Update on Color Flow Imaging in Obstetrics
Abstract
:1. Introduction
2. Color Flow Imaging Modes
3. Safety and Optimization
4. Clinical Applications in Obstetrics
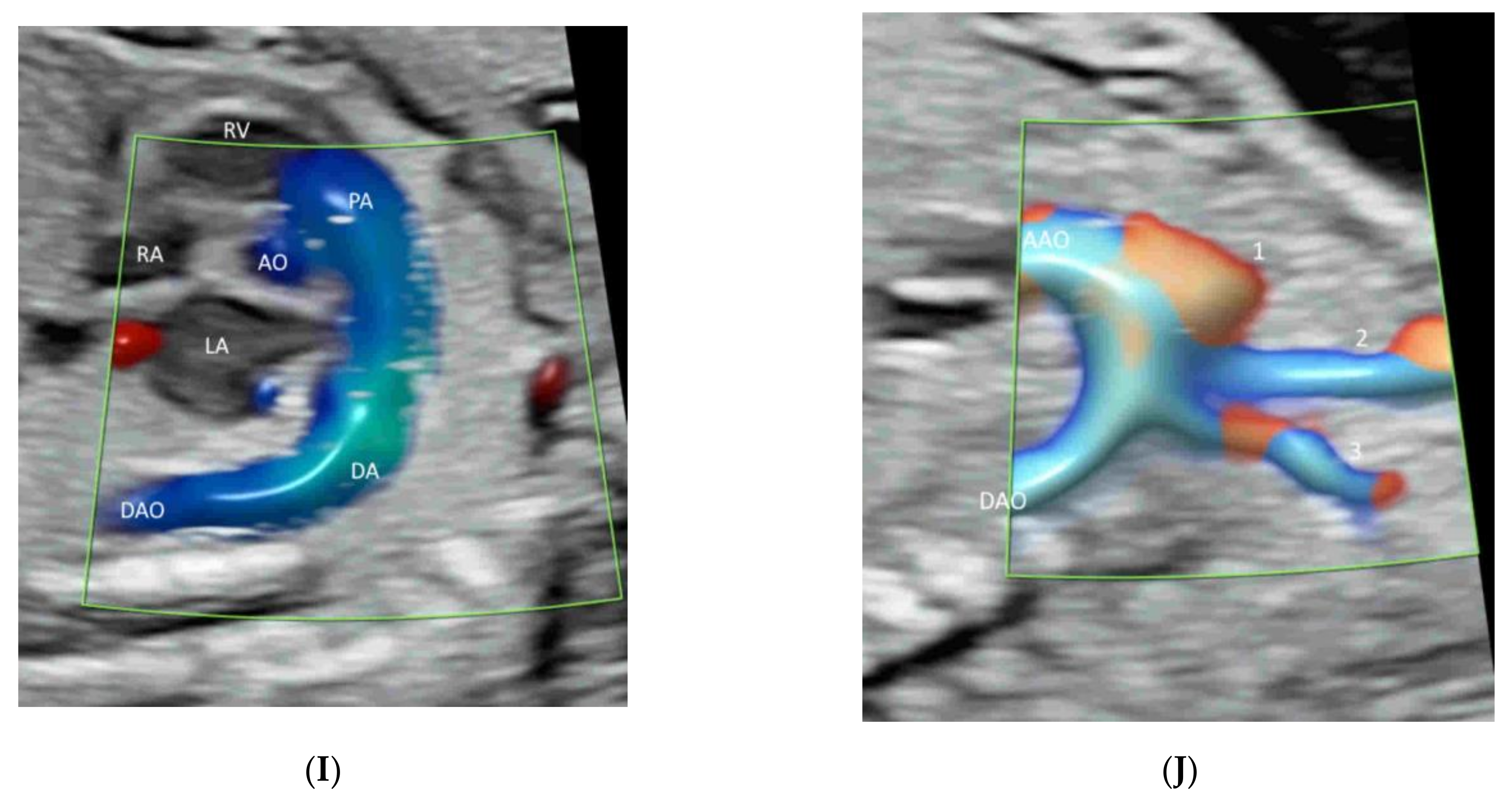
4.1. Fetal Heart
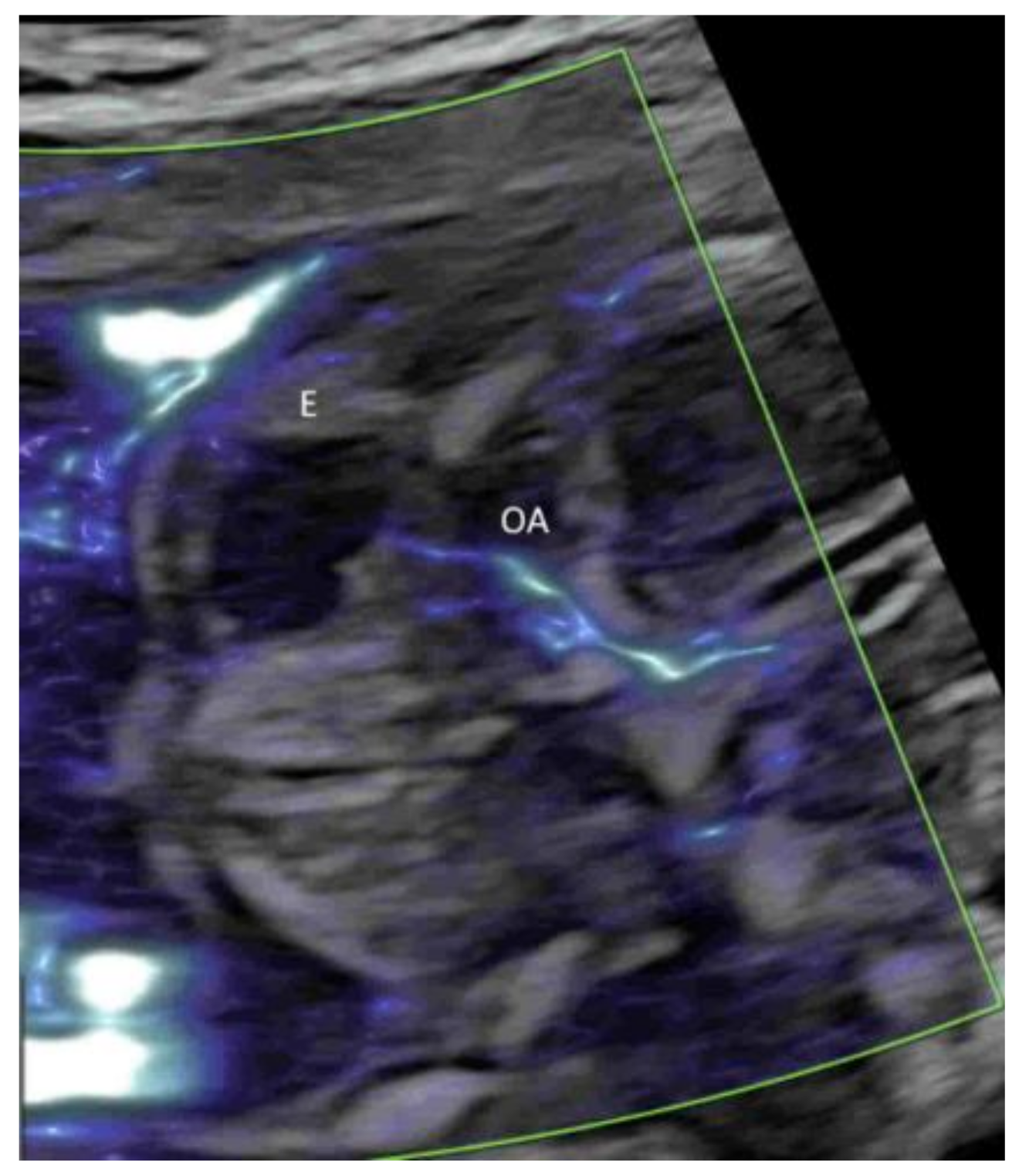
4.2. Fetal Brain
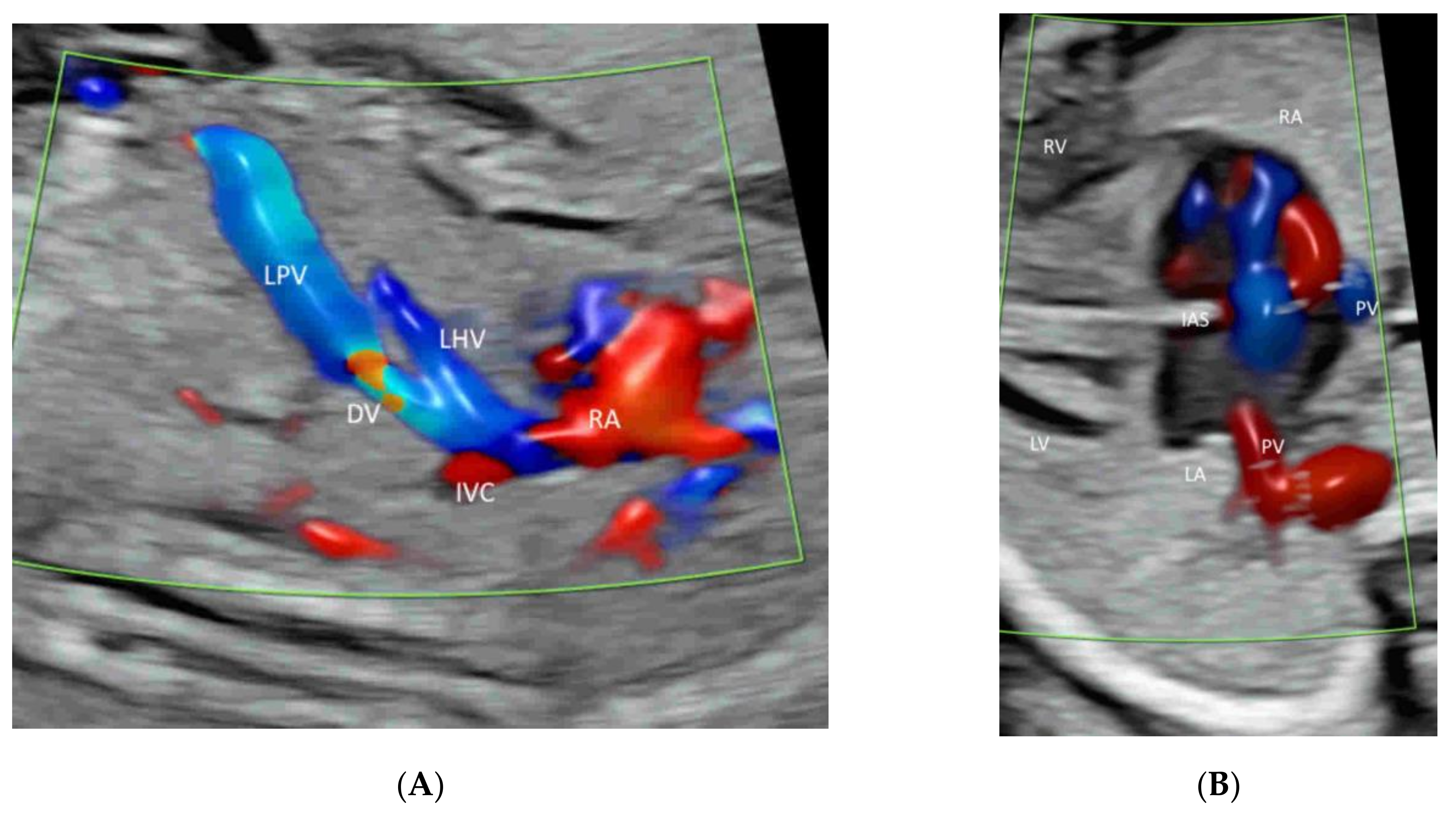
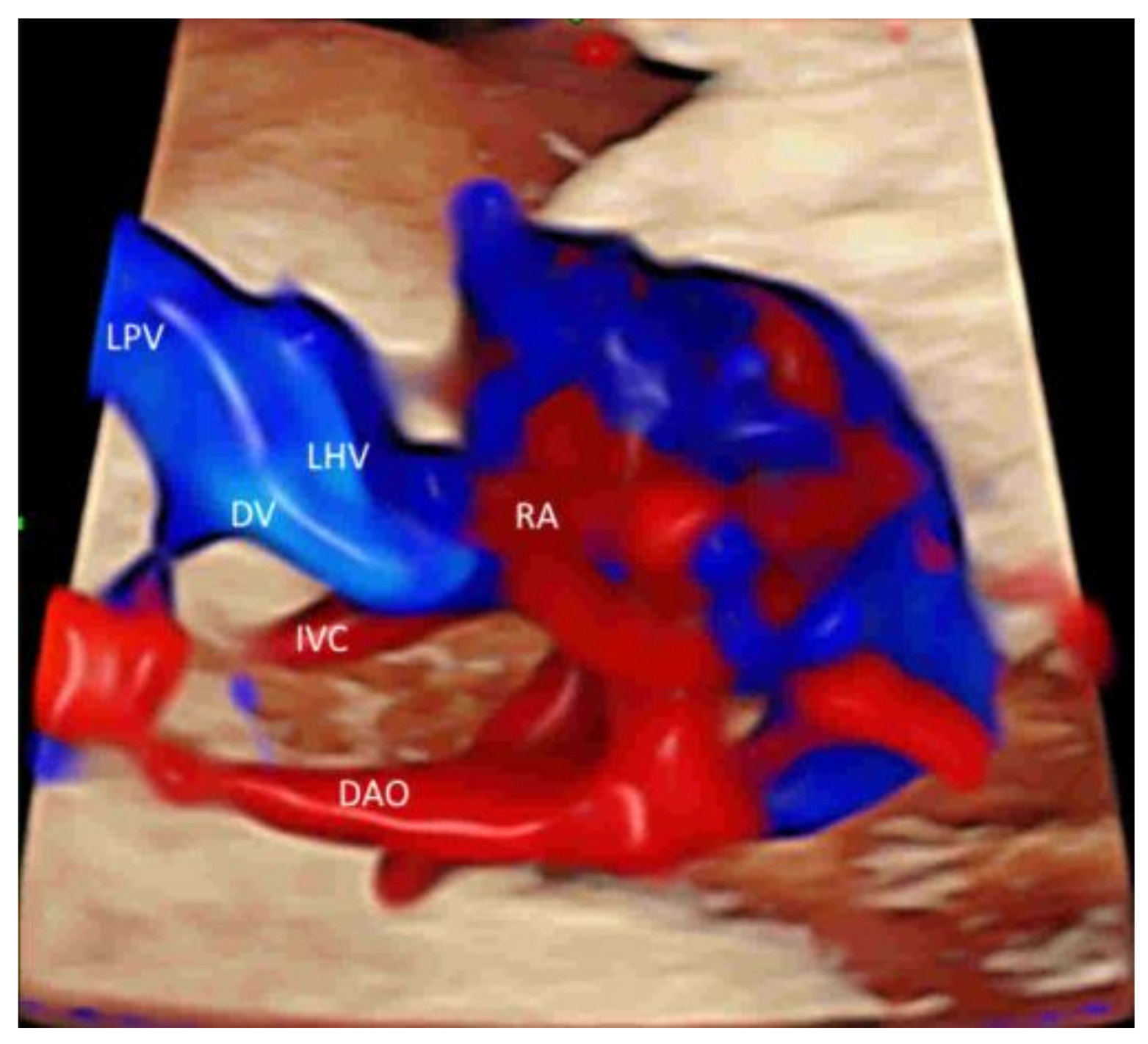
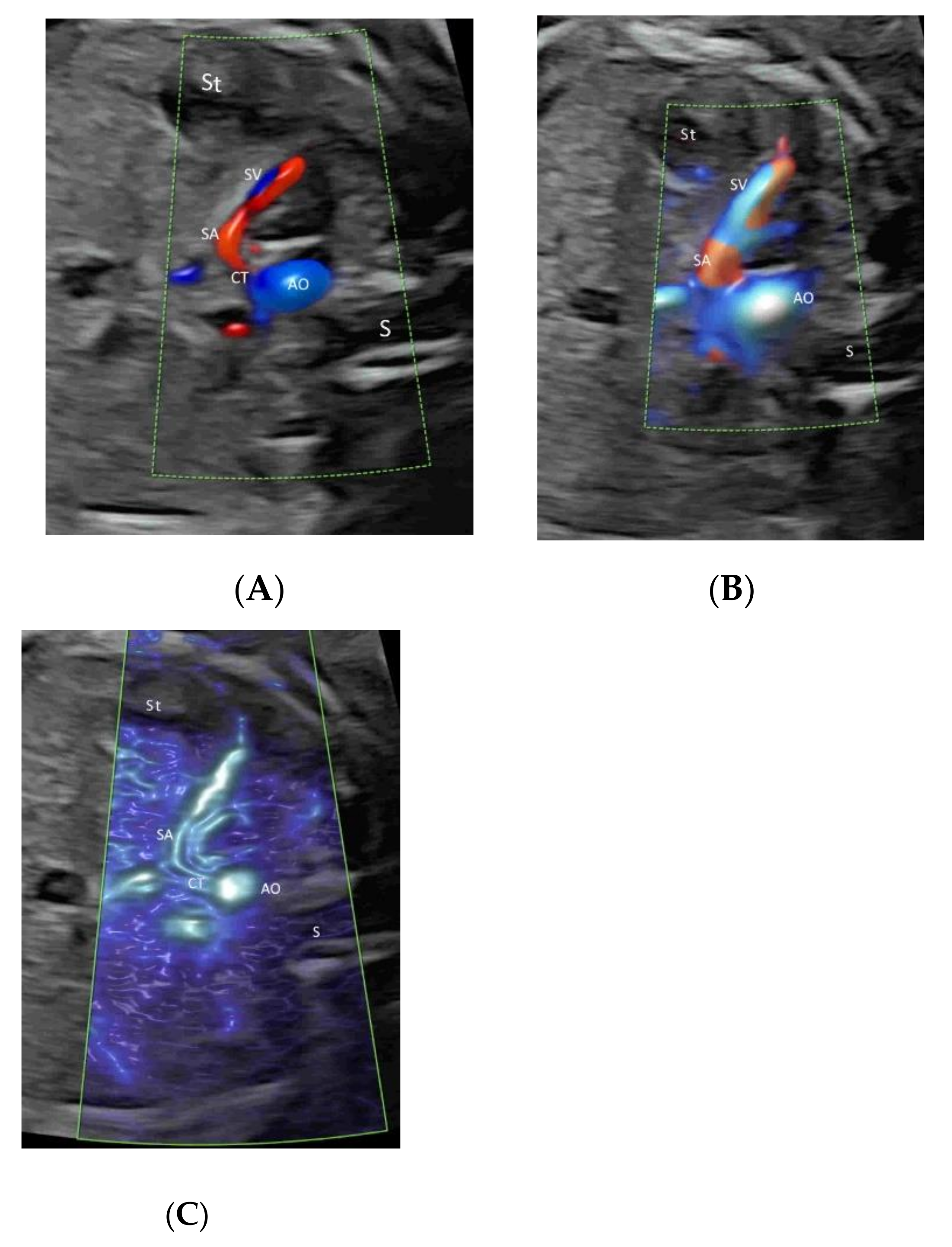
4.3. Fetal Abdomen and Other Fetal Vessels
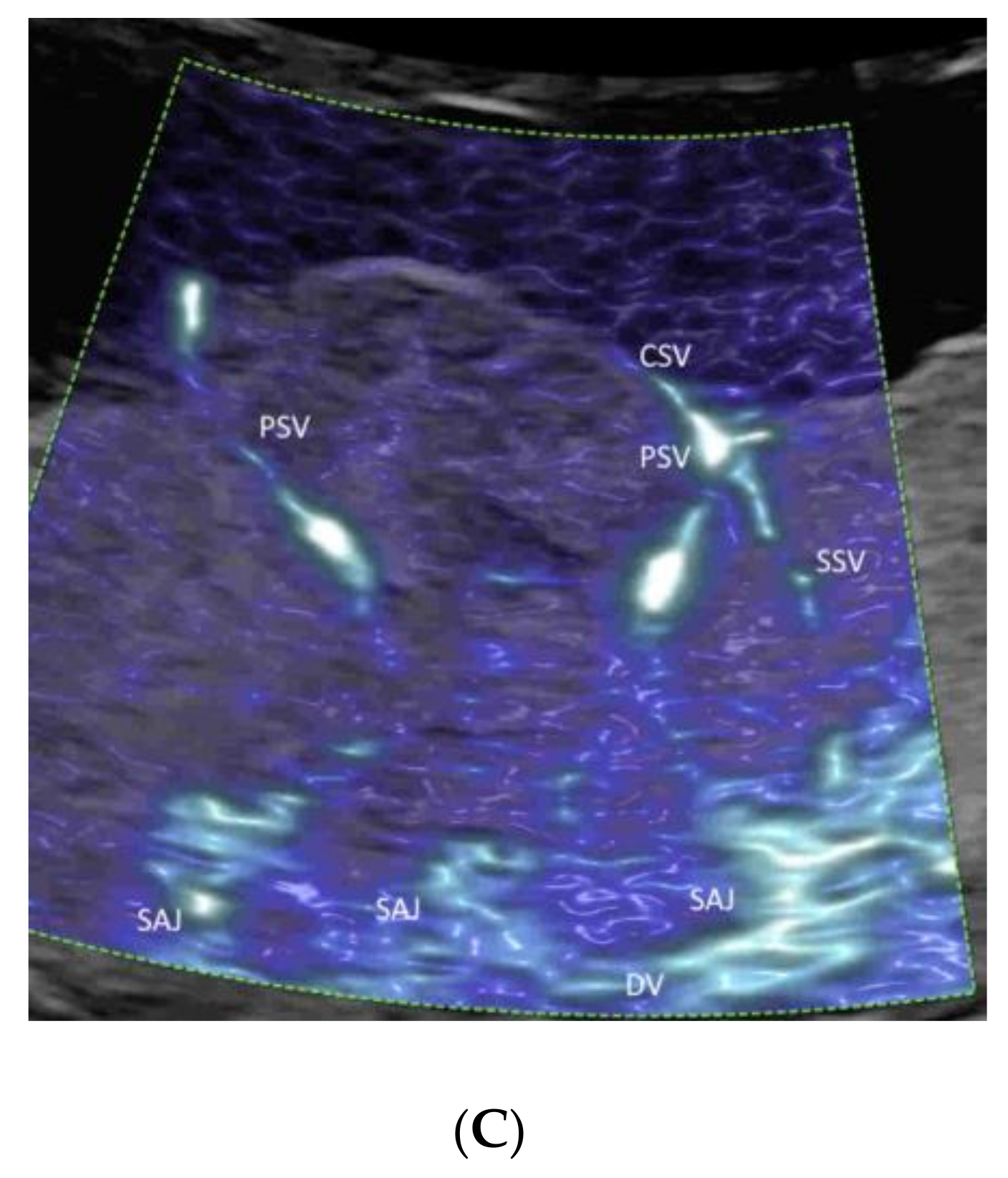
4.4. Placenta
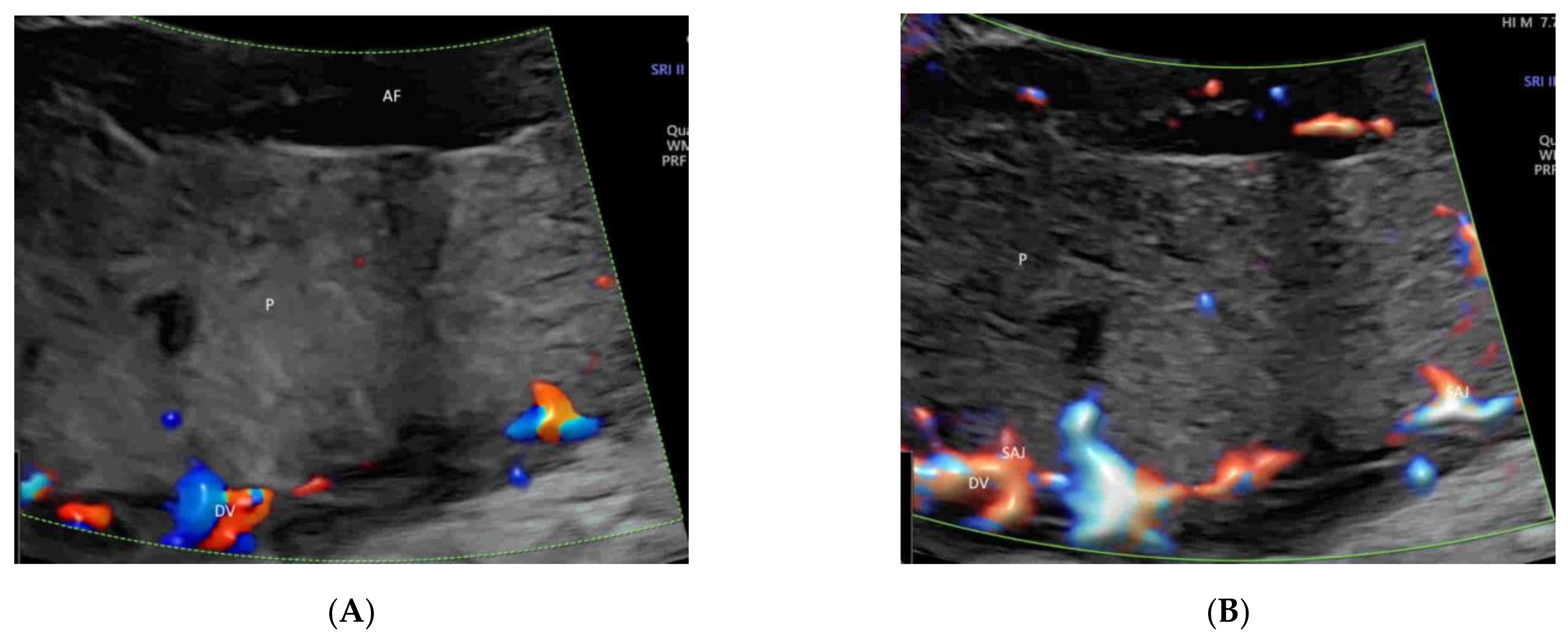
4.5. Umbilical Cord
4.6. First Trimester
4.7. Twins
5. Pitfalls of CFI
6. Special Indications of New Modes of CFI
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhide, A.; Acharya, G.; Baschat, A.; Bilardo, C.M.; Brezinka, C.; Cafici, D.; Ebbing, C.; Hernandez-Andrade, E.; Kalache, K.; Kingdom, J.; et al. ISUOG Practice Guidelines (updated): Use of Doppler velocimetry in obstetrics. Ultrasound Obstet. Gynecol. 2021, 58, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, W.; Rojas, I.; Robert, J.A.; Schnapp, C.; Alcalde, J.L. Prenatal detection of velamentous insertion of the umbilical cord: A prospective color Doppler ultrasound study. Ultrasound Obstet. Gynecol. 2003, 21, 564–569. [Google Scholar] [CrossRef]
- Wiechec, M.; Knafel, A.; Nocun, A. Prenatal detection of congenital heart defects at the 11- to 13-week scan using a simple color Doppler protocol including the 4-chamber and 3-vessel and trachea views. J. Ultrasound Med. 2015, 34, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Chaoui, R.; Abuhamad, A.; Martins, J.; Heling, K.S. Recent development in three and four dimension fetal echocardiography. Fetal Diagn. Ther. 2020, 47, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Nadel, A.S. Addition of color Doppler to the routine obstetric sonographic survey aids in the detection of pulmonic stenosis. Fetal Diagn. Ther. 2010, 28, 175–179. [Google Scholar] [CrossRef]
- Grosvenor, A.; Silver, R.; Porter, T.F.; Zempolich, K. Optimal management of placenta accreta. Am. J. Obstet. Gynecol. 2006, 195, S82. [Google Scholar] [CrossRef]
- Committee on Obstetric Practice. Committee opinion no. 529: Placenta accreta. Obstet. Gynecol. 2012, 120, 207–211. [Google Scholar] [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Bilardo, C.M.; Chalouhi, G.E.; Ghi, T.; Kagan, K.O.; Lau, T.K.; Papageorghiou, A.T.; Raine-Fenning, N.J.; Stirnemann, J.; et al. ISUOG Practice Guidelines: Performance of first-trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. 2013, 41, 102–113. [Google Scholar]
- Syngelaki, A.; Hammami, A.; Bower, S.; Zidere, V.; Akolekar, R.; Nicolaides, K.H. Diagnosis of fetal non-chromosomal abnormalities on routine ultrasound examination at 11–13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2019, 54, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. AIUM Practice Parameter for the Performance of Detailed Second- and Third-Trimester Diagnostic Obstetric Ultrasound Examinations. J. Ultrasound Med. 2019, 38, 3093–3100. [Google Scholar] [CrossRef]
- Anonymous. AIUM Practice Parameter for the Performance of Fetal Echocardiography. J. Ultrasound Med. 2020, 39, E5–E16. [Google Scholar]
- Paladini, D.; Malinger, G.; Birnbaum, R.; Monteagudo, A.; Pilu, G.; Salomon, L.J.; Timor-Tritsch, I.E. ISUOG Practice Guidelines (updated): Sonographic examination of the fetal central nervous system. Part 2: Performance of targeted neurosonography. Ultrasound Obstet. Gynecol. 2021, 57, 661–671. [Google Scholar] [CrossRef]
- International Society of Ultrasound in Obstetrics and Gynecology; Carvalho, J.S.; Allan, L.D.; Chaoui, R.; Copel, J.A.; DeVore, G.R.; Hecher, K.; Lee, W.; Munoz, H.; Paladini, D.; et al. ISUOG Practice Guidelines (updated): Sonographic screening examination of the fetal heart. Ultrasound Obstet. Gynecol. 2013, 41, 348–359. [Google Scholar] [CrossRef]
- Wang, Z.; Tyson, M.; Casey, C. LV Function Evaluation Using LV eFlow. White paper, Hitachi Aloka Medical America. Available online: http://www.hitachi-aloka.com/assets/pdf/white-paper-LV-eFlow%20pdf (accessed on 15 December 2021).
- Wu, G.; Xie, T.R.; Dimaano, M.M.; Alghrouz, M.I.; Ahmad, M. High-definition blood flow imaging in the assessment of left ventricular function: Initial experience and comparison with contrast echocardiography. Echocardiography 2019, 36, 546–557. [Google Scholar] [CrossRef]
- Karaca, L.; Oral, A.; Kantarci, M.; Sade, R.; Ogul, H.; Bayraktutan, U.; Okur, A.; Yüce, I. Comparison of the superb microvascular imaging technique and the color Doppler techniques for evaluating children’s testicular blood flow. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1947–1953. [Google Scholar]
- Wu, L.; Yen, H.H.; Soon, M.S. Spoke-wheel sign of focal nodular hyperplasia revealed by superb micro-vascular ultrasound imaging. QJM Int. J. Med. 2015, 108, 669–670. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Li, G.; Li, J.; Ren, W.D. The diagnostic value of superb microvascular imaging (SMI) in detecting blood flow signals of breast lesions. Medicine 2015, 94, e1502. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ota, Y.; Nagai, M.; Kusaka, G.; Tanaka, Y.; Naritaka, H. Ultrasonography monitoring with superb microvascular imaging technique in brain tumor surgery. World Neurosurg. 2017, 97, 749.e11–749.e20. [Google Scholar] [CrossRef]
- Athanasopoulos, N.; Seale, A.N.; Kilby, M.D. SlowflowHD for the Examination of the Fetal Heart in the First Trimester. Available online: https://www.womens-health.net/img/first-trimester/SlowFlow_HD_Whitepaper_JB83959XXf_v9.pdf (accessed on 15 December 2021).
- Wang, Y.; Zhang, Y. Fetal Vascular Rings and Pulmonary Slings: Strategies for Two- and Three- Dimensional Echocardiographic Diagnosis. J. Am. Soc. Echocardiogr. 2021, 34, 336–351. [Google Scholar] [CrossRef]
- Gindes, L.; Pretorius, L.H.; Romine, L.E.; Kfir, M.; D’Agostini, D.; Hull, A.; Achiron, R. Three-Dimensional Ultrasonographic Depiction of Fetal Abdominal Blood Vessels. J. Ultrasound Med. 2009, 28, 977–988. [Google Scholar] [CrossRef]
- Ito, M.; AboEllail, M.A.M.; Yamamoto, K.; Kanenishi, K.; Tanaka, H.; Masaoka, H.; Hata, T. HDlive Flow silhouette mode and spatiotemporal image correlation for diagnosing congenital heart disease. Ultrasound Obstet. Gynecol. 2017, 50, 411–415. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Chen, L.Z.; Yin, S.W.; Cai, A.L.; Yang, Z.Y. Non-invasive dynamic observation of placental vascular anastomoses in monochorionic twins: Assessment using three-dimensional sonography combined with tomographic ultrasound imaging. Placenta 2020, 95, 84–90. [Google Scholar] [CrossRef]
- Paladini, D. Sonography in obese and overweight pregnant women: Clinical, medicolegal and technical issues. Ultrasound Obstet. Gynecol. 2009, 33, 720–729. [Google Scholar] [CrossRef]
- Del Bianco, A.; Russo, S.; Lacerenza, N.; Rinaldi, M.; Rinaldi, G.; Nappi, L.; Greco, P. Four chamber view plus three-vessel and trachea view for a complete evaluation of the fetal heart during the second trimester. J. Perinat. Med. 2006, 34, 309–312. [Google Scholar] [CrossRef]
- He, Y.H.; Liu, K.; Gu, X.Y.; Zhang, Y.; Han, J.C.; Liu, X.W.; Li, Z.A. The application of high definition flow imaging in fetal hemodynamics. Clin. Exp. Obstet. Gynecol. 2015, 42, 11–17. [Google Scholar] [PubMed]
- Molina, F.S.; Faro, C.; Sotiriadis, A.; Dagklis, T.; Nicolaides, K.H. Heart stroke volume and cardiac output by four-dimensional ultrasound in normal fetuses. Ultrasound Obstet. Gynecol. 2008, 32, 181–187. [Google Scholar] [CrossRef]
- Paladini, D.; Volpe, P.; Sglavo, G.; Vassallo, M.; De Robertis, V.; Marasini, M.; Russo, M.G. Transposition of the great arteries in the fetus: Assessment of the spatial relationships of the arterial trunks by four-dimensional echocardiography. Ultrasound Obstet. Gynecol. 2008, 31, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Volpe, P.; Campobasso, G.; De Robertis, V.; Di Paolo, S.; Caruso, G.; Stanziano, A.; Volpe, N.; Gentile, M. Two- and four-dimensional echocardiography with B-flow imaging and spatiotemporal image correlation in prenatal diagnosis of isolated total anomalous pulmonary venous connection. Ultrasound Obstet. Gynecol. 2007, 30, 830–837. [Google Scholar] [CrossRef]
- Volpe, P.; Tuo, G.; De Robertis, V.; Campobasso, G.; Marasini, M.; Tempesta, A.; Gentile, M.; Rembouskos, G. Fetal interrupted aortic arch: 2D-4D echocardiography, associations and outcome. Ultrasound Obstet. Gynecol. 2010, 35, 302–309. [Google Scholar] [CrossRef]
- Pooh, R.K. Normal anatomy by three-dimensional ultrasound in the second and third trimesters. Semin. Fetal Neonatal Med. 2012, 17, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, J.; Yamada, H.; Kawasaki, E.; Matsumoto, T.; Takahashi, S.; Suzuki, N. Application of superb micro-vascular imaging (SMI) in obstetrics. J. Matern. Fetal Neonatal Med. 2018, 31, 261–263. [Google Scholar] [CrossRef]
- Paladini, D.; Deloison, B.; Rossi, A.; Chalouhi, G.E.; Gandolfo, C.; Sonigo, P.; Buratti, S.; Millischer, A.E.; Tuo, G.; Ville, Y.; et al. Vein of Galen aneurysmal malformation (VGAM) in the fetus: Retrospective analysis of perinatal prognostic indicators in a two-center series of 49 cases. Ultrasound Obstet. Gynecol. 2017, 50, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Yagel, S.; Cohen, S.M.; Valsky, D.V.; Shen, O.; Lipschuetz, M.; Messing, B. Systematic examination of the fetal abdominal precordial veins: A cohort study. Ultrasound Obstet. Gynecol. 2015, 45, 578–583. [Google Scholar] [CrossRef] [Green Version]
- Yagel, S.; Cohen, S.M.; Valsky, D.V. Simplifying imaging of the abdominal fetal precordial venous system. Ultrasound Obstet. Gynecol. 2019, 53, 571–575. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, M. Prenatal diagnosis of an aberrant ductus venosus draining into the coronary sinus using two- and three-dimensional echocardiography: A case report. BMC Pregnancy Childbirth 2021, 21, 392. [Google Scholar] [CrossRef]
- Leung, K.Y. Imaging of fetal precordial venous system by four-dimensional ultrasound with spatiotemporal image correlation technology. J. Clin. Ultrasound 2021. [Google Scholar] [CrossRef]
- Bethune, M.; Alibrahim, E.; Davies, B.; Yong, E. A pictorial guide for the second trimester ultrasound. Australas. J. Ultrasound Med. 2013, 16, 98–113. [Google Scholar] [CrossRef] [Green Version]
- Aboellail, M.A.; Ito, M.; Hata, T.; Kanenishi, K.; Mori, N.; Nitta, E.; Miyake, T. Advances in Color Doppler in Obstetrics. J. South Asian Fed. Obstet. Gynecol. 2019, 11, 1–12. [Google Scholar] [CrossRef]
- Sepulveda, W.; Sepulveda, F.; Corral, E.; Gutierrez, J. Giant hepatic hemangioma in the fetus: Case reports and updated review of the literature. J. Matern. Fetal Neonatal Med. 2021, 34, 2554–2566. [Google Scholar] [CrossRef]
- Abuhamad, A.Z.; Robinson, J.N.; Bogdan, D.; Tannous, R.J. Color Doppler of the splenic artery in the prenatal diagnosis of heterotaxic syndromes. Am. J. Perinatol. 1999, 16, 0469–0474. [Google Scholar] [CrossRef]
- Tenkumo, C.; Hanaoka, U.; AboEllail, M.A.M.; Ishimura, M.; Morine, M.; Maeda, K.; Hata, T. HDlive Flow with HDlive silhouette mode in diagnosis of fetal hepatic hemangioma. Ultrasound Obstet. Gynecol. 2017, 49, 541–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gucciardo, L.; Uyttebroek, A.; De Wever, I.; Renard, M.; Claus, F.; Devlieger, R.; Lewi, L.; De Catte, L.; Deprest, J. Prenatal assessment and management of sacrococcygeal teratoma. Prenat. Diagn. 2011, 31, 678–688. [Google Scholar] [CrossRef]
- Ivanitskaya, O.; Andreeva, E.; Odegova, N. Prenatal diagnosis of Klippel-Trenaunay syndrome: Series of four cases and review of the literature. Ultrasound 2020, 28, 91–102. [Google Scholar] [CrossRef]
- Garcia-Flores, J.; Cruceyra, M.; Cañamares, M.; Garicano, A.; Espada, M.; Nieto, O.; Tamarit, I.; de la Cuesta, R.S. Sonographic Evaluation of Fetal Adrenal Gland in Gestational Diabetes: Relation to Fetal Growth and Maternal Biochemical Markers. J. Ultrasound Med. 2017, 36, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, Z.F.; Aalipour, S.; Sheikh, M.; Shafaat, M.; Hantoushzadeh, S.; Borna, S.; Khazardoost, S. Ultrasound measurement of fetal adrenal gland in fetuses with intrauterine growth restriction, an early predictive method for adverse outcomes. J. Matern. Fetal Neonatal Med. 2019, 32, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- De Luca, J.; Rousseau, T.; Durand, C.; Sagot, P.; Sapin, E. Diagnostic and therapeutic dilemma with large prenatally detected cystic adrenal masses. Fetal Diagn. Ther. 2002, 17, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.; Kim, J.H.; Jang, K.M.; Yoo, S.Y.; Lim, G.Y.; Kim, M.J.; Kim, O.H. Comparison of Clinico-Radiological Features between Congenital Cystic Neuroblastoma and Neonatal Adrenal Hemorrhagic Pseudocyst. Korean J. Radiol. 2011, 12, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.O.; Vines, S.K.; Aquilina, J.; Wathen, N.C.; Harrington, K. Are placental lakes of any clinical significance? Placenta 2002, 23, 685–690. [Google Scholar] [CrossRef]
- Schiffer, V.; van Haren, A.; De Cubber, L.; Bons, J.; Coumans, A.; van Kuijk, S.M.; Spaanderman, M.; Al-Nasiry, S. Ultrasound evaluation of the placenta in healthy and placental syndrome pregnancies: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 262, 45–56. [Google Scholar] [CrossRef]
- Colpaert, R.M.; Ramseyer, A.M.; Luu, T.; Quick, C.M.; Frye, L.T.; Magann, E.F. Diagnosis and Management of Placental Mesenchymal Disease. A Review of the Literature. Obstet. Gynecol. Surv. 2019, 74, 611–622. [Google Scholar]
- Pagani, G.; Cali, G.; Acharya, G.; Trisch, I.T.; Palacios-Jaraquemada, J.; Familiari, A.; Buca, D.; Manzoli, L.; Flacco, M.E.; Fanfani, F.; et al. Diagnostic accuracy of ultrasound in detecting the severity of abnormally invasive placentation: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2018, 97, 25–37. [Google Scholar] [CrossRef]
- Calì, G.; Foti, F.; Minneci, G. 3D power Doppler in the evaluation of abnormally invasive placenta. J. Perinat. Med. 2017, 45, 701–709. [Google Scholar] [CrossRef]
- Amer, H.Z.M.; Heller, D.S. Chorangioma and related vascular lesions of the placenta–A review. Fetal Pediatr. Pathol. 2010, 29, 199–206. [Google Scholar] [CrossRef]
- Mack, L.M.; Mastrobattista, J.M.; Gandhi, R.; Castro, E.C.; Burgess, A.P.H.; Lee, W. Characterization of Placental Microvasculature Using Superb Microvascular Imaging. J. Ultrasound Med. 2019, 38, 2485–2491. [Google Scholar] [CrossRef]
- Sainz, J.A.; Carrera, J.; Borrero, C.; García-Mejido, J.A.; Fernández-Palacín, A.; Robles, A.; Sosa, F.; Arroyo, E. Study of the Development of Placental Microvascularity by Doppler SMI (Superb Microvascular Imaging): A Reality Today. Ultrasound Med. Biol. 2020, 46, 3257–3267. [Google Scholar] [CrossRef]
- Hata, T.; Kanenishi, K.; Yamamoto, K.; AboEllail, M.A.M.; Mashima, M.; Mori, N. Microvascular imaging of thick placenta with fetal growth restriction. Ultrasound Obstet. Gynecol. 2018, 51, 837–839. [Google Scholar] [CrossRef] [Green Version]
- García-Jiménez, R.; Arroyo, E.; Borrero, C.; Garcia-Mejido, J.A.; Sosa, F.; Fernández-Palacín, A.; Sainz, J.A. Evaluation of Placental Micro-vascularization by Superb Micro-vascular Imaging Doppler in Cases of Intra-uterine Growth Restriction: A First Step. Ultrasound Med. Biol. 2021, 47, 1631–1636. [Google Scholar] [CrossRef]
- Chen, Y.H.; Liu, X.; Xu, C.M.; Yan, S.P.; Hu, Q.; Long, F.W.; Qin, G.C. Standardization of diagnosis for coiling of the umbilical cord around fetal neck by ultrasound. Int. J. Gynecol. Obstet. 2019, 147, 96–101. [Google Scholar] [CrossRef]
- Abuhamad, A. Three-dimensional ultrasound with color Doppler imaging of an umbilical cord true knot. Ultrasound Obstet. Gynecol. 2014, 43, 360. [Google Scholar] [CrossRef]
- Hasbun, J.; Alcalde, J.L.; Sepulveda, W. Three-dimensional power Doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: Value and limitations. J. Ultrasound Med. 2007, 26, 1215–1220. [Google Scholar] [CrossRef]
- Minnella, G.P.; Crupano, F.M.; Syngelaki, A.; Zidere, V.; Akolekar, R.; Nicolaides, K.H. Diagnosis of major heart defects by routine first-trimester ultrasound examination: Association with increased nuchal translucency, tricuspid regurgitation and abnormal flow in ductus venosus. Ultrasound Obstet. Gynecol. 2020, 55, 637–644. [Google Scholar] [CrossRef]
- Wong, A.E.; Sepulveda, W. Acardiac anomaly: Current issues in prenatal assessment and treatment. Prenat. Diagn. 2005, 25, 796–806. [Google Scholar] [CrossRef]
- Winkler, P.; Helmke, K.; Mahl, M. Major pitfalls in doppler investigations. Pediatr. Radiol. 1990, 20, 304–310. [Google Scholar] [CrossRef]
- Martins, W.; Lima, J.C.; Welsh, A.W.; Araujo, E.; Miyague, A.H.; Filho, F.M.; Raine-Fenning, N.J. Three-dimensional Doppler evaluation of single spherical samples from the placenta: Intra- and interobserver reliability. Ultrasound Obstet. Gynecol. 2012, 40, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramowicz, J.S. Obstetric ultrasound: Where are we and where are we going? Ultrasonography 2021, 40, 57–74. [Google Scholar] [CrossRef]
- Leung, K.Y. Applications of advanced ultrasound technology in obstetrics. Diagnostics 2021, 11, 1217. [Google Scholar] [CrossRef]
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, K.-y.; Wan, Y.-L. Update on Color Flow Imaging in Obstetrics. Life 2022, 12, 226. https://doi.org/10.3390/life12020226
Leung K-y, Wan Y-L. Update on Color Flow Imaging in Obstetrics. Life. 2022; 12(2):226. https://doi.org/10.3390/life12020226
Chicago/Turabian StyleLeung, Kwok-yin, and Yung-Liang Wan. 2022. "Update on Color Flow Imaging in Obstetrics" Life 12, no. 2: 226. https://doi.org/10.3390/life12020226
APA StyleLeung, K.-y., & Wan, Y.-L. (2022). Update on Color Flow Imaging in Obstetrics. Life, 12(2), 226. https://doi.org/10.3390/life12020226





