Dexmedetomidine Co-Administered with Lidocaine Decreases Nociceptive Responses and Trigeminal Fos Expression without Motor Dysfunction and Hypotension in a Murine Orofacial Formalin Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Drugs
2.3. Formalin-Induced Orofacial Pain Test
2.4. Fos Immunohistochemistry
2.5. Image Analysis
2.6. Rota-Rod Test
2.7. Assessment of Systolic Pressure and Heartbeat
2.8. Statistical Analysis
3. Results
3.1. Dexmedetomidine Reduces Orofacial Formalin-Induced Nociceptive Responses
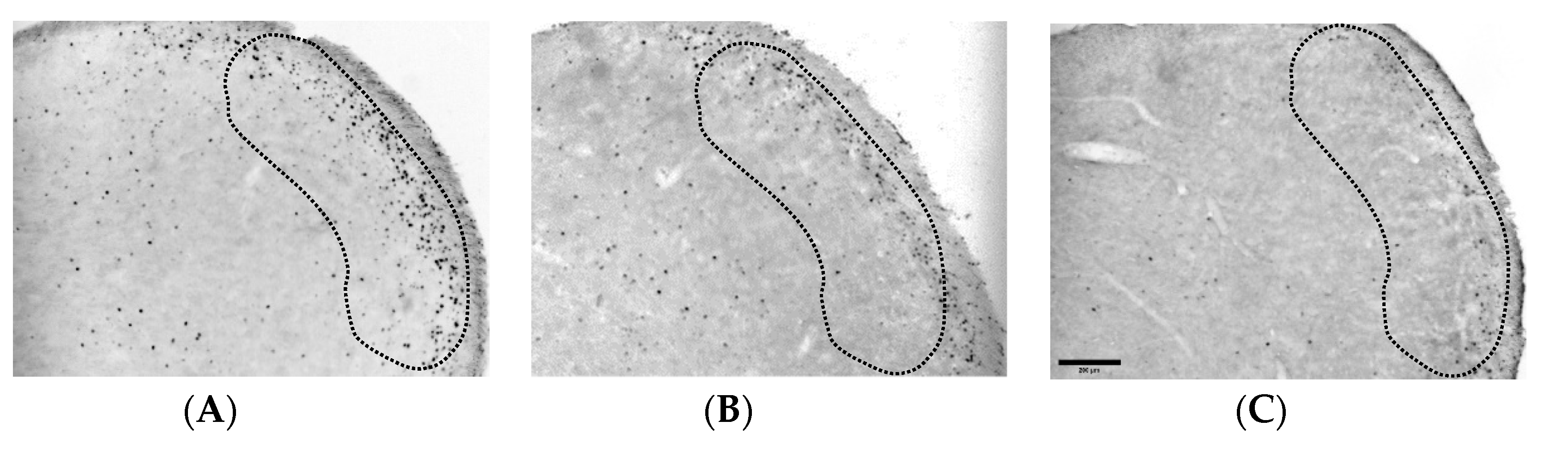
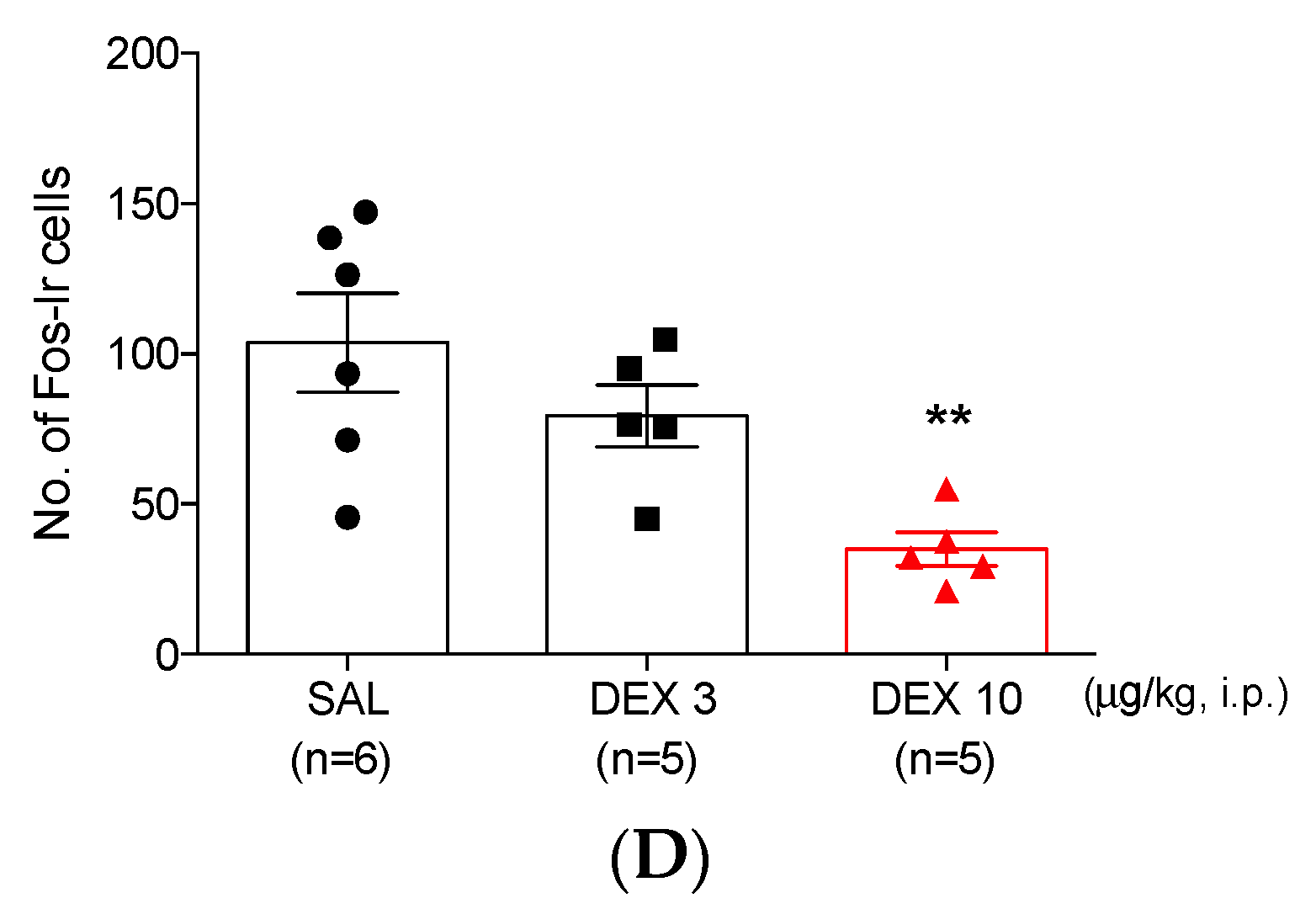
3.2. Dexmedetomidine Reduces the Increase of Fos-ir Cells in TNC
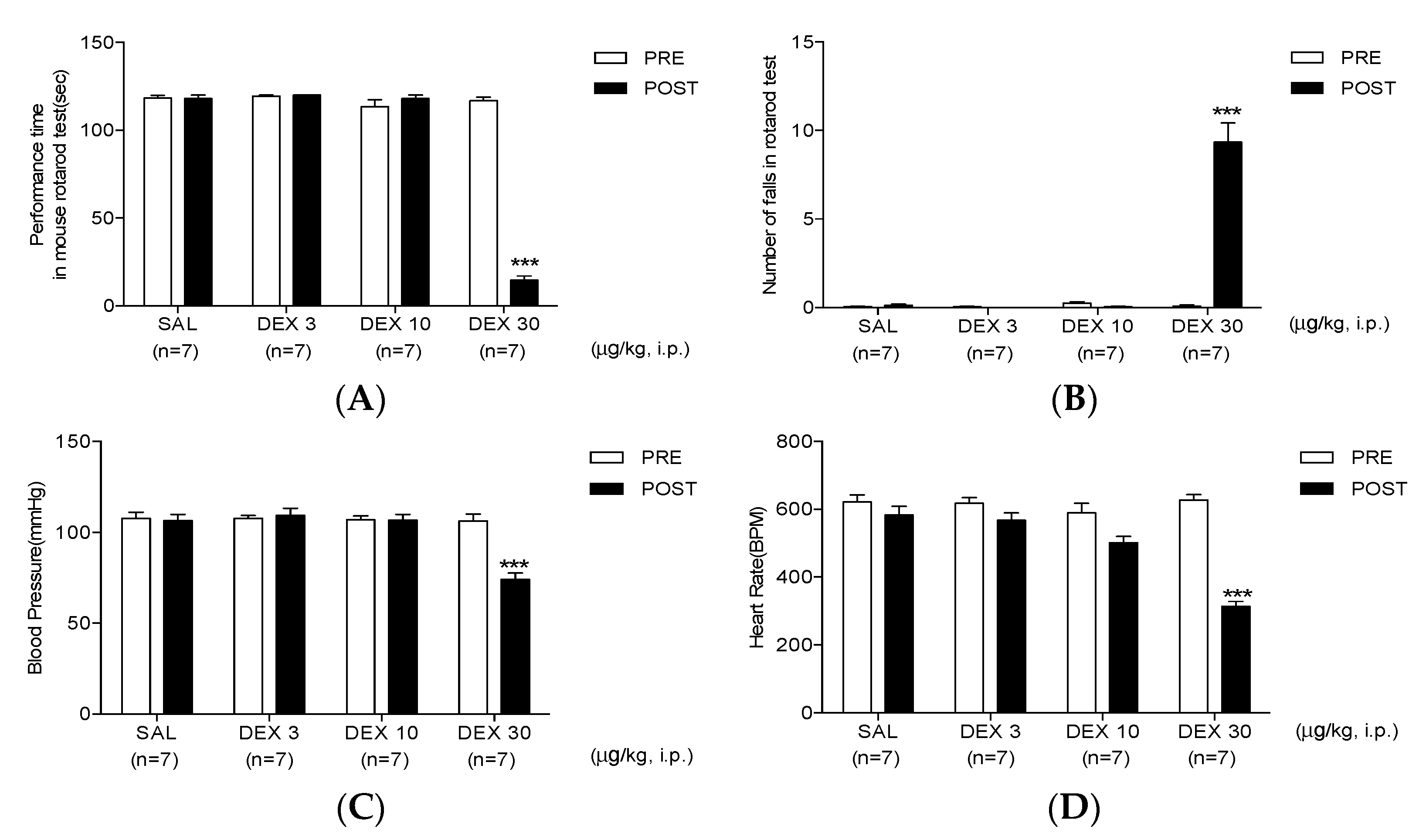
3.3. High-Dose Dexmedetomidine Induces Motor Dysfunction and Decreases Both Systolic Pressure and Heart Rate
3.4. Co-Administration of Dexmedetomidine with 0.5% Lidocaine Reduces Orofacial Formalin-Induced Nociceptive Responses
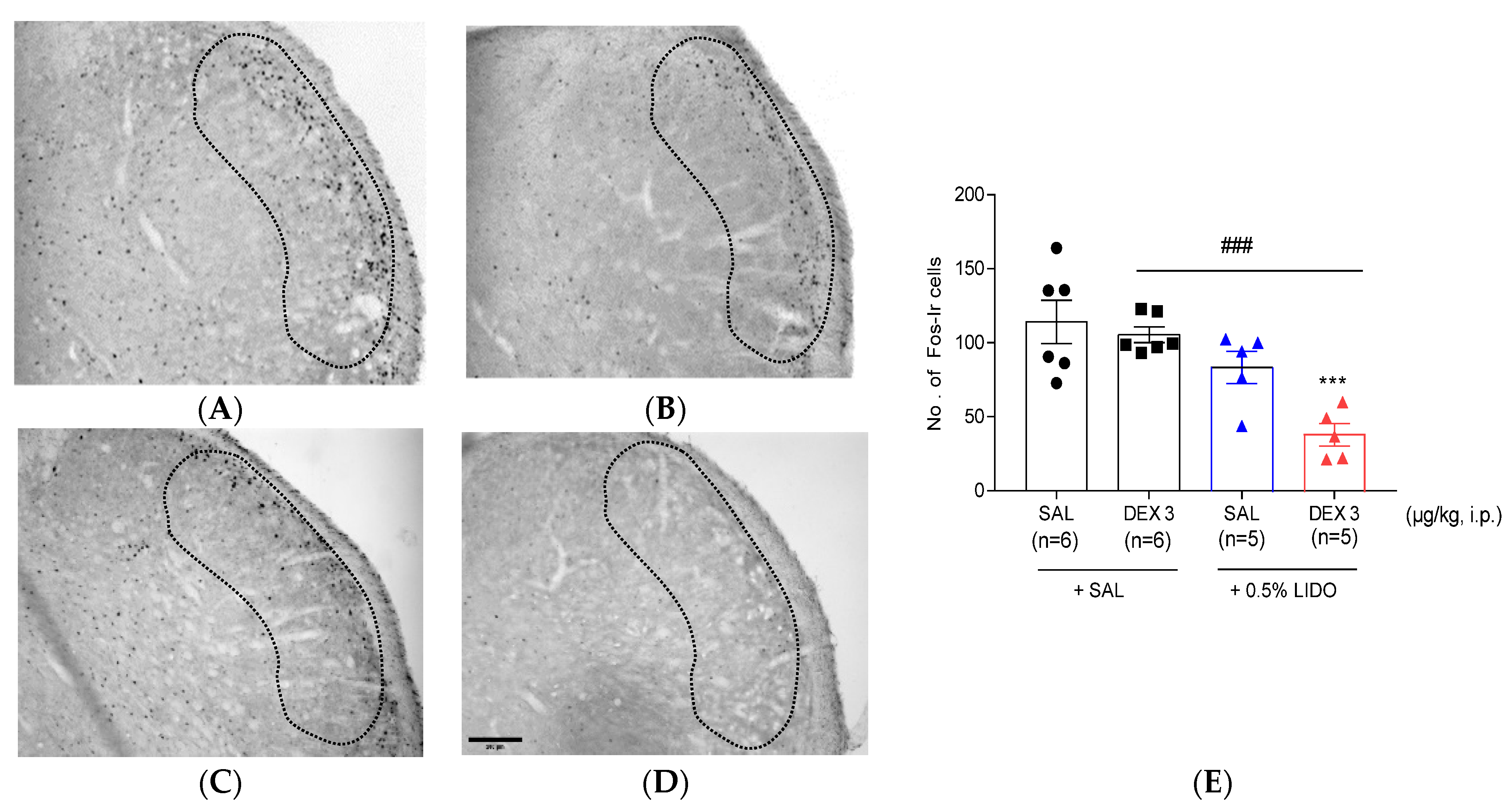
3.5. Co-Administration of Dexmedetomidine with 0.5% Lidocaine Reduces Fos-ir Cells
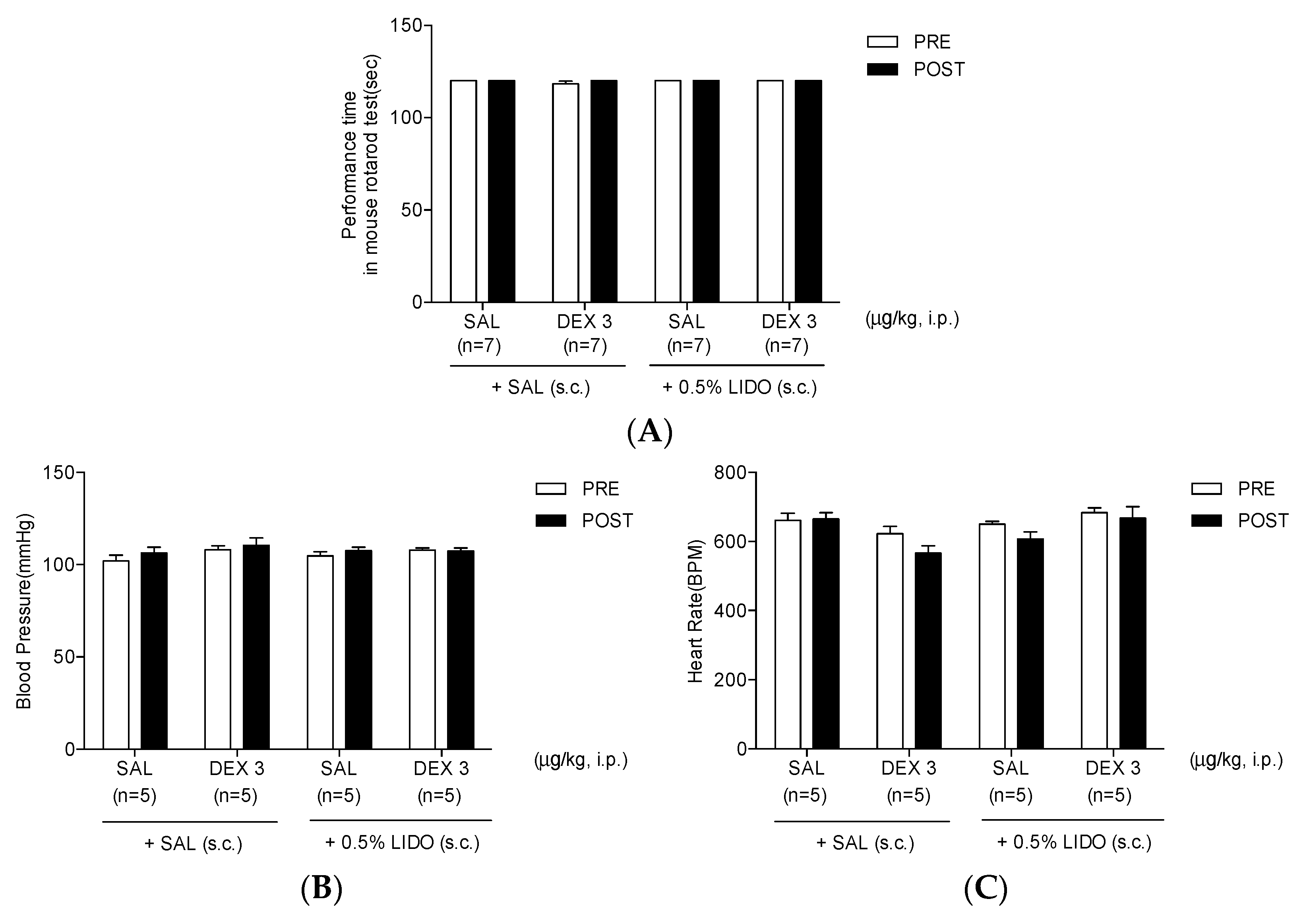
3.6. Co-Administration of Dexmedetomidine with 0.5% Lidocaine Does Not Affect Motor Performance, Blood Pressure, and Heart Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raboisson, P.; Dallel, R. The orofacial formalin test. Neurosci. Biobehav. Rev. 2004, 28, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Miranda, H.F.; Sierralta, F.; Prieto, J.C. Synergism between NSAIDs in the orofacial formalin test in mice. Pharmacol. Biochem. Behav. 2009, 92, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.; Navarro, C.; Noriega, V.; Pinardi, G.; Sierralta, F.; Prieto, J.C.; Miranda, H.F. Synergism between COX-3 inhibitors in two animal models of pain. Inflammopharmacology 2010, 18, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Grosu, I.; Lavand’homme, P. Use of dexmedetomidine for pain control. F1000 Med. Rep. 2010, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- Kalso, E.A.; Poyhia, R.; Rosenberg, P.H. Spinal antinociception by dexmedetomidine, a highly selective alpha 2-adrenergic agonist. Pharmacol. Toxicol. 1991, 68, 140–143. [Google Scholar] [CrossRef]
- Yaksh, T.L.; Pogrel, J.W.; Lee, Y.W.; Chaplan, S.R. Reversal of nerve ligation-induced allodynia by spinal alpha-2 adrenoceptor agonists. J. Pharmacol Exp. Ther. 1995, 272, 207–214. [Google Scholar]
- Zhang, H.; Zhou, F.; Li, C.; Kong, M.; Liu, H.; Zhang, P.; Zhang, S.; Cao, J.; Zhang, L.; Ma, H. Molecular mechanisms underlying the analgesic property of intrathecal dexmedetomidine and its neurotoxicity evaluation: An in vivo and in vitro experimental study. PLoS ONE 2013, 8, e55556. [Google Scholar] [CrossRef]
- Nazarian, A.; Christianson, C.A.; Hua, X.Y.; Yaksh, T.L. Dexmedetomidine and ST-91 analgesia in the formalin model is mediated by alpha2A-adrenoceptors: A mechanism of action distinct from morphine. Br. J. Pharmacol. 2008, 155, 1117–1126. [Google Scholar] [CrossRef]
- Buerkle, H.; Yaksh, T.L. Pharmacological evidence for different alpha 2-adrenergic receptor sites mediating analgesia and sedation in the rat. Br. J. Anaesth. 1998, 81, 208–215. [Google Scholar] [CrossRef]
- Endo, T.; Gabka, J.; Taubenheim, L. Intraligamentary anesthesia: Benefits and limitations. Quintessence Int. 2008, 39, e15–e25. [Google Scholar]
- Catterall, W.A. Molecular mechanisms of gating and drug block of sodium channels. Novartis Found. Symp. 2002, 241, 206–218, discussion 218–232. [Google Scholar] [PubMed]
- Hermanns, H.; Hollmann, M.W.; Stevens, M.F.; Lirk, P.; Brandenburger, T.; Piegeler, T.; Werdehausen, R. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: A narrative review. Br. J. Anaesth. 2019, 123, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Schnaider, T.B.; Vieira, A.M.; Brandao, A.C.; Lobo, M.V. Intraoperative analgesic effect of epidural ketamine, clonidine or dexmedetomidine for upper abdominal surgery. Rev. Bras. Anestesiol. 2005, 55, 525–531. [Google Scholar] [PubMed]
- Kanazi, G.E.; Aouad, M.T.; Jabbour-Khoury, S.I.; Al Jazzar, M.D.; Alameddine, M.M.; Al-Yaman, R.; Bulbul, M.; Baraka, A.S. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol. Scand. 2006, 50, 222–227. [Google Scholar] [CrossRef]
- El-Hennawy, A.M.; Abd-Elwahab, A.M.; Abd-Elmaksoud, A.M.; El-Ozairy, H.S.; Boulis, S.R. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br. J. Anaesth. 2009, 103, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Simon, A.; Valencia, M.; Cacho-Asenjo, E.; Honorato-Cia, C.; Nunez-Cordoba, J.M.; Manzanilla, O.; Aldaz, A.; Panadero, A.; Guridi, J.; Alegre, M. Effects of dexmedetomidine on subthalamic local field potentials in Parkinson’s disease. Br. J. Anaesth. 2021, 127, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ard, J.; Doyle, W.; Bekker, A. Awake craniotomy with dexmedetomidine in pediatric patients. J. Neurosurg. Anesthesiol. 2003, 15, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Munro, H.M.; Tirotta, C.F.; Felix, D.E.; Lagueruela, R.G.; Madril, D.R.; Zahn, E.M.; Nykanen, D.G. Initial experience with dexmedetomidine for diagnostic and interventional cardiac catheterization in children. Paediatr. Anaesth. 2007, 17, 109–112. [Google Scholar] [CrossRef]
- Shukry, M.; Kennedy, K. Dexmedetomidine as a total intravenous anesthetic in infants. Paediatr. Anaesth. 2007, 17, 581–583. [Google Scholar] [CrossRef]
- Bornhof, M.; Ihmsen, H.; Schwilden, H.; Yeomans, D.C.; Tzabazis, A. The orofacial formalin test in mice revisited-effects of formalin concentration, age, morphine and analysis method. J. Pain. 2011, 12, 633–639. [Google Scholar] [CrossRef]
- Yeo, J.H.; Kim, S.J.; Roh, D.H. Rapamycin reduces orofacial nociceptive responses and microglial p38 mitogen-activated protein kinase phosphorylation in trigeminal nucleus caudalis in mouse orofacial formalin model. Korean J. Physiol. Pharmacol. 2021, 25, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.G.; la Motte, R.H. Behavioral differentiation between itch and pain in mouse. Pain 2008, 139, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Roh, D.H.; Kim, H.W.; Yoon, S.Y.; Kang, S.Y.; Kwon, Y.B.; Cho, K.H.; Han, H.J.; Ryu, Y.H.; Choi, S.M.; Lee, H.J.; et al. Bee venom injection significantly reduces nociceptive behavior in the mouse formalin test via capsaicin-insensitive afferents. J. Pain 2006, 7, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Roh, D.H.; Kwon, Y.B.; Kim, H.W.; Seo, H.S.; Han, H.J.; Lee, H.J.; Beitz, A.J.; Lee, J.H. Acupoint stimulation with diluted bee venom (apipuncture) potentiates the analgesic effect of intrathecal clonidine in the rodent formalin test and in a neuropathic pain model. J. Pain 2009, 10, 253–263. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Yu, N.; Jia, C.; Wang, S. Mechanisms of dexmedetomidine in neuropathic pain. Front. Neurosci. 2020, 14, 330. [Google Scholar] [CrossRef]
- Asano, T.; Dohi, S.; Ohta, S.; Shimonaka, H.; Iida, H. Antinociception by epidural and systemic alpha(2)-adrenoceptor agonists and their binding affinity in rat spinal cord and brain. Anesth Analg. 2000, 90, 400–407. [Google Scholar] [CrossRef]
- Kimura, M.; Saito, S.; Obata, H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neurosci. Lett. 2012, 529, 70–74. [Google Scholar] [CrossRef]
- Li, S.S.; Zhang, W.S.; Ji, D.; Zhou, Y.L.; Li, H.; Yang, J.L.; Xiong, Y.C.; Zhang, Y.Q.; Xu, H. Involvement of spinal microglia and interleukin-18 in the anti-nociceptive effect of dexmedetomidine in rats subjected to CCI. Neurosci. Lett. 2014, 560, 21–25. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Roh, D.H.; Yeo, J.H.; Woo, J.; Han, S.H.; Kim, K.S. Analgesic efficacy of alpha2 adrenergic receptor agonists depends on the chronic state of neuropathic pain: Role of regulator of g protein signaling 4. Neuroscience 2021, 455, 177–194. [Google Scholar] [CrossRef]
- Roh, D.H.; Kim, H.W.; Yoon, S.Y.; Seo, H.S.; Kwon, Y.B.; Han, H.J.; Beitz, A.J.; Lee, J.H. Intrathecal clonidine suppresses phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit in spinal dorsal horn neurons of rats with neuropathic pain. Anesth. Analg. 2008, 107, 693–700. [Google Scholar] [CrossRef]
- Im, S.T.; Jo, Y.Y.; Han, G.; Jo, H.J.; Kim, Y.H.; Park, C.K. Dexmedetomidine inhibits voltage-gated sodium channels via alpha2-adrenoceptors in trigeminal ganglion neurons. Mediators Inflamm. 2018, 2018, 1782719. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Yeom, M.Y.; Kang, E.S.; Kang, J.W.; Song, H.K. The antinociceptive effect of dexmedetomidine modulates spleen cell immunity in mice. Int. J. Med. Sci. 2014, 11, 226–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, M.; Shao, A.; Tang, X.; Feng, M.; Wang, J.; Qiu, Y. MiR-34a affects dexmedetomidine-inhibited chronic inflammatory visceral pain by targeting to HDAC2. BMC Anesthesiol. 2019, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Liu, Z.; Yu, L. Dexmedetomidine attenuates lipopolysaccharide induced acute lung injury by targeting NLRP3 via miR-381. J. Biochem. Mol. Toxicol. 2018, 32, e22211. [Google Scholar] [CrossRef]
- Ter Horst, G.J.; Meijler, W.J.; Korf, J.; Kemper, R.H. Trigeminal nociception-induced cerebral Fos expression in the conscious rat. Cephalalgia 2001, 21, 963–975. [Google Scholar] [CrossRef]
- Mitsikostas, D.D.; Sanchez del Rio, M. Receptor systems mediating c-fos expression within trigeminal nucleus caudalis in animal models of migraine. Brain Res. Brain Res. Rev. 2001, 35, 20–35. [Google Scholar] [CrossRef]
- Bhatt, D.K.; Gupta, S.; Ploug, K.B.; Jansen-Olesen, I.; Olesen, J. mRNA distribution of CGRP and its receptor components in the trigeminovascular system and other pain related structures in rat brain, and effect of intracerebroventricular administration of CGRP on Fos expression in the TNC. Neurosci. Lett. 2014, 559, 99–104. [Google Scholar] [CrossRef]
- Brand, P.A.; Paris, A.; Bein, B.; Meybohm, P.; Scholz, J.; Ohnesorge, H.; Tonner, P.H. Propofol sedation is reduced by delta9-tetrahydrocannabinol in mice. Anesth. Analg. 2008, 107, 102–106. [Google Scholar] [CrossRef]
- Kaplancikli, Z.A.; Altintop, M.D.; Turan-Zitouni, G.; Ozdemir, A.; Can, O.D. Synthesis and analgesic activity of some acetamide derivatives. J. Enzyme Inhib. Med. Chem. 2012, 27, 275–280. [Google Scholar] [CrossRef]
- Quintans Jde, S.; Menezes, P.P.; Santos, M.R.; Bonjardim, L.R.; Almeida, J.R.; Gelain, D.P.; Araujo, A.A.; Quintans-Junior, L.J. Improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in beta-cyclodextrin. Phytomedicine 2013, 20, 436–440. [Google Scholar] [CrossRef]
- Kasap, M.; Can, O.D. Opioid system mediated anti-nociceptive effect of agomelatine in mice. Life Sci. 2016, 163, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.H.; Yoon, S.Y.; Kim, S.J.; Oh, S.B.; Lee, J.H.; Beitz, A.J.; Roh, D.H. Clonidine, an alpha-2 adrenoceptor agonist relieves mechanical allodynia in oxaliplatin-induced neuropathic mice; potentiation by spinal p38 MAPK inhibition without motor dysfunction and hypotension. Int. J. Cancer 2016, 138, 2466–2476. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Lee, J.Y.; Roh, D.H.; Oh, S.B. Pharmacopuncture with scolopendra subspinipes suppresses mechanical allodynia in oxaliplatin-induced neuropathic mice and potentiates clonidine-induced anti-allodynia without hypotension or motor impairment. J. Pain 2018, 19, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.K.; Cheung, C.W.; Chong, Y.K. Alpha-2 agonists in acute pain management. Expert. Opin. Pharmacother. 2010, 11, 2849–2868. [Google Scholar] [CrossRef]
- Zhang, Z.; Ferretti, V.; Guntan, I.; Moro, A.; Steinberg, E.A.; Ye, Z.; Zecharia, A.Y.; Yu, X.; Vyssotski, A.L.; Brickley, S.G.; et al. Neuronal ensembles sufficient for recovery sleep and the sedative actions of alpha2 adrenergic agonists. Nat. Neurosci. 2015, 18, 553–561. [Google Scholar] [CrossRef]
- Hunter, J.C.; Fontana, D.J.; Hedley, L.R.; Jasper, J.R.; Lewis, R.; Link, R.E.; Secchi, R.; Sutton, J.; Eglen, R.M. Assessment of the role of alpha2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br. J. Pharmacol. 1997, 122, 1339–1344. [Google Scholar] [CrossRef]
- Rangel, R.A.; Marinho, B.G.; Fernandes, P.D.; de Moura, R.S.; Lessa, M.A. Pharmacological mechanisms involved in the antinociceptive effects of dexmedetomidine in mice. Fundam Clin. Pharmacol. 2014, 28, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Votava, M.; Hess, L.; Sliva, J.; Krsiak, M.; Agova, V. Dexmedetomidine selectively suppresses dominant behaviour in aggressive and sociable mice. Eur. J. Pharmacol. 2005, 523, 79–85. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Casasola, O.A.; Mayoral, V. The topical 5% lidocaine medicated plaster in localized neuropathic pain: A reappraisal of the clinical evidence. J. Pain Res. 2016, 9, 67–79. [Google Scholar] [CrossRef]
- Baron, R.; Allegri, M.; Correa-Illanes, G.; Hans, G.; Serpell, M.; Mick, G.; Mayoral, V. The 5% lidocaine-medicated plaster: Its inclusion in international treatment guidelines for treating localized neuropathic pain, and clinical evidence supporting its use. Pain Ther. 2016, 5, 149–169. [Google Scholar] [CrossRef]
- Gudin, J.; Nalamachu, S. Utility of lidocaine as a topical analgesic and improvements in patch delivery systems. Postgrad. Med. 2020, 132, 28–36. [Google Scholar] [CrossRef]
- Yang, X.; Wei, X.; Mu, Y.; Li, Q.; Liu, J. A review of the mechanism of the central analgesic effect of lidocaine. Medicine 2020, 99, e19898. [Google Scholar] [CrossRef] [PubMed]
- Abbadie, C.; Taylor, B.K.; Peterson, M.A.; Basbaum, A.I. Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: Studies with remifentanil and lidocaine. Pain 1997, 69, 101–110. [Google Scholar] [CrossRef]
- Hao, S.; Takahata, O.; Iwasaki, H. Antinociceptive interaction between spinal clonidine and lidocaine in the rat formalin test: An isobolographic analysis. Anesth. Analg. 2001, 92, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.P.; Zhao, J.J.; Wang, W.X.; Liu, Y.; Wu, H.F.; Chen, C.; Yu, L.; Gui, J.B. Coapplication of lidocaine and membrane-impermeable lidocaine derivative QX-222 produces divergent effects on evoked and spontaneous nociceptive behaviors in mice. BioMed Res. Int. 2014, 2014, 628729. [Google Scholar] [CrossRef] [PubMed]
- Yamane, A.; Higuchi, H.; Tomoyasu, Y.; Ishii-Maruhama, M.; Maeda, S.; Miyawaki, T. Effect of dexmedetomidine injected into the oral mucosa in combination with lidocaine on local anesthetic potency in humans: A crossover double-blind study. J. Oral Maxillofac Surg. 2015, 73, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.J.; Gao, Y.; Zhao, L.; Jin, D.; Wang, D.; Wang, D.Q. Co-application of lidocaine and QX-572 induces divergent pain behaviours in mice. J. Pharm. Pharmacol. 2015, 67, 1272–1278. [Google Scholar] [CrossRef]
- Bittencourt, A.L.; Takahashi, R.N. Mazindol and lidocaine are antinociceptives in the mouse formalin model: Involvement of dopamine receptor. Eur. J. Pharmacol. 1997, 330, 109–113. [Google Scholar] [CrossRef]
- Singh, V.; Thepra, M.; Kirti, S.; Kumar, P.; Priya, K. Dexmedetomidine as an additive to local anesthesia: A step to development in dentistry. J. Oral Maxillofac. Surg. 2018, 76, 2091.e1–2091.e7. [Google Scholar] [CrossRef]
- Alizargar, J.; Etemadi, S.M.; Kaviani, N.; Wu, S.V.; Jafarzadeh, K.; Ranjbarian, P.; Hsieh, N.C. Injection of lidocaine alone versus lidocaine plus dexmedetomidine in impacted third molar extraction surgery, a double-blind randomized control trial for postoperative pain evaluation. Pain Res. Manag. 2021, 2021, 6623792. [Google Scholar] [CrossRef]
- Takeda, M.; Ikeda, M.; Tanimoto, T.; Lipski, J.; Matsumoto, S. Changes of the excitability of rat trigeminal root ganglion neurons evoked by alpha(2)-adrenoreceptors. Neuroscience 2002, 115, 731–741. [Google Scholar] [CrossRef]
- Gu, X.Y.; Liu, B.L.; Zang, K.K.; Yang, L.; Xu, H.; Pan, H.L.; Zhao, Z.Q.; Zhang, Y.Q. Dexmedetomidine inhibits tetrodotoxin-resistant Nav1.8 sodium channel activity through Gi/o-dependent pathway in rat dorsal root ganglion neurons. Mol. Brain 2015, 8, 15. [Google Scholar] [CrossRef] [PubMed]
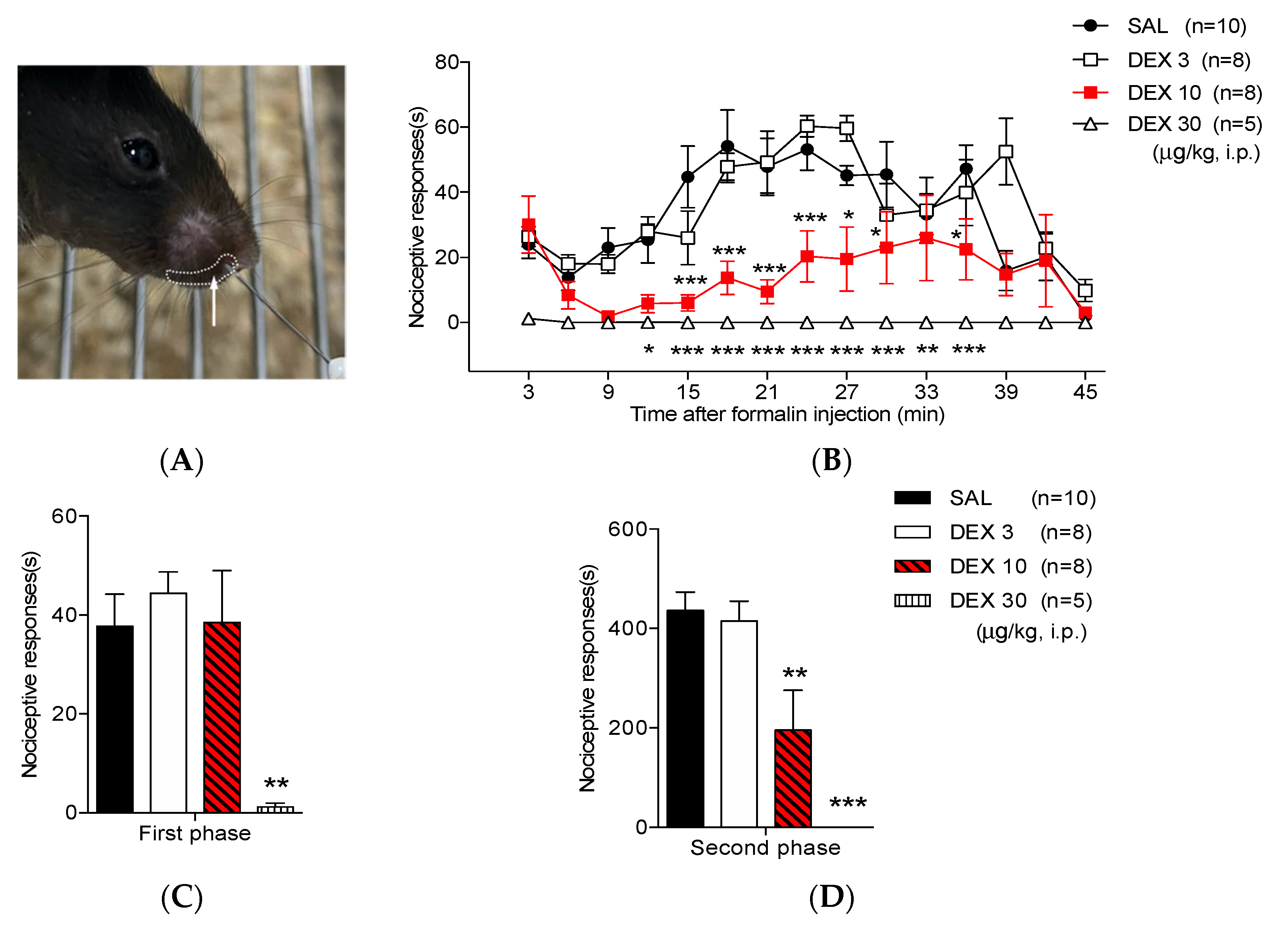

| Treatment | First Phase (s) | p-Value | Second Phase (s) | p-Value |
|---|---|---|---|---|
| SAL | 37.77 ± 6.49 | NA | 436.36 ± 36.81 | NA |
| DEX 3 | 44.43 ± 4.3 | 0.9989 | 415.34 ± 39.51 | 0.9805 |
| DEX 10 | 38.49 ± 10.49 | 0.9176 | 195.20 ± 80.15 ** | 0.0045 |
| DEX 30 | 1.23 ± 0.75 ** | 0.0018 | 0 ± 0 *** | 0.0001 |
| Treatment | First Phase (s) | p-Value | Second Phase (s) | p-Value |
|---|---|---|---|---|
| SAL + SAL | 44.54 ± 4.22 | NA | 486.32 ± 47.44 | NA |
| DEX 3 + SAL | 45.35 ± 4.85 | 0.7067 | 495.05 ± 52.61 | 0.9999 |
| SAL + LIDO | 17.47 ± 2.97 **, ## | ** 0.0064, ## 0.0011 | 477.77 ± 79.28 | 0.9935 |
| DEX 3 + LIDO | 14.62 ± 3.09 **, ### | ** 0.0041, ### 0.0007 | 232.88 ± 26.20 **, ## | ** 0.0043, ## 0.0076 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, J.-H.; Roh, D.-H. Dexmedetomidine Co-Administered with Lidocaine Decreases Nociceptive Responses and Trigeminal Fos Expression without Motor Dysfunction and Hypotension in a Murine Orofacial Formalin Model. Life 2022, 12, 215. https://doi.org/10.3390/life12020215
Yeo J-H, Roh D-H. Dexmedetomidine Co-Administered with Lidocaine Decreases Nociceptive Responses and Trigeminal Fos Expression without Motor Dysfunction and Hypotension in a Murine Orofacial Formalin Model. Life. 2022; 12(2):215. https://doi.org/10.3390/life12020215
Chicago/Turabian StyleYeo, Ji-Hee, and Dae-Hyun Roh. 2022. "Dexmedetomidine Co-Administered with Lidocaine Decreases Nociceptive Responses and Trigeminal Fos Expression without Motor Dysfunction and Hypotension in a Murine Orofacial Formalin Model" Life 12, no. 2: 215. https://doi.org/10.3390/life12020215
APA StyleYeo, J.-H., & Roh, D.-H. (2022). Dexmedetomidine Co-Administered with Lidocaine Decreases Nociceptive Responses and Trigeminal Fos Expression without Motor Dysfunction and Hypotension in a Murine Orofacial Formalin Model. Life, 12(2), 215. https://doi.org/10.3390/life12020215






