Promising Assays for Examining a Putative Role of Ribosomal Heterogeneity in COVID-19 Susceptibility and Severity
Abstract
1. Interaction of SARS-CoV-2 with Ribosome
2. Heterogeneity of Ribosome
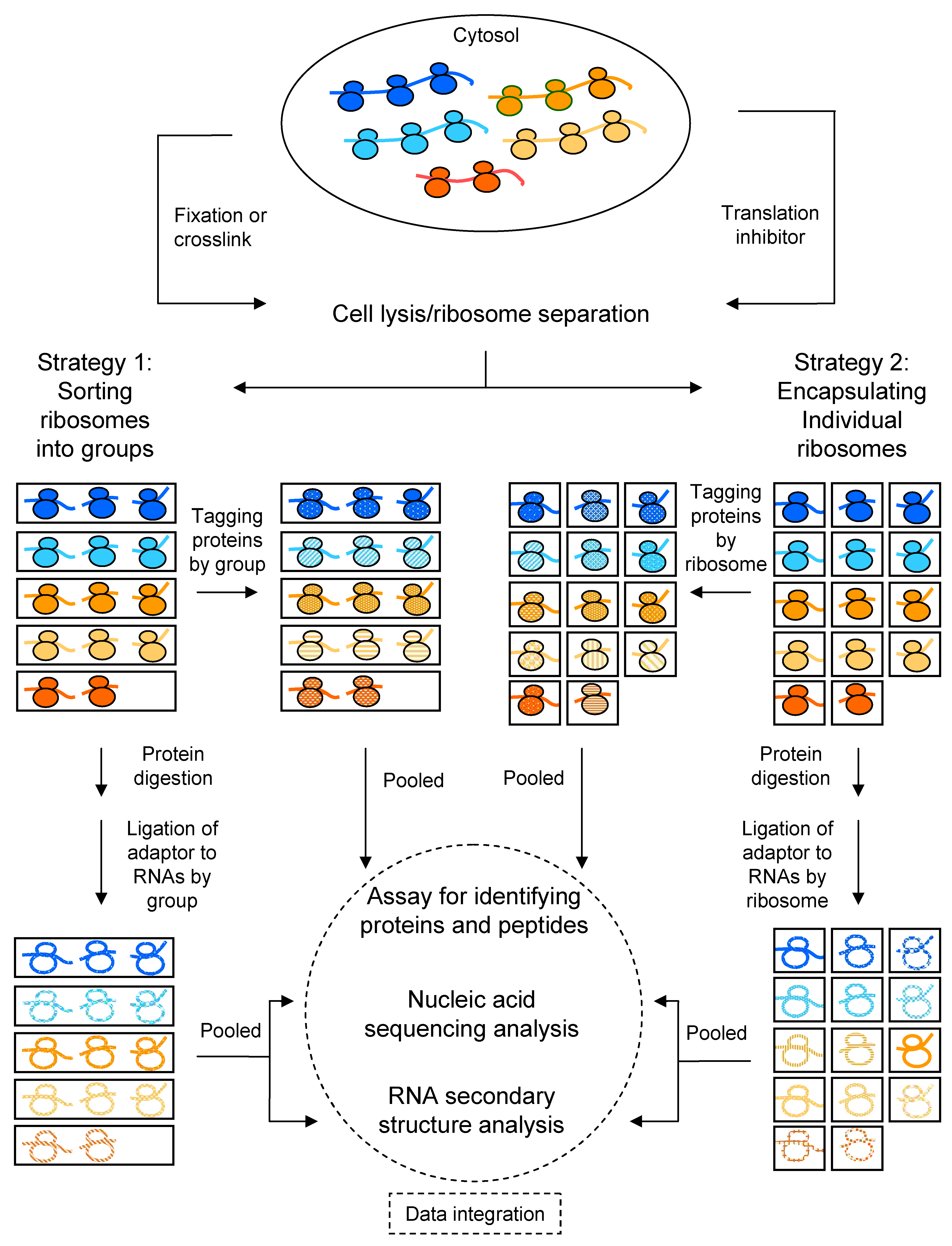
3. Detection of Ribosomal Heterogeneity
3.1. Chemical Probing of Ribosomal Components or rRNA Structures
3.2. Proximity Ligation to Generate rRNA-mRNA Chimeras for Sequencing
3.3. Nanopore Gating of Individual Ribosomes
3.4. Nanopore RNA Sequencing and/or Structural Analyses
3.5. Single-ribosome Mass Spectrometry
3.6. Microfluidic Droplets for Separating Ribosomes or Indexing rRNAs/mRNAs
4. Final Notes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simeoni, M.; Cavinato, T.; Rodriguez, D.; Gatfield, D. I(nsp1)ecting SARS-CoV-2–ribosome interactions. Commun. Biol. 2021, 4, 715. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Wong, J.P.; Damania, B. SARS-CoV-2 dependence on host pathways. Science 2021, 371, 884–885. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.K.; Blanco, M.R.; Bruce, E.A.; Honson, D.D.; Chen, L.M.; Chow, A.; Bhat, P.; Ollikainen, N.; Quinodoz, S.A.; Loney, C.; et al. SARS-CoV-2 disrupts splicing, translation, and protein trafficking to suppress host defenses. Cell 2020, 183, 1325–1339. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Y.; Avi, G.A.; Nachshon, A.; Winkler, R.; Fisher, T.; Rozman, B.; Mizrahi, O.; Lubelsky, Y.; Zuckerman, B.; Slobodin, B.; et al. SARS-CoV-2 uses a multipronged strategy to impede host protein synthesis. Nature 2021, 594, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Lapointea, C.P.; Groselya, R.; Johnsona, A.G.; Wanga, J.; Fernándezc, I.S.; Puglisi, J.D. Dynamic competition between SARS-CoV-2 NSP1 and mRNA on the human ribosome inhibits translation initiation. Proc. Natl. Acad. Sci. USA 2021, 118, e2017715118. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.A.; Belk, J.A.; Qi, Y.; Yasumoto, Y.; Wei, J.; Alfajaro, M.M.; Shi, Q.; Mumbach, M.R.; Limaye, A.; DeWeirdt, P.C.; et al. Discovery and functional interrogation of SARS-CoV-2 RNA-host protein interactions. Cell 2021, 184, 2394–2411. [Google Scholar] [CrossRef]
- Labeau, A.; Lefevre-Utile, A.; Bonnet-Madin, L.; Fery-Simonian, L.; Soumelis, V.; Lotteau, V.; Vidalain, P.-O.; Amara, A.; Meertens, L. Characterization and functional interrogation of 1 SARS-CoV-2 RNA interactome. bioRxiv 2021. [Google Scholar]
- Lee, S.; Lee, Y.-S.; Choi, Y.; Son, A.; Park, Y.; Lee, K.-M.; Kim, J.; Kim, J.-S.; Kim, V.N. The SARS-CoV-2 RNA interactome. Mol. Cell 2021, 81, 2838–2850. [Google Scholar] [CrossRef]
- Schmidt, N.; Lareau, C.A.; Keshishian, H.; Ganskih, S.; Schneider, C.; Hennig, T.; Melanson, R.; Werner, S.; Wei, Y.; Zimmer, M.; et al. The SARS-CoV-2 RNA–protein interactome in infected human cells. Nat. Microbiol. 2021, 6, 339–353. [Google Scholar] [CrossRef]
- Bhatt, P.R.; Scaiola, A.; Loughran, G.; Leibundgut, M.; Kratzel, A.; Meurs, R.; Dreos, R.; O’Connor, K.M.; McMillan, A.; Bode, J.W.; et al. Structural basis of ribosomal frameshifting during translation of the SARS-CoV-2 RNA genome. Science 2021, 372, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Ziv, O.; Price, J.; Shalamova, L.; Kamenova, T.; Goodfellow, I.; Weber, F.; Miska, E.A. The short-and long-range RNA-RNA Interactome of SARS-CoV-2. Mol. Cell 2020, 80, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Cai, Z.; Xiao, X.; Rao, J.; Chen, J.; Hu, N.; Yang, M.; Xing, X.; Wang, Y.; Li, M.; et al. The architecture of the SARS-CoV-2 RNA genome inside virion. Nat. Commun. 2021, 12, 3917. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Penumutchu, S.; Nguyen, K.; Mbonye, U.; Tolbert, B.S.; Karn, J.; Komar, A.A.; Mazumder, B. A structurally conserved RNA element within SARS-CoV-2 ORF1a RNA and S mRNA regulates translation in response to viral S protein-induced signaling in human lung cells. J. Virol. 2021, 96, 1. [Google Scholar] [CrossRef] [PubMed]
- Kamel, W.; Noerenberg, M.; Cerikan, B.; Chen, H.; Jarvelin, A.I.; Kammoun, M.; Lee, J.Y.; Shuai, N.; Garcia-Moreno, M.; Andrejeva, A.; et al. Global analysis of protein-RNA interactions in SARS-CoV-2-infected cells reveals key regulators of infection. Mol. Cell 2021, 81, 2851–2867. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Svetlov, V.; Wolf, Y.I.; Koonin, E.V.; Nudler, E.; Artsimovitch, I. Allosteric activation of SARS-CoV-2 RNA-dependent RNA polymerase by remdesivir triphosphate and other phosphorylated nucleotides. mBio 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.B.; Ghalei, H.; Ward, E.A.; Potts, E.L.; Karbstein, K. Rps26 directs mRNA-specific translation by recognition of Kozak sequence elements. Nat. Struct. Mol. Biol. 2017, 24, 700–707. [Google Scholar] [CrossRef]
- Sample, P.J.; Wang, B.; Reid, D.W.; Presnyak, V.; McFadyen, I.; Morris, D.R.; Seelig, G. Human 5′UTR design and variant effect prediction from a massively parallel translation assay. Nat. Biotechnol. 2019, 37, 803–809. [Google Scholar] [CrossRef]
- Shi, Z.; Fujii, K.; Kovary, K.M.; Genuth, N.R.; Rost, H.L.; Teruel, M.N.; Barna, M. Heterogeneous ribosomes preferentially translate distinct subpools of mRNAs genome-wide. Mol. Cell 2017, 67, 71–83. [Google Scholar] [CrossRef]
- Simsek, D.; Tiu, G.C.; Flynn, R.A.; Byeon, G.W.; Leppek, K.; Xu, A.F.; Chang, H.Y.; Barna, M. The mammalian ribo-interactome reveals ribosome functional diversity and heterogeneity. Cell 2017, 169, 1051–1065. [Google Scholar] [CrossRef]
- Leppek, K.; Byeon, G.W.; Fujii, K.; Barna, M. VELCRO-IP RNA-seq reveals ribosome expansion segment function in translation genome-wide. Cell Rep. 2021, 34, 108629. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.D.; Pagano, J.F.B.; Girard, G.; Ensink, W.A.; van Olst, M.; van Leeuwen, S.; Nehrdich, U.; Spaink, H.P.; Rauwerda, H.; Jonker, M.J.; et al. Expression of distinct maternal and somatic 5.8S, 18S, and 28S rRNA types during zebrafish development. RNA 2017, 28, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, J. Ribosome heterogeneity in stem cells and development. J. Cell Biol. 2020, 219, e202001108. [Google Scholar] [CrossRef] [PubMed]
- Gay, D.M.; Lund, A.H.; Jansson, M.D. Translational control through ribosome heterogeneity and functional specialization. Trends Biochem. Sci. 2022, 47, 66–81. [Google Scholar] [CrossRef]
- Aw, J.G.A.; Shen, Y.; Wilm, A.; Sun, M.; Lim, X.N.; Boon, K.-L.; Tapsin, S.; Chan, Y.-S.; Tan, C.-P.; Sim, A.Y.L.; et al. In Vivo mapping of eukaryotic RNA interactomes reveals principles of higher-order organization and regulation. Mol. Cell 2016, 62, 603–617. [Google Scholar] [CrossRef]
- Ziv, O.; Gabryelska, M.M.; Lun, A.T.L.; Gebert, L.F.R.; Sheu-Gruttadauria, J.; Meredith, L.W.; Liu, Z.-Y.; Kwok, C.K.; Qin, C.-F.; MacRae, I.J.; et al. COMRADES determines in vivo RNA structures and interactions. Nat. Methods 2018, 15, 785–788. [Google Scholar] [CrossRef]
- Christy, T.W.; Giannetti, C.A.; Laederach, A.; Weeks, K.M. Identifying proximal RNA interactions from cDNA-encoded crosslinks with ShapeJumper. PLoS Comput. Biol. 2021, 17, e1009632. [Google Scholar] [CrossRef]
- Sharma, E.; Sterne-Weiler, T.; O’Hanlon, D.; Blencowe, B.J. Global mapping of human RNA-RNA interactions. Mol. Cell 2016, 62, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Vigilante, A.; Darbo, E.; Zirra, A.; Militti, C.; D’Ambrogio, A.; Luscombe, N.M.; Ule, J. hiCLIP reveals the in vivo atlas of mRNA secondary structures recognized by Staufen 1. Nature 2015, 519, 491–494. [Google Scholar] [CrossRef]
- Tüting, C.; Iacobucci, C.; Ihling, C.H.; Kastritis1, P.L.; Sinz, A. Structural analysis of 70S ribosomes by cross-linking/mass spectrometry reveals conformational plasticity. Sci. Rep. 2020, 10, 12618. [Google Scholar] [CrossRef]
- Urdaneta, E.C.; Vieira-Vieira, C.H.; Hick, T.; Wessels, H.-H.; Figini, D.; Moschall, R.; Medenbach, J.; Ohler, U.; Granneman, S.; Selbach, M.; et al. Purification of cross-linked RNA-protein complexes by phenol-toluol extraction. Nat. Commun. 2019, 10, 990. [Google Scholar] [CrossRef]
- Velema, W.A.; Park, H.S.; Kadina, A.; Orbai, L.; Kool, E.T. Trapping transient RNA complexes by chemically reversible acylation. Angew. Chem. Int. Ed. 2020, 59, 22017–22022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, K.; Bai, J.; Velema, W.A.; Yu, C.; van Damme, R.; Lee, W.H.; Corpuz, M.L.; Chen, J.-F.; Lu, Z. Optimized photochemistry enables efficient analysis of dynamic RNA structuromes and interactomes in genetic and infectious diseases. Nat. Commun. 2021, 12, 2344. [Google Scholar] [CrossRef] [PubMed]
- Kudla, G.; Granneman, S.; Hahn, D.; Beggs, J.D.; Tollervey, D. Cross-linking, ligation, and sequencing of hybrids reveals RNA–RNA interactions in yeast. Proc. Natl. Acad. Sci. USA 2011, 108, 10010–10015. [Google Scholar] [CrossRef] [PubMed]
- Trendel, J.; Schwarz, T.; Horos, R.; Prakash, A.; Bateman, A.; Hentze, M.W.; Krijgsveld, J. The human RNA-binding proteome and its dynamics during translational arrest. Cell 2019, 176, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Cao, C.; Ji, L.; Ye, R.; Wang, D.; Xia, C.; Wang, S.; Du, Z.; Hu, N.; Yu, X.; et al. RIC-seq for global in situ profiling of RNA–RNA spatial interactions. Nature 2020, 582, 432–437. [Google Scholar] [CrossRef]
- Slavin, M.; Zamel, J.; Zohar, K.; Eliyahu, T.; Braitbard, M.; Brielle, E.; Baraz, L.; Stolovich-Rain, M.; Friedman, A.; Wolf, D.G.; et al. Targeted in situ cross-linking mass spectrometry and integrative modeling reveal the architectures of three proteins from SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2021, 118, e2103554118. [Google Scholar] [CrossRef]
- Kudla, G.; Wan, Y.; Helwak, A. RNA Conformation Capture by Proximity Ligation. Annu. Rev. Genom. Hum. Genet. 2020, 21, 81–100. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, H.Y. The RNA base-pairing problem and base-pairing solutions. Cold Spring Harb. Perspect. Biol. 2018, 10, a034926. [Google Scholar] [CrossRef]
- Qian, X.; Zhao, J.; Yeung, P.Y.; Zhang, Q.C.; Kwok, C.K. Revealing lncRNA structures and interactions by sequencing-based approaches. Trends Biochem. Sci. 2019, 44, 33–52. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Zaleta-Rivera, K.; Huang, X.; Dai, X.; Zhong, S. RNA, action through interactions. Trends Genet. 2018, 34, 867–882. [Google Scholar] [CrossRef]
- Wheeler, E.C.; Van Nostrand, E.L.; Yeo, G.W. Advances and challenges in the detection of transcriptome-wide protein–RNA interactions. WIREs RNA 2018, 9, e1436. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D., III; Assmann, S.M.; Bevilacqua, P.C. Probing RNA structure in vivo. Curr. Opin. Struct. Biol. 2019, 59, 151–158. [Google Scholar] [CrossRef]
- Granneman, S.; Kudla, G.; Petfalski, E.; Tollervey, D. Identification of protein binding sites on U3 snoRNA and pre-rRNA by UV cross-linking and high-throughput analysis of cDNAs. Proc. Natl. Acad. Sci. USA 2009, 106, 9613–9618. [Google Scholar] [CrossRef]
- Ramani, V.; Qiu, R.; Shendure, J. High-throughput determination of RNA structure by proximity ligation. Nat. Biotechnol. 2015, 33, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Panopoulos, P.; Mauro, V.P. Antisense masking reveals contributions of mRNA-rRNA base pairing to translation of Gtx and FGF2 mRNAs. J. Biol. Chem. 2008, 283, 33087–33093. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Jackson, N.L.; Shcherbakov, O.D.; Choi, H.; Blume, S.W. The human IGF1R IRES likely operates through a Shine-Dalgarno-like interaction with the G961 loop (E-site) of the 18S rRNA and is kinetically modulated by a naturally polymorphic polyU loop. J. Cell Biochem. 2010, 110, 531–544. [Google Scholar]
- Martin, F.; Menetret, J.-F.; Simonetti, A.; Myasnikov, A.G.; Vicens, Q.; Prongidi-Fix, L.; Natchiar, S.K.; Klaholz, B.P.; Eriani, G. Ribosomal 18S rRNA base pairs with mRNA during eukaryotic translation initiation. Nat. Commun. 2016, 7, 12622. [Google Scholar] [CrossRef]
- Matsuda, D.; Mauro, V.P. Base pairing between hepatitis C virus RNA and 18S rRNA is required for IRES-dependent translation initiation in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 15385–15389. [Google Scholar] [CrossRef]
- Leppek, K.; Fujii, K.; Quade, N.; Susanto, T.T.; Boehringer, D.; Lenarcic, T.; Xue, S.; Genuth, N.R.; Ban, N.; Barna, M. Gene- and species-specific hox mRNA translation by ribosome expansion segments. Mol. Cell 2020, 80, 980–995. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Shin, B.-S.; Kim, J.-R.; Dever, T.E.; Puglisi, J.D.; Fernández, I.S. Structural basis for the transition from translation initiation to elongation by an 80S-eIF5B complex. Nat. Commun. 2020, 11, 5003. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Tidu, A.; Eriani, G.; Martin, F. Secondary structure of the SARS-CoV-2 5′-UTR. RNA Biol. 2021, 18, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Schubert, K.; Karousis, E.D.; Jomaa, A.; Scaiola, A.; Echeverria, B.; Gurzeler, L.A.; Leibundgut, M.; Thie, V.; Mühlemann, O.; Ban, N. SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to inhibit translation. Nat. Struct. Mol. Biol. 2020, 27, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.S.; Balasubramaniam, A.; Sallee, F.R.; Parker, S.L. The expansion segments of 28S Ribosomal RNA extensively match human messenger RNAs. Front. Genet. 2018, 9, 66. [Google Scholar] [CrossRef]
- Bastide, A.; David, A. Interaction of rRNA with mRNA and tRNA in translating mammalian ribosome: Functional implications in health and disease. Biomolecules 2018, 8, 100. [Google Scholar] [CrossRef]
- Armachea, J.-P.; Jarascha, A.; Angera, A.M.; Villab, E.; Beckera, T.; Bhushana, S.; Jossinetc, F.; Habeckd, M.; Dindara, G.; Franckenberga, S.; et al. Cryo-EM structure and rRNA model of a translating eukaryotic 80S ribosome at 5.5-Å resolution. Proc. Natl. Acad. Sci. USA 2010, 107, 19748–19753. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, L.; Melander, Y.; Nygard, O. Probing the structure of mouse Ehrlich ascites cell 5.8S, 18S and 28S ribosomal RNA in situ. Nucleic Acids Res. 1994, 22, 1374–1382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anger, A.M.; Armache, J.-P.; Berninghausen, O.; Habeck, M.; Subklewe, M.; Wilson, D.N.; Beckmann, R. Structures of the human and Drosophila 80S ribosome. Nature 2013, 497, 80–85. [Google Scholar] [CrossRef]
- Holmberg, L.; Nygard, O. Mapping of nuclease-sensitive sites in native reticulocyte ribosomes: An analysis of the accessibility of ribosomal RNA to enzymatic cleavage. Eur. J. Biochem. 1997, 247, 160–168. [Google Scholar] [CrossRef]
- Culviner, P.H.; Nocedal, I.; Fortune, S.M.; Laub, M.T. Global analysis of the specificities and targets of endoribonucleases from Escherichia coli toxin-antitoxin systems. Mbio 2021, 12, 1. [Google Scholar] [CrossRef]
- Han, Y.; Lee, E.-J. Substrate specificity of bacterial endoribonuclease toxins. BMB Rep. 2020, 53, 611–621. [Google Scholar] [CrossRef]
- Masuda, H.; Inouye, M. Toxins of prokaryotic toxin-antitoxin systems with sequence-specific endoribonuclease activity. Toxins 2017, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Zavialov, A.V.; Pavlov, M.Y.; Elf, J.; Gerdes, K.; Ehrenberg, M. the bacterial toxin rele displays codon-specific cleavage of mRNAs in the ribosomal a site. Cell 2003, 112, 131–140. [Google Scholar] [CrossRef]
- Querido, J.B.; Sokabe, M.; Kraatz, S.; Gordiyenko, Y.; Skehel, J.M.; Fraser, C.S.; Ramakrishnan, V. Structure of a human 48S translational initiation complex. Science 2020, 369, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- MacLean, N. Ribosome numbers in a fission yeast. Nature 1965, 207, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Zelena, J. Ribosomes in myelinated axons of dorsal root ganglia. Z. Zellforsch. Microsk. Anat. 1972, 124, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Turowski, T.W.; Tollervey, D. Cotranscriptional events in eukaryotic ribosome synthesis. WIREs RNA 2015, 6, 129–139. [Google Scholar] [CrossRef]
- Britten, R.J.; Roberts, R.B. High-resolution density gradient sedimentation analysis. Science 1960, 131, 32–33. [Google Scholar] [CrossRef]
- Warner, J.R.; Knopf, P.M.; Rich, A. A multiple ribosomal structure in protein synthesis. Proc. Natl. Acad. Sci. 1963, 49, 122–129. [Google Scholar] [CrossRef]
- Heiman, M.; Kulicke, R.; Fenster, R.J.; Greengard, P.; Heintz, N. Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP). Nat. Protocols 2014, 9, 1282–1291. [Google Scholar] [CrossRef]
- Inada, T.; Winstall, E.; Tarun, S.Z.; Yates, J.R.; Schieltz, D.; Sachs, A.B. One-step affinity purification of the yeast ribosome and its associated proteins and mRNAs. RNA 2002, 8, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Larance, M.; Harney, D.J.; Sundaramoorthy, R.; Ly, T.; Owen-Hughes, T.; Lamond, A.I. Efficient analysis of mammalian polysomes in cells and tissues using Ribo Mega-SEC. Elife 2018, 7, e36530. [Google Scholar] [CrossRef] [PubMed]
- Rudenkoa, M.I.; Holmesc, M.R.; Ermolenkob, D.N.; Luntc, E.J.; Gerhardtb, S.; Nollerb, H.F.; Deamera, D.W.; Hawkinsc, A.; Schmidt, H. Controlled gating and electrical detection of single 50S ribosomal subunits through a solid-state nanopore in a microfluidic chip. Biosens. Bioelectron. 2011, 29, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Stott, M.A.; Harrington, M.; Li, Y.; Sampad, M.J.N.; Lancaster, L.; Yuzvinsky, T.D.; Noller, H.F.; Hawkins, A.R.; Schmidt, H. On demand delivery and analysis of single molecules on a programmable nanopore-optofluidic device. Nat. Commun. 2019, 10, 3712. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, M.; Leach, A.R.; Hopes, T.; Aspden, J.L.; Actis, P. Ribosome fingerprinting with a solid-state nanopore. ACS Sens. 2020, 5, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Garalde, D.R.; Snell, E.A.; Jachimowicz, D.; Sipos, B.; Lloyd, J.H.; Bruce, M.; Pantic, N.; Admassu, T.; James, P.; Warland, A.; et al. Highly parallel direct RN A sequencing on an array of nanopores. Nat. Methods 2018, 15, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Mathewson, N.J.; Manage, S.A.H.; Burrows, C.J. Nanopore dwell time analysis permits sequencing and conformational assignment of pseudouridine in SARS-CoV-2. ACS Cent. Sci. 2021, 7, 1707–1717. [Google Scholar] [CrossRef]
- Smith, A.M.; Jain, M.; Mulroney, L.; Garalde, D.R.; Akeson, M. Reading canonical and modified nucleobases in 16S ribosomal RNA using nanopore native RNA sequencing. PLoS ONE 2019, 14, e0216709. [Google Scholar] [CrossRef]
- Jain, M.; Olsen, H.E.; Akeson, M.; Abu-Shumays, R. Adaptation of Human Ribosomal RNA for Nanopore Sequencing of Canonical and Modified Nucleotides. In RNA Modifications: Methods and Protocols, Methods in Molecular Biology; McMahon, M., Ed.; Springer Science+Business Media, LLC: New York, NY, USA, 2021; Volume 2298, pp. 53–74. [Google Scholar]
- Aw, J.G.A.; Lim, S.W.; Wang, J.X.; Lambert, F.R.P.; Tan, W.T.; Shen, Y.; Zhang, Y.; Kaewsapsak, P.; Li, C.; Ng, S.B.; et al. Determination of isoform-specific RNA structure with nanopore long reads. Nat. Biotechnol. 2021, 39, 336–346. [Google Scholar] [CrossRef]
- Henley, R.Y.; Ashcroft, B.A.; Farrell, I.; Cooperman, B.S.; Lindsay, S.M.; Wanunu, M. Electrophoretic deformation of individual transfer rna molecules reveals their identity. Nano Lett. 2016, 16, 138–144. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Zhao, C.; Tian, K.; Shi, R.; Du, X.; Burcke, A.J.; Wang, J.; Chen, S.-J.; Gu, L.-Q. Nanopore electric snapshots of an RNA tertiary folding pathway. Nat. Commun. 2017, 8, 1458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, X.; Zhang, S.; Liu, Y.; Wang, S.; Fan, P.; Du, X.; Yan, S.; Zhang, P.; Chen, H.-Y.; et al. Structural-profiling of low molecular weight RNAs by nanopore trapping/translocation using Mycobacterium smegmatis porin A. Nat. Commun. 2021, 12, 3368. [Google Scholar] [CrossRef]
- Rostom, A.A.; Fucini, P.; Benjamin, D.R.; Juenemann, R.; Nierhaus, K.H.; Hartl, F.U.; Dobson, C.M.; Robinson, C.V. Detection and selective dissociation of intact ribosomes in a mass spectrometer. Proc. Natl. Acad. Sci. USA 2000, 97, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, M.; Fort, K.L.; Boll, D.; Reinhardt-Szyba, M.; Routh, A.; Makarov, A.; Heck, A.J.R. High-fidelity mass analysis unveils heterogeneity in intact ribosomal particles. Nat. Methods 2017, 14, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-H.; Tamara, S.; Heck, A.J.R. Single-particle mass analysis of intact ribosomes by mass photometry and Orbitrap-based charge detection mass spectrometry. iScience 2021, 24, 103211. [Google Scholar] [CrossRef]
- Wegener, M.; Ennen, I.; Walhorn, V.; Anselmetti, D.; Hütten, A.; Dietz, K.-J. Magnetic tracking of protein synthesis in microfluidic environments—Challenges and perspectives. Nanomaterials 2019, 9, 0585. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Kim, S.-H.; Horsfall, L.; Abbaspourrad, A.; Rosser, S.J.; Cooper, J.; Weitz, D.A. Protein expression, aggregation, and triggered release from polymersomes as artificial cell-like structures. Angew. Chem. Int. Ed. 2012, 51, 6416–6420. [Google Scholar] [CrossRef] [PubMed]
- Datlinger, P.; Rendeiro, A.F.; Boenke, T.; Senekowitsch, M.; Krausgruber, T.; Barreca, D.; Bock, C. Ultra-high-throughput single-cell RNA sequencing and perturbation screening with combinatorial fluidic indexing. Nat. Methods 2021, 18, 635–642. [Google Scholar] [CrossRef]
- Parker, C.G.; Pratt, M.R. Click chemistry in proteomic investigations. Cell 2020, 180, 605–632. [Google Scholar] [CrossRef]
| Technique | Chemical Probe | Irradiation | Probing Structure a | Ref. |
|---|---|---|---|---|
| SPLASH (Sequencing of psoralen crosslinked, ligated, and selected hybrids) | Psoralen-PEG3-Biotin | UV-A (365 nm) | rRNA-rRNA, mRNA-rRNA, snRNA-rRNA, snoRNA-rRNA, and mRNA-mRNA | [25] |
| COMRADES (Cross-linking of matched RNAs and deep sequencing) | Psoralen-triethylene glycol azide | UV-A (365 nm) | rRNA-rRNA and interaction of viral RNA with cellular RNAs | [26] |
| SHAPE-JuMP (Selective 2′-hydroxyl acylation, primer extension and juxtaposed merged pairs) | Trans bis-isatoic anhydride | RNA-RNA | [27] | |
| LIGR-seq (Ligation of interacting RNA and high-throughput Sequencing) | 4′-aminomethyltrioxalen (AMT) | UV-A (365 nm) | snRNA-snRNA, snoRNA-mRNA, and rRNA-rRNA | [28] |
| hiCLIP (RNA hybrid, individual-nucleotide resolution, UV crosslinking and immunoprecipitation | Formaldehyde | UV-C (254 nm) | Protein-protein and RNA-protein RNA-protein | [29] |
| XL-MS (Crosslinking/ mass spectrometry) | disuccinimidyl diacetic urea | Protein-protein | [30] | |
| PTex (Phenol-Toluol extraction) crosslinked RNA-protein | With/without 2-iminothiolane | UV-C (254 nm) | RNA-protein | [31] |
| Chemically Reversible Acylation | bis-nicotinic azide reversible interaction | RNA-RNA | [32] | |
| PARIS 2 (Psoralen analysis of RNA interactions and structures, second version) | amotosalen or 4′-aminomethyl trioxalen | UV-A (365 nm) | rRNA-rRNA, mRNA-rRNA, and snoRNA-rRNA | [33] |
| CLASH (Crosslinking, ligation, and sequencing of hybrids) | UV-C (254 nm) | snoRNA-rRNA and RNA-protein | [34] | |
| XRNAX (Protein-Crosslinked RNA Extraction) | UV-C (254 nm) | RNA-protein | [35] | |
| RIC-seq (RNA in situ conformational sequencing) | Formaldehyde | rRNA-rRNA, snoRNA-rRNA, rRNA-mRNA, and snoRNA-mRNA | [36] | |
| In situ CLMS (In situ cross-linking and mass spectrometry) | Formaldehyde Disuccinimidyl suberate | Protein-protein Protein-protein | [37] |
| Method | Crosslink (for Purification or Enrichment) | Fragmentation (for Purification or Enrichment) | Ligation (for Purification or Enrichment) | Library Preparation and Sequencing | Cautions a | Ref. |
|---|---|---|---|---|---|---|
| SPLASH | Psoralen-PEG3-Biotin/UV-A (Trizol RNA extraction) | MgCl2, pH 8.3, 95 °C (urea gel to obtain 90–110 nucleotides [nts] followed by streptavidin bead purification) | T4 PNKinase followed by T4 RNA ligase I (Trizol) | Crosslink-reversal by UV-C, 3′adaptor ligation, urea gel to obtain 110–130 nts, cDNA synthesis and circularization, PCR (200–300 bp), and next-gen Seq | Low cell permeability of biopsoralen; UV-C damage to RNAs | [25] |
| CLASH | UV-C (IgG bead RNAs-protein complex purification after cell lysis) | RNase A and T1 single-stranded endo-RNase (Nickel bead to bind His-tag snoRNP) | On-bead 3′-phosphate removal, followed by 3′ and 5′ adaptor ligation by T4 RNA ligase (gel isolation of the RNA-protein complex) | Proteinase K digestion, RNA extraction, cDNA synthesis, PCR (>60 bp), TA cloning, next-gen Seq | Detection of interaction for a specific protein | [34,44] |
| RPL (RNA proximity ligation) | None | Endogenous RNase for yeast cell; RNase A and T1 for human cell | T4 PNKinase followed by T4 RNA ligase I (Trizol) | Modified Illumina TruSeq RNA kit (RNA fragmentation, cDNA synthesis, and PCR) and pair-end 80/101-bp reads in a next-gen Seq | Low abundance of mRNAs | [45] |
| LIGR-seq | AMT/UV-A (Trizol/DNase I treatment) | S1 endonuclease after rRNA depletion (phenol-chloroform extraction) | circRNA ligase (3′-5′ exo-RNase R digestion followed by phenol and chloroform extraction) | Crosslink-reversal by UV-C, precipitation of RNAs, modified Clontech SMARTER library prep, gel isolation of >200 bp products for next-gen Seq | Reduction of rRNA due to depletion step | [28] |
| COMRADES | Psoralen-triethylene glycol azide/UV-A (Qiagen RNeasy lysis/purification, followed by on-bead isolation with antisense probes) | RNase III db-stranded endo-RNase (Zymo RNA Concentrator, chemical linking of biotin-alkyne to azide on crosslinked RNAs and on-bead isolation) | RNA ligase 1 (Zymo RNA Clean and Concentrator) | Crosslink-reversal by UV-C, 5′- and 3′-adaptors ligation, cDNA synthesis, PCR, and on-gel size selection for pair-end 150-bp reads in a next-gen Seq | Detection of interaction for a specific RNA | [12] |
| RIC-seq | Formaldehyde (quenched, washed, and cell permeabilization) | In-cell micrococcal endo-exonuclease digestion (cell wash, 3′dephosphorylation of fragmented RNAs, ligation of pCp-biotin, removal of 3′phosphate of pCp-ligated RNAs, cell wash, 5′phosphorylation, and cell wash) | In-cell T4 RNA ligase ligation (cell wash and lysis by proteinase K, Trizol extraction, DNase I treated, fragmentation of RNA in MgCl2 at 94 °C, on-bead enrichment of biotin-labeled RNA chimeras, and phenol and chloroform extraction) | Strand-specific cDNA synthesis (hexamers to generate the first strand, RNase H-truncated RNA strand as primer to yield a dUTP-containing second strand, 3′adenylation, ligation of 3′ and 5′adaptors, and digestion of the dUTP strand), PCR and on-gel isolation of 200–450 bp products for next-gen Seq with pair-end reads of about 260 bp | Enriched interactions: mRNA-mRNA > ncRNA-ncRNA > mRNA-ncRNA > pseudogene- pseudogene (ncRNA includes lncRNA, snoRNA, and eRNA) | [36] |
| PARIS2 | Water-soluble amotosalen/UV-A (cell or tissue wash, cell lysis with Guanidine Isothiocyanate followed by Proteinase K and DNase digestions with phenol/isopropanol extraction after each digestion) | ShortCut RNase III double-stranded endoribonuclease (RNA precipitation and 2D urea-gel to isolate RNA fragments of 50 nts or higher) | T4 RNA ligase 1 (RNA precipitation) | Crosslink-reversal with little RNA damage (UV-C plus acridine orange singlet quencher), 3′adaptor ligation, SuperScript-IV to generate cDNAs, RNase H/A/T1 to remove RNAs, CircLigase™ II to circularize cDNAs, PCR, and on-gel isolation of 175 bp or higher for next-gen Seq | Low efficiency of proximity ligation by T4 RNA ligase (≈10% gapped or chimeric reads); bias toward uridine crosslink by AMT or amotosalen | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiao, Y.-H. Promising Assays for Examining a Putative Role of Ribosomal Heterogeneity in COVID-19 Susceptibility and Severity. Life 2022, 12, 203. https://doi.org/10.3390/life12020203
Shiao Y-H. Promising Assays for Examining a Putative Role of Ribosomal Heterogeneity in COVID-19 Susceptibility and Severity. Life. 2022; 12(2):203. https://doi.org/10.3390/life12020203
Chicago/Turabian StyleShiao, Yih-Horng. 2022. "Promising Assays for Examining a Putative Role of Ribosomal Heterogeneity in COVID-19 Susceptibility and Severity" Life 12, no. 2: 203. https://doi.org/10.3390/life12020203
APA StyleShiao, Y.-H. (2022). Promising Assays for Examining a Putative Role of Ribosomal Heterogeneity in COVID-19 Susceptibility and Severity. Life, 12(2), 203. https://doi.org/10.3390/life12020203






