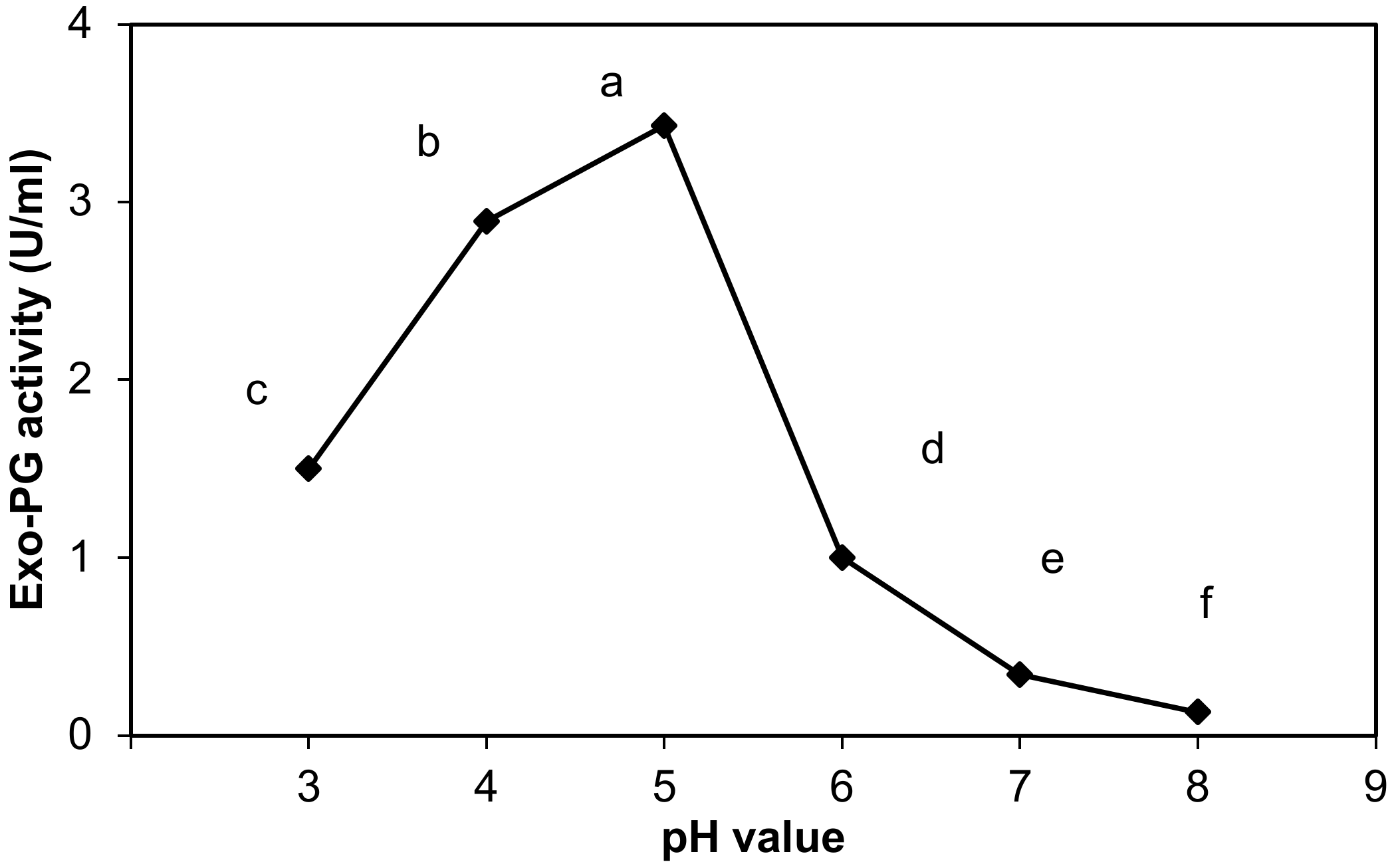
3.2.2. Effect of pH on the Exo-Polygalacturonase Activity
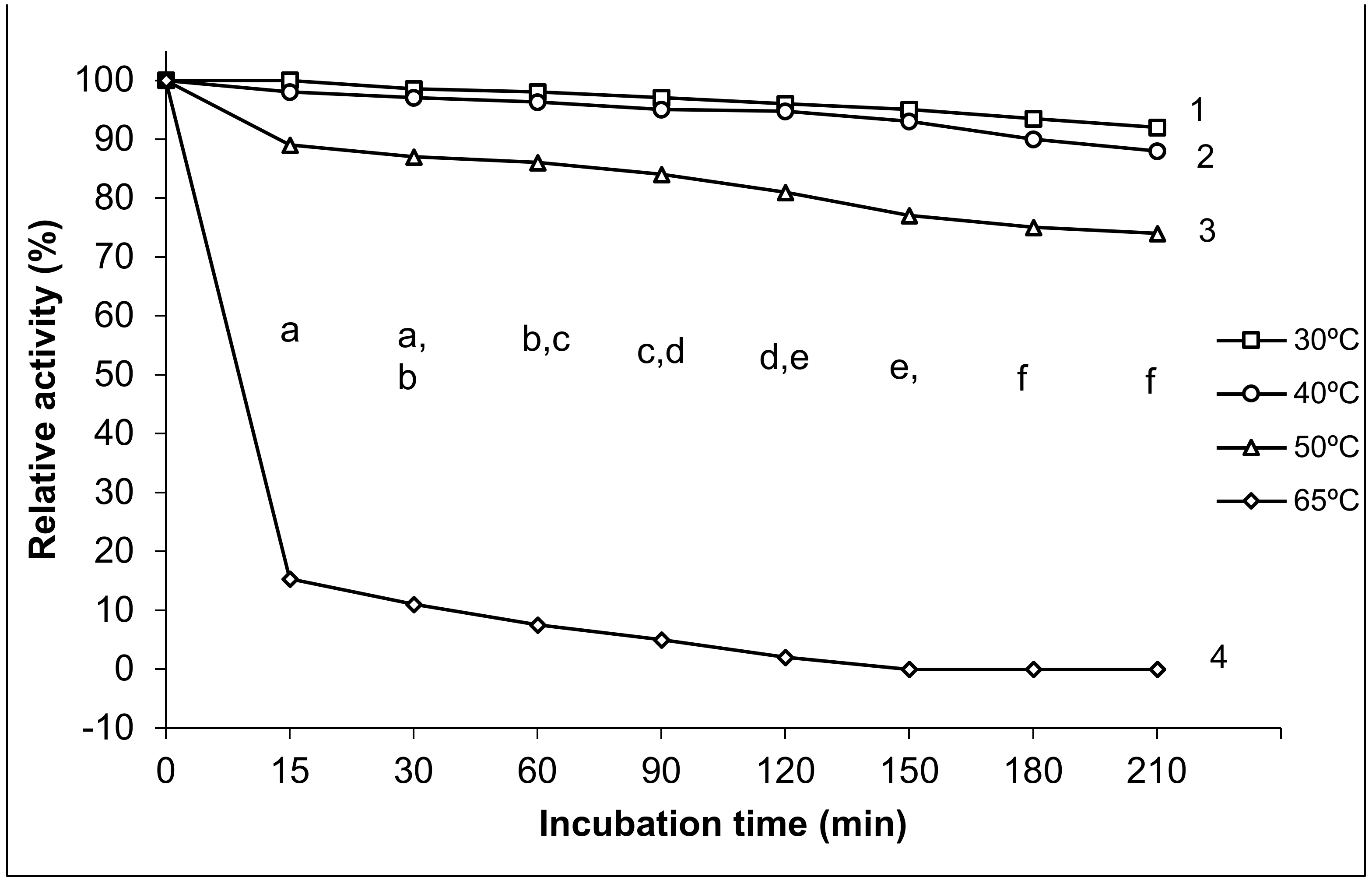
3.2.3. Thermal Stability of Exo-Polygalacturonase Activity
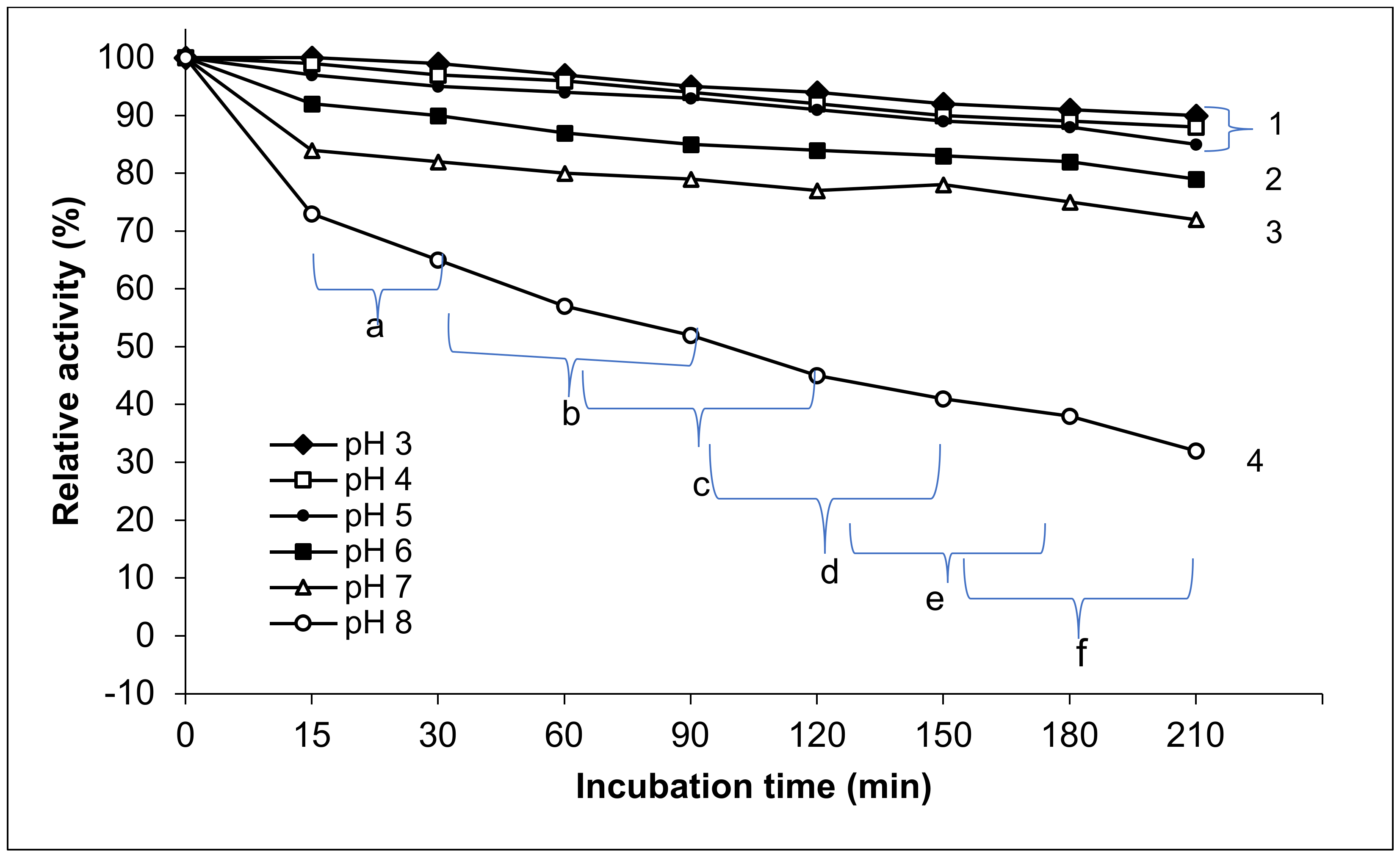
3.2.4. pH Stability of Exo-Polygalacturonase Activity
3.2.5. Kinetic Constants of Exo-Polygalacturonase Activity
4. Discussion
4.1. Purification of Exo-Polygalacturonase Enzyme
4.2. Characterization of Partially Purified Exo-Polygalacturonase
4.2.1. Effect of Temperature on Exo-Polygalacturonase Activity
4.2.2. Effect of pH on the Exo-Polygalacturonase Activity
4.2.3. Thermal Stability of Exo-Polygalacturonase Activity
4.2.4. pH Stability of Exo-Polygalacturonase Activity
4.2.5. Kinetic Constants of Exo-Polygalacturonase Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alkorta, I.; Garbisu, C.; Liama, M.J.; Serra, J.S. Industrial applications of pectic enzymes: A review. Process. Biochem. 1998, 33, 21–28. [Google Scholar] [CrossRef]
- Kashyab, D.R.; Vohra, P.K.; Chopra, S.; Tewari, R. Application of pectinases in the commercial sector: A review. Bioresour. Technol. 2001, 77, 215–227. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Kapoor, M.; Rustagi, R. Enhanced production of an alkaline pectinase from Streptomyces sp. RCK-SC by whole-cell immobilization and solid-state cultivation. World J. Microbiol. Biotechnol. 2004, 20, 257–263. [Google Scholar] [CrossRef]
- Li, Z.M.; Jin, B.; Zhang, H.X. purification and characterization of three alkaline endo- polygalacturonase from a newly isolated Bacillus gibsonii. Chin. J. Process. Eng. 2008, 8, 690–695. [Google Scholar]
- Palagiri, S.; Mayukha, M.; Sagar, G.; Chourasiya, R.; Sibi, G. Production of Pectinases and Pectinolytic Enzymes: Microorganisms, Cultural Conditions and Substrates. Adv. Biotechnol. Microbiol. 2019, 14, 53–59. [Google Scholar] [CrossRef]
- Singh, S.A.; Ramakrishna, M.; Rao, A.G.A. Optimization of down-stream processing parameters for the recovery of pectinase from the fermented broth of Aspergillus carbonarious. Process. Biochem. 1999, 35, 411–417. [Google Scholar] [CrossRef]
- Prathyusha, K.; Suneetha, V. Bacterial pectinases and their potent biotechnological application in fruit processing/juice production industry: A review. J. Phytol. Res. 2011, 3, 6–19. [Google Scholar]
- Solis, S.; Flores, M.E.; Huitron, C. Isolation of endo- polygalacturonases hyperproducing mutant of Aspergillus sp. CH-Y-1043. Biotechnol. Lett. 1990, 12, 751–756. [Google Scholar] [CrossRef]
- Blanco, P.; Sieiro, C.; Villa, T.G. Production of pectic enzymes in yeast. FEMS Microbiol. Lett. 1999, 175, 1–9. [Google Scholar] [CrossRef]
- Favela-Torres, E.; Volke-Sepulveda, T.; Viniegra-Gonzalez, G. Production of hydrolytic depolymerising pectinases: A review. Food Technol. Biotechnol. 2006, 44, 221–227. [Google Scholar]
- Jing, L.; Wang, B.; Long, F.; Yue, B.; Zhang, M.; Zng, Q. Study on the composition of substrate and ferment condition of Penicillium oxalicum Curri & Thom production pectinase. J. Shenyang Agric. Univ. 2008, 39, 38–43. [Google Scholar]
- Reid, I.; Ricard, M. Pectinase in paper making Solving retention problems in mechanical pulps bleached with hydrogen peroxide. Enzym. Microb. Technol. 2000, 26, 115–123. [Google Scholar]
- Kapoor, M.; Beg, Q.K.; Bushan, B.; Sing, K.; Dadhich, K.S.; Hoondal, G.S. Application of an alkaline and thermostable polygalacturonase from Bacillus sp. MG-CP-2 in degumming of ramie (Boehmeria nivea) and sunn hemp (Crotaloria juneea) bast fibers. Process. Biochem. 2001, 36, 803–807. [Google Scholar] [CrossRef]
- Hoondal, G.S.; Tiwari, R.P.; Tewari, R.; Dahiya, N.; Beg, O.K. Microbial alkaline pectinases and their industrial applications: A review. Appl. Microbiol. Biotechnol. 2002, 59, 409–418. [Google Scholar]
- Voragen, A.; Wolters, H.; Verdonschot-Kroef, T.; Rombouts, F.M.; Pilnik, W. Effect of Juice-Releasing Enzymes on Juice Quality. In Proceedings of the International Fruit Juice Symposium, Zurich, Switzerland, 15–18 October 1986; Juris Durk Verlag: Zurich, Switzerland, 1986; pp. 453–462. [Google Scholar]
- Li, J.; Zhou, P.; Liu, H.; Lin, J.; Gong, Y.; Xiao, W.; Liu, Z. Monosaccharides and ethanol production from superfine ground sugarcane bagasse using enzyme cocktail. Bioresources 2014, 9, 2529–2540. [Google Scholar] [CrossRef][Green Version]
- Said, S.; Fonseca, M.G.V.; Siessere, V. Pectinase production by Penicillium frequentans. World J. Microbiol. Biotechnol. 1991, 7, 607–608. [Google Scholar] [CrossRef]
- Ismail, A.S. Utilization of orange peels for the production of multienzyme complexes by some fungal strains. Process. Biochem. 1996, 31, 645–650. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Ismail, A.S.; El-Abd, M.A.; Hegazy, E.M.; El-Diwany, A.I. Effective technological pectinases by Aspergillus carneus NRC1 utilizing Egyptian orange juice industry scarps. Int. Biodeterior. Biodegrad. 2009, 63, 12–18. [Google Scholar] [CrossRef]
- Heerd, D.; Diercks, S.; Fernandes-Lahore, M. Efficient polygalacturonase production from solid-state culture of Aspergillus sojae under optimized conditions. Springer Plus 2014, 3, 742–755. [Google Scholar] [CrossRef]
- Viikari, L.; Tenkanen, M.; Suuranakki, M. Biotechnology Set; Wiley: Hoboken, NJ, USA, 2001; pp. 523–546. [Google Scholar] [CrossRef]
- FAO. 2011. Available online: http://www.fao.org (accessed on 11 January 2022).
- OECD/FAO, 2021. OECD-FAO Agricultural Outlook (Edition 2021). Available online: https://www.oecd-ilibrary.org/content/data/4bde2d83-en (accessed on 11 August 2021).
- Foster, B.L.; Dale, B.E.; Doran-Peterson, J.B. Enzymatic hydrolysis of ammonia-treated sugar beet pulp. Appl. Biochem. Biotechnol. 2001, 91–93, 269–282. [Google Scholar] [CrossRef]
- Jacob, N. Pectinolytic enzymes. In Biotechnology for Agro-Industrial Residues Utilization; Springer: Amsterdam, The Netherlands, 2009; pp. 383–396. [Google Scholar] [CrossRef]
- Zhang, C.H.; Li, Z.M.; Peng, X.W.; Jia, Y.; Zhang, H.X.; Bai, Z.H. Separation, purification and characterization of three endo- polygalacturonase from newly isolated Penicillum oxalicum. Chin. J. Process. Eng. 2009, 9, 242–249. [Google Scholar]
- Lekha, P.K.; Lonsane, B.K. Comparative titres, location and properties of tannin acyl hydrolase produced by Aspergillus niger PKL104 in solid-state, liquid surface and submerged fermentations. Process. Biochem. 1991, 29, 497–503. [Google Scholar] [CrossRef]
- Gummadi, S.N.; Panda, T. Purification and biochemical properties of microbial pectinases: A review. Process. Biochem. 2003, 38, 987–996. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Pande, V.; Arif, M. Production, Purification, and Characterization of Polygalacturonase from Rhizomucor pusillus Isolated from Decomposting Orange Peels. Enzym. Res. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Meena, K.K.; Jaipal, M.K.; Singh, U. Production kinetics and characterization of pectinase enzyme from Aspergillus niger. South Asian J. Food Technol. Environ. 2015, 1, 131–135. [Google Scholar] [CrossRef]
- Almowallad, S.A. Studies on Fungal Pectinase Enzymes. Master’s Thesis, Assiut University, Assiut, Egypt, May 2008. [Google Scholar]
- Almowallad, S.A.; Aljobair, M.O.; Alkuraieef, A.N.; Aljahani, A.H.; Alsuhaibani, A.M.; Alsayadi, M.M. Utilization of agro- industrial orange peel and sugar beet pulp waste for fungal endo-polygalacturonase production. Saudi J. Biol. Sci. 2022, 29, 963–969. [Google Scholar] [CrossRef]
- Almowallad, S.A. Studies on Partial Purification and Characterization of Fungal Pectinases Enzymes and Their Application in Food Processing. Ph.D. Thesis, Assiut University, Assiut, Egypt, October 2012. [Google Scholar]
- Paterson, R.R.; Bridge, P.D.; Crosswaite, M.J.; Howksorth, D.L. A reapprasial of terverticillate pencillia using biochemical, physiological and morphological features III. An evaluation of pectinase and amylase isenozymes for species characterization. J. Gen Microbiol. 1989, 135, 2979–2991. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for the determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Baracat, M.C.; Valentim, C.; Muchovej, J.J.; Silvia, D.O. Selection of pectinolytic fungi for degumming natural fibers. Biotechnol. Lett. 1989, 11, 809–902. [Google Scholar] [CrossRef]
- Moyo, S.; Gashe, B.A.; Collison, E.K.; Mpuchane, S. Optimizing growth conditions for the pectinolytic activity of Kluyveromyces wickerhamii by using response surface methodology. Int. J. Food Microbiol. 2003, 85, 87–100. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ramachandran, S. Isolation, Purification and Characterization of Pectinase from Penicillium Citrinum of Pectinase: Characteristics and Applications. Ph.D. Thesis, Cairo University, Cairo, Egypt, October 2005. [Google Scholar]
- Hara, T.; Lim, J.Y.; Fujio, Y.; Ueda, S. Purification and some properties of exo- polygalacturonase from Aspergillus niger cultured in the medium containing Satsuma mandarin peel. Nipp. Shok. Kog. Gakk. 1984, 31, 581–586. [Google Scholar] [CrossRef][Green Version]
- Hebert, G.A.; Pelham, P.L.; Pittman, B. Determination of the optimal ammonium sulfate concentration for the fractionation of Rabbit, Sheep, Horse and Goat Antisera. Appl. Microbiol. 1973, 25, 26–36. [Google Scholar] [CrossRef]
- Mansour, S.M. Studies on Pectinolytic Enzymes of Fungi. Ph.D. Thesis, Ain Shams University, Ain Shams, Egypt, November 1996. [Google Scholar]
- Arbaisah, S.M.; Asbi, B.A.; Junainah, A.H.; Jamilah, B. Purification and properties of pectinesterase from soursop (Anona muricata) pulp. Food Chem. 1997, 59, 33–40. [Google Scholar] [CrossRef]
- Farinas, C.S.; Scarpelini, L.M.; Miranda, E.A.; Bertucci Neto, V. Evaluation of operational parameters on the precipitation of endoglucanase and xylanase produced by solid state fermentation of Aspergillus niger. Braz. J. Chem. Eng. 2011, 28, 17–26. [Google Scholar] [CrossRef]
- Kumar, A.; Galaev, I.Y.; Mattiasson, B. Precipitation of proteins: Nonspecific and specific. In Isolation and Purification of Proteins; Hutti-Kaul, R., Mattiasson, B., Eds.; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Jalil, M.T.M.; Ibrahim, D. Partial purification and characterization of pectinase produced by Aspergillus niger LFP-1 grown on pomelo peels as a substrate. Top. Life Sci. Res. 2021, 32, 1–22. [Google Scholar] [CrossRef]
- Coelho, M.A.Z.; Medronho, R.C.; Leite, S.G.F.; Couri, S. Partial purification of a polygalacturonase produced by solid state cultures of Aspergillus niger 3T5P. Braz. J. Microbiol. 1995, 26, 318–322. [Google Scholar]
- Doukani, K. Microbial Production of Pectinase: Characteristics and Applications. Ph.D. Thesis, Cairo University, Cairo, Egypt, September 2009. [Google Scholar]
- Lee, T.H.; Kim, B.Y.; Chung, Y.R.; Lee, S.Y.; Lee, C.W.; Kim, J.W. Purification and characterization of an exo- polygalacturonase from Botrytis cinerea. J. Microbiol. 1997, 35, 134–140. [Google Scholar]
- Kaur, G.; Kumar, S.; Satyanarayana, T. Production, characterization and application of a thermostable exo- polygalacturonase of thermophilic mould Sporotrichum thermophile Apinis. Bioresour. Technol. 2004, 94, 239–243. [Google Scholar] [CrossRef]
- Khatri, B.P.; Bhattarai, T.; Shrestha., S.; Maharjan, J. lkaline thermostable pectinase enzyme from Aspergillus niger strain MCAS2 isolated from Manaslu Conservation Area, Gorkha, Nepal. Springer Plus 2015, 4, 488. [Google Scholar] [CrossRef]
- Ire, F.S.; Vinking, E.G. Production, purification and characterization of polygalacturonase from Aspergillus niger in solid state and submerged fermentation using banana peels. J. Adv. Biol. Biotechnol. 2016, 10, 1–15. [Google Scholar] [CrossRef]
- Raak, N.; Abbate, R.A.; Lederer, A.; Rohm, H.; Jaros, D. Size separation techniques for the characterization of cross-linked casein: A review of methods and their applications. Separations 2018, 5, 14. [Google Scholar] [CrossRef]
- Barense, R.I.; Chellegatti, M.A.S.; Fonseca, M.J.V.; Said, S. Partial Purification and characterization of exo- polygalacturonase II and III from Penicillium frequentans. Braz. J. Microbiol. 2001, 32, 327–330. [Google Scholar] [CrossRef]
- Chellegatti, M.A.S.; Fonseca, M.J.V.; Said, S. Purification and partial characterization of exo- polygalacturonase from Penicillium frequentans. Microbiol. Res. 2002, 157, 19–24. [Google Scholar] [CrossRef]
- Ahmed, I.; Zia, M.A.; Hussain, M.A.; Akram, Z.; Naveed, M.T.; Nowrouzi, A. Bioprocessing of citrus waster peel for induced pectinase production by Aspergillus niger; its purification and characterization. J. Radiat. Res. Appl. Sci. 2016, 9, 148–154. [Google Scholar] [CrossRef]
- Dinu, D.; Nechifor, M.T.; Stoian, G.; Costache, M.; Dinischiotu, A. Enzyme with new biochemical properties in the pectinolytic complex produced by Aspergillus niger MIUG 16. J. Biotechnol. 2007, 131, 128–137. [Google Scholar] [CrossRef]
- Sharma, D.C.; Satyanarayan, T. Production and application of pectinolytic enzymes of Sporotrichum thermopohile and Bacillus pumilus. In Biotechnolgical Approaches for Sustainable Development; Reddy, M.S., Khanna, S., Eds.; Allied Publishers: New Delhi, India, 2004; pp. 164–169. [Google Scholar]
- Kulkarni, N.S.; Jaiswal, J.V.; Bodhankar, M.G. Influence of agro-waste amendment on soil microbial population in relation to plant growth response. J. Environ. Biol. 2007, 28, 623–626. [Google Scholar]
- Gewali, M.; Maharjan, J.; Thapa, S.; Shrestha, J.K. Studies on polygalacturonase from Aspergillus flavus. Sci. World 2007, 5, 19–22. [Google Scholar] [CrossRef]
- Ahmed, A.; Sohail, M. Characterization of pectinase from Geotrichum candidum AA15 and its potential application in orange juice clarification. J. King Saud Univ. Sci. 2020, 32, 955–961. [Google Scholar] [CrossRef]
- Maciel, M.D.C.; Herculano, P.N.; Porto, T.S.; Teixeira, M.F.S.; Moreira, K.A.; Souza-Motta, C.M. Production and partial characterization of pectinases from forage palm by Aspergillus niger URM4645. Afr. J. Biotechnol. 2011, 10, 2469–2475. [Google Scholar]
- Kumar, S.S.; Palanivelu, P. Purification and characterization of an exo- polygalacturonase from the thermophilic fungus, Thermomyces lanuginosus. World J. Microbiol. Biotechnol. 1999, 15, 643–646. [Google Scholar] [CrossRef]
- Galiotou-Panayotou, M.P.R.; Kapantai, M.; Kalantzi, O. Growth conditions of Aspergillus sp. ATHUM-3428 for polygalacturonase production. Appl. Microbiol. Biotechnol. 1997, 47, 425–429. [Google Scholar] [CrossRef] [PubMed]
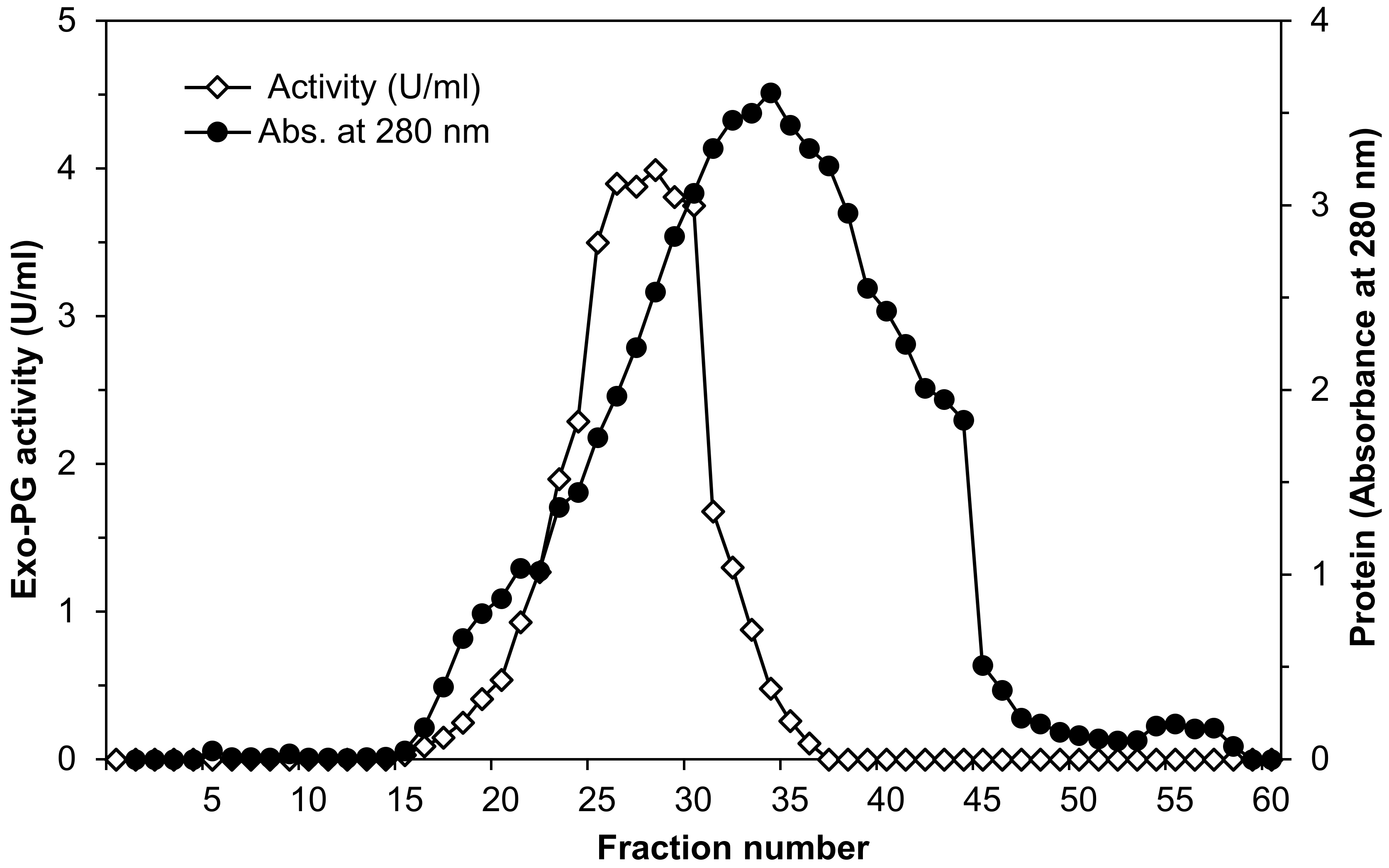

| Purification Steps | Total Activity (Units) | Total Protein (mg) | Specific Activity (U/mg) | Yield (%) | Purification (Fold) |
|---|---|---|---|---|---|
| Crude enzyme | 141 | 699 | 0.2 | 100 | 1 |
| Ammonium sulphate precipitation 20–80% | 109 | 78 | 1.4 | 77 | 7 |
| Acetone precipitation 66% | 89 | 58.5 | 1.52 | 63 | 7.6 |
| Gel filtration chromatography | 81 | 14.4 | 5.63 | 57 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almowallad, S.A.; Alshammari, G.M.; Alsayadi, M.M.; Aljafer, N.; Al-Sanea, E.A.; Yahya, M.A.; Al-Harbi, L.N. Partial Purification and Characterization of Exo-Polygalacturonase Produced by Penicillium oxalicum AUMC 4153. Life 2022, 12, 284. https://doi.org/10.3390/life12020284
Almowallad SA, Alshammari GM, Alsayadi MM, Aljafer N, Al-Sanea EA, Yahya MA, Al-Harbi LN. Partial Purification and Characterization of Exo-Polygalacturonase Produced by Penicillium oxalicum AUMC 4153. Life. 2022; 12(2):284. https://doi.org/10.3390/life12020284
Chicago/Turabian StyleAlmowallad, Shamsan A., Ghedeir M. Alshammari, Muneer M. Alsayadi, Naofel Aljafer, Ekram A. Al-Sanea, Mohammed Abdo Yahya, and Laila Naif Al-Harbi. 2022. "Partial Purification and Characterization of Exo-Polygalacturonase Produced by Penicillium oxalicum AUMC 4153" Life 12, no. 2: 284. https://doi.org/10.3390/life12020284
APA StyleAlmowallad, S. A., Alshammari, G. M., Alsayadi, M. M., Aljafer, N., Al-Sanea, E. A., Yahya, M. A., & Al-Harbi, L. N. (2022). Partial Purification and Characterization of Exo-Polygalacturonase Produced by Penicillium oxalicum AUMC 4153. Life, 12(2), 284. https://doi.org/10.3390/life12020284






