Mitragyna Species as Pharmacological Agents: From Abuse to Promising Pharmaceutical Products
Abstract
:1. Introduction
2. Research Methodology
3. Historical Background and Botanical Origins
4. Habitat and Cultivation of Mitragyna Plants
5. The Genus Mitragyna in Traditional Medicine Uses
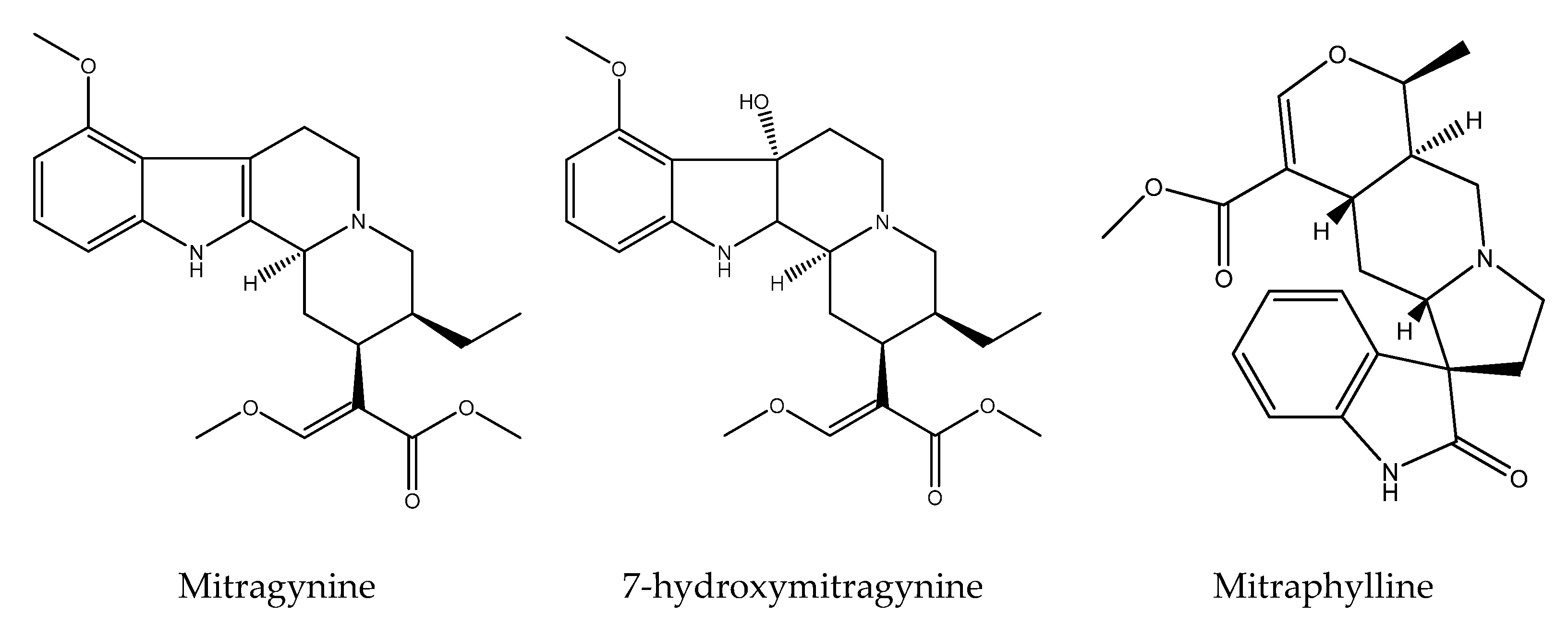
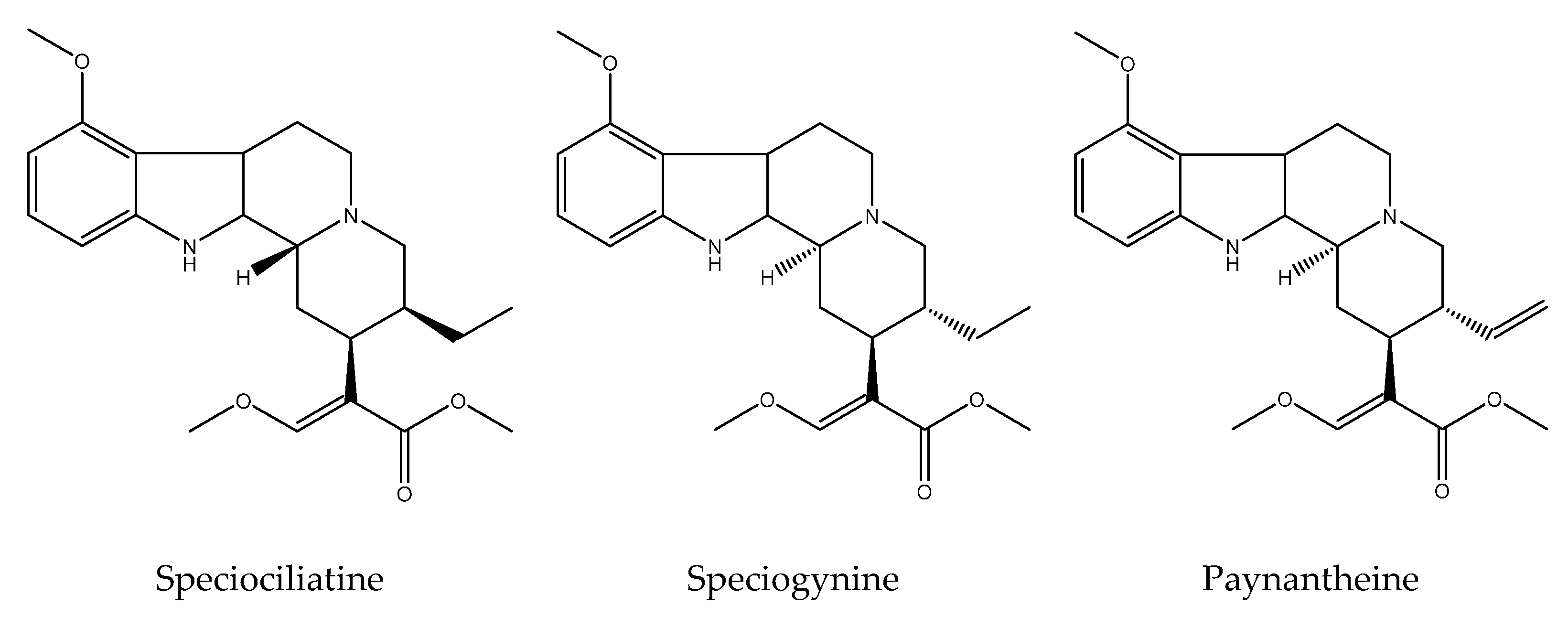
6. Phytochemistry of the Genus Mitragyna
7. Pharmacology and Toxicity of the Genus Mitragyna
7.1. Pharmacological Aspect of M. speciosa
7.2. Toxicological Aspect of M. speciosa
8. The Abuse and Addiction of the Mitragyna Species
8.1. Abuse and Adverse Effects of M. speciosa
8.2. Addiction Effects of M. speciosa
8.3. Addiction Withdrawal Symptoms of Opioid Dependence
9. Legal Issues of Mitragyna Plants
10. The Prospective Potential of Mitragyna Species as a Pharmaceutical Product
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Löfstrand, S.D.; Krüger, Å.; Razafimandimbison, S.G.; Bremer, B. Phylogeny and generic delimitations in the sister tribes Hymenodictyeae and Naucleeae (Rubiaceae). Syst. Bot. 2014, 39, 304–315. [Google Scholar] [CrossRef]
- Beckett, A.H.; Shellard, E.J.; Tackie, A.N. The Mitragyna species of Ghana. J. Pharm. Pharmacol. 1963, 15, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Shellard, E.J.; Houghton, P.J.; Resha, M. The mitragyna species of Asia. Part XXXI. The alkaloids of Mitragyna speciosa Korth from Thailand. Planta Med. 1978, 34, 26–36. [Google Scholar] [CrossRef]
- Huysmans, S.; Robbrecht, E.; Smets, E. Are the genera Hallea and Mitragyna (Rubiaceae-Coptosapelteae) pollen morphologically distinct? Blumea 1994, 39, 321–340. [Google Scholar]
- Singh, D.; Narayanan, S.; Vicknasingam, B. Traditional and non-traditional uses of Mitragynine (Kratom): A survey of the literature. Brain Res. Bull. 2016, 126, 41–46. [Google Scholar] [CrossRef]
- Shaik Mossadeq, W.M.; Sulaiman, M.R.; Tengku Mohamad, T.A.; Chiong, H.S.; Zakaria, Z.A.; Jabit, M.L.; Baharuldin, M.T.H.; Israf, D.A. Anti-inflammatory and antinociceptive effects of Mitragyna speciosa Korth methanolic extract. Med. Princ. Pract. 2009, 18, 378–384. [Google Scholar] [CrossRef]
- Tohar, N.; Shilpi, J.A.; Sivasothy, Y.; Ahmad, S.; Awang, K. Chemical constituents and nitric oxide inhibitory activity of supercritical carbon dioxide extracts from Mitragyna speciosa leaves. Arab. J. Chem. 2019, 12, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Kumarnsit, E.; Keawpradub, N.; Nuankaew, W. Acute and long-term effects of alkaloid extract of Mitragyna speciosa on food and water intake and body weight in rats. Fitoterapia 2006, 77, 339–345. [Google Scholar] [CrossRef]
- Watanabe, K.; Yano, S.; Horie, S.; Yamamoto, L.T. Inhibitory effect of mitragynine, an alkaloid with analgesic effect from Thai medicinal plant Mitragyna speciosa, on electrically stimulated contraction of isolated guinea-pig ileum through the opioid receptor. Life Sci. 1997, 60, 933–942. [Google Scholar] [CrossRef]
- Reanmongkol, W.; Keawpradub, N.; Sawangjaroen, K. Effects of the extracts from Mitragyna speciosa Korth. leaves on analgesic and behavioral activities in experimental animals. Songklanakarin J. Sci. Technol. 2007, 29, 39–48. [Google Scholar]
- Carpenter, J.M.; Criddle, C.A.; Craig, H.K.; Ali, Z.; Zhang, Z.; Khan, I.A.; Sufka, K.J. Comparative effects of Mitragyna speciosa extract, mitragynine, and opioid agonists on thermal nociception in rats. Fitoterapia 2016, 109, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Kruegel, A.C.; Uprety, R.; Grinnell, S.G.; Langreck, C.; Pekarskaya, E.A.; Le Rouzic, V.; Ansonoft, M.; Gassaway, M.M.; Pintar, J.E.; Pasternak, G.W.; et al. 7-Hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent. Sci. 2019, 5, 992–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idayu, N.F.; Hidayat, M.T.; Moklas, M.A.M.; Sharida, F.; Raudzah, A.R.N.; Shamima, A.R.; Apryani, E. Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomedicine 2011, 18, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Moklas, M.A.M.; Suliman, N.A.; Taib, C.N.M.; Baharuldin, M.T.H. Sedative, cognitive impairment and anxiolytic effects of acute Mitragyna speciosa in rodents. J. US China Med. Sci. 2013, 10, 37–44. [Google Scholar]
- Obeng, S.; Kamble, S.H.; Reeves, M.E.; Restrepo, L.F.; Patel, A.; Behnke, M.; Chear, N.J.-Y.; Ramanathan, S.; Sharma, A.; Leon, F.; et al. Investigation of the adrenergic and opioid binding affinities, metabolic stability, plasma protein binding properties, and functional effects of selected indole-based kratom alkaloids. J. Med. Chem. 2020, 63, 433–439. [Google Scholar] [CrossRef]
- Johnson, L.E.; Balyan, L.; Magdalany, A.; Saeed, F.; Salinas, R.; Wallace, S.; Veltri, C.A.; Swogger, M.T.; Walsh, Z.; Grundmann, O. The potential for kratom as an antidepressant and antipsychotic. Yale J. Biol. Med. 2020, 93, 283–289. [Google Scholar]
- Vijeepallam, K.; Pandy, V.; Murugan, D.D.; Naidu, M. Methanolic extract of Mitragyna speciosa Korth leaf inhibits ethanol seeking behaviour in mice: Involvement of antidopaminergic mechanism. Metab. Brain Dis. 2019, 34, 1713–1722. [Google Scholar] [CrossRef]
- Apryani, E.; Hidayat, M.T.; Moklas, M.A.M.; Fakurazi, S.; Idayu, N.F. Effects of mitragynine from Mitragyna speciosa Korth leaves on working memory. J. Ethnopharmacol. 2010, 129, 357–360. [Google Scholar] [CrossRef]
- Chittrakarn, S.; Sawangjaroen, K.; Prasettho, S.; Janchawee, B.; Keawpradub, N. Inhibitory effects of kratom leaf extract (Mitragyna speciosa Korth.) on the rat gastrointestinal tract. J. Ethnopharmacol. 2008, 116, 173–178. [Google Scholar] [CrossRef]
- Jebunnessa; Uddin, S.B.; Mahabub-Uz-Zaman, M.; Akter, R.; Ahmed, N.U. Antidiarrheal activity of ethanolic bark extract of Mitragyna diversifolia. Bangladesh J. Pharmacol. 2009, 4, 144–146. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, S.; Azizi, J.B.; Ramanathan, S.; Ismail, S.; Sasidharan, S.; Said, M.I.M.; Mansor, S.M. Evaluation of antioxidant and antibacterial activities of aqueous, methanolic and alkaloid extracts from Mitragyna speciosa (rubiaceae family) leaves. Molecules 2009, 14, 3964–3974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuniarti, R.; Nadia, S.; Alamanda, A.; Zubir, M.; Syahputra, R.A.; Nizam, M. Characterization, phytochemical screenings and antioxidant activity test of kratom leaf ethanol extract (Mitragyna speciosa Korth) using DPPH method. J. Phys. Conf. Ser. 2020, 1462, 12026. [Google Scholar] [CrossRef]
- Takayama, H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem. Pharm. Bull. 2004, 52, 916–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firmansyah, A.; Sundalian, M.; Taufiq, M. Kratom (Mitragyna speciosa Korth) for a new medicinal: A review of pharmacological and compound analysis. Biointerface Res. Appl. Chem. 2021, 11, 9704–9718. [Google Scholar]
- Raffa, R.B.; Beckett, J.R.; Brahmbhatt, V.N.; Ebinger, T.M.; Fabian, C.A.; Nixon, J.R.; Orlando, S.T.; Rana, C.A.; Tejani, A.H.; Tomazic, R.J. Orally active opioid compounds from a non-poppy source. J. Med. Chem. 2013, 56, 4840–4848. [Google Scholar] [CrossRef]
- Abdullah, H.M.A.; Haq, I.; Lamfers, R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: Is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019, 12, 17–20. [Google Scholar] [CrossRef]
- Oberbarnscheidt, T. Kratom—A lethal drug on the rise. J. Addict. Prev. 2019, 7, 1–6. [Google Scholar]
- Williams, R.S.; Nikitin, D. The internet market for Kratom, an opioid alternative and variably legal recreational drug. Int. J. Drug Policy 2020, 78, 102715. [Google Scholar] [CrossRef]
- Pompei, P. The legal highs of novel drugs of abuse. J. Drug Abus. 2016, 2, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Shekar, S.P.; Rojas, E.E.; D’Angelo, C.C.; Gillenwater, S.R.; Galvis, N.P.M. Legally lethal kratom: A herbal supplement with overdose potential. J. Psychoactive Drugs 2019, 51, 28–30. [Google Scholar] [CrossRef]
- Mallow, M.S. Ketum abuse in Malaysia: Its legal status and proposed solution. Perdana Int. J. Acad. Res. 2020, 7, 29–37. [Google Scholar]
- Warner, M.L.; Kaufman, N.C.; Grundmann, O. The pharmacology and toxicology of kratom: From traditional herb to drug of abuse. Int. J. Legal Med. 2016, 130, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Suwanlert, S. A study of kratom eaters in Thailand. Bull. Narc. 1975, 27, 21–27. [Google Scholar] [PubMed]
- Prozialeck, W.C.; Avery, B.A.; Boyer, E.W.; Grundmann, O.; Henningfield, J.E.; Kruegel, A.C.; McMahon, L.R.; McCurdy, C.R.; Swogger, M.T.; Veltri, C.A.; et al. Kratom policy: The challenge of balancing therapeutic potential with public safety. Int. J. Drug Policy 2019, 70, 70–77. [Google Scholar] [CrossRef]
- Forrester, M.B. Kratom exposures reported to texas poison centers. J. Addict. Dis. 2013, 32, 396–400. [Google Scholar] [CrossRef]
- Post, S.; Spiller, H.A.; Chounthirath, T.; Smith, G.A. Kratom exposures reported to United States poison control centers: 2011–2017. Clin. Toxicol. 2019, 57, 847–854. [Google Scholar] [CrossRef]
- Chittrakarn, S.; Penjamras, P.; Keawpradub, N. Quantitative analysis of mitragynine, codeine, caffeine, chlorpheniramine and phenylephrine in a kratom (Mitragyna speciosa Korth.) cocktail using high-performance liquid chromatography. Forensic Sci. Int. 2012, 217, 81–86. [Google Scholar] [CrossRef]
- Singh, D.; Narayanan, S.; Vicknasingam, B.; Corazza, O.; Santacroce, R.; Roman-Urrestarazu, A. Changing trends in the use of kratom (Mitragyna speciosa) in Southeast Asia. Hum. Psychopharmacol. 2017, 32, e2582. [Google Scholar] [CrossRef] [Green Version]
- Tayabali, K.; Bolzon, C.; Foster, P.; Patel, J.; Kalim, M.O. Kratom: A dangerous player in the opioid crisis. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Beckett, A.H.; Shellard, E.J.; Phillipson, J.D.; Lee, C.M. The mitragyna species of Asia. Part. VI. Oxindole alkaloid from the leaves of Mitragyna speciosa Korth. Planta Med. 1966, 14, 266–276. [Google Scholar] [CrossRef]
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Ruhsam, M.; Moat, J.; Brummitt, N.A. A global assessment of distribution, diversity, edemism, and taxonomic effort in the Rubiaceae. Ann. Missouri Bot. Gard. 2009, 96, 68–78. [Google Scholar] [CrossRef]
- Manns, U.; Bremer, B. Towards a better understanding of intertribal relationships and stable tribal delimitations within Cinchonoideae s.s. (Rubiaceae). Mol. Phylogenet. Evol. 2010, 56, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Puff, C. Flora of Thailand: Rubiaceae; Faculty Centre of Botany, Universitat Wien: Vienna, Austria, 2007; Available online: https://homepage.univie.ac.at/christian.puff/_FTH-RUB/FTH-RUB_HOME.htm (accessed on 24 December 2020).
- Razafimandimbison, S.G.; Bremer, B. Phylogeny and classification of Naucleeae s.l. (Rubiceae) inferred from molecular (ITS, rbcL, and trnT-F) and morphological data. Am. J. Bot. 2002, 89, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Haviland, G.D. A revision of the tribe Naucleae (Nat. Ord. Rubiaceae). J. Linn. Soc. 1897, 33, 1–4. [Google Scholar] [CrossRef]
- Ridsdale, C.E. A revision of Mitragyna and Uncaria (Rubiaceae). Blumea 1978, 24, 43–100. [Google Scholar]
- Razafimandimbison, S.G.; Bremer, B. Tribal delimitation from inference molecular and morphological data. Natl. Plantentuin Belg. 2001, 71, 515–538. [Google Scholar]
- Kamala, F.D.; Baas, P.; Beeckman, H. Mitragyna ledermannii (K. Krause) Ridsdale: Anatomie. Rota Ressour. Vég. Afr. Trop. 2012, 7, 526. [Google Scholar]
- Eisenman, S.W. The botany of Mitragyna speciosa (Korth.) Havil. and related species. In Kratom and Other Mitragynines: The Chemistry and Pharmacology of Opioids from a Non-Opium Source; Raffa, R.B., Ed.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2015; pp. 57–76. [Google Scholar]
- Brown, P.N.; Lund, J.A.; Murch, S.J. A botanical, phytochemical and ethnomedicinal review of the genus Mitragyna Korth: Implications for products sold as kratom. J. Ethnopharmacol. 2017, 202, 302–325. [Google Scholar] [CrossRef]
- Razafimandimbison, S.G.; Kellogg, E.; Bremer, B. Recent origin and phylogenetic utility of divergent ITS putative pseudogenes: A case study from Naucleae (Rubiaceae). Syst Biol. 2004, 53, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Ranggasamy, R.; Ghafar, Z.A.; Jean, S.W.; Hussain, N.H.; Japri, N.; Badron, U.H.; Juan, T.; Wasiman, M.I.; Ismail, Z. Herbal monograph methodology for identification of Mitragyna speciosa (Korth.) Havil leaves. J. Pharmacogn. Phytochem. 2015, 4, 256–262. [Google Scholar]
- Hassan, Z.; Muzaimi, M.; Navaratnam, V.; Yusoff, N.H.M.; Suhaimi, F.W.; Vadivelu, R.; Vicknasingam, B.K.; Amato, D.; von Horsten, S.; Ismail, N.I.W.; et al. From Kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse, and addiction. Neurosci. Biobehav. Rev. 2013, 37, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Macko, E.; Weisbach, J.A.; Douglas, B. Some observations on the pharmacology of mitragynine. Arch. Int. Pharmacolodyn. Ther. 1972, 198, 145–161. [Google Scholar]
- Gong, F.; Gu, H.; Xu, Q.; Kang, W. Genus Mitragyna: Ethnomedicinal uses and pharmacological studies. Phytopharmacology 2012, 3, 263–272. [Google Scholar]
- Nilus, R.; Fah, L.Y.; Hastie, A. Species selection trial in burnt peat swamp vegetation in southwest coast of Sabah, Malaysia. Rehabil. Trop. Rainfor. Ecosyst. 2011, 75–88. [Google Scholar]
- Zhang, M.; Sharma, A.; Leon, F.; Avery, B.; Kjolgren, R.; McCurdy, C.R.; Pearson, B.J. Effects of nutrient fertility on growth and alkaloidal content in Mitragyna speciosa (Kratom). Front. Plant Sci. 2020, 11, 597696. [Google Scholar] [CrossRef] [PubMed]
- Wahyono, S.; Widowati, L.; Handayani, L.; Sampurno, O.D.; Haryanti, S.; Fauzi, F.; Ratnawati, G.; Budiarti, M.S. Kratom, Prospek Kesehatan dan Sosial Ekonomi; Lembaga Penerbit Badan Penelitian dan Pengembangan Kesehatan: Jakarta, Indonesia, 2019.
- Adjétey, T.A.K.; Dje, M.K.; Vangah-Manda, M.; Adoubryn, K.D.; Kone, L.P.; Kone, M.; Guede-Guina, F. Antimalarial activity of Mitragyna ciliata (Aubrev and Pellegr) (Rubiaceae): Preliminary study. S. Afr. J. Bot. 2007, 73, 226–229. [Google Scholar] [CrossRef] [Green Version]
- Henrietta, A.O.; Veronica, I.O.; Okpuzor, J.; Adedayo, T.; Esue, S. Mitragyna ciliata and its trypanocidal activity. Afr. J. Biotechnol. 2007, 6, 2310–2313. [Google Scholar]
- Konkon, N.G.; Adjoungoua, A.L.; Manda, P.; Simaga, D.; N’Guessan, K.E.; Kone, B.D. Toxicological and phytochemical screening study of Mitragyna inermis (willd.) O ktze (Rubiaceae), antidiabetic plant. Int. J. Med. Plants Res. 2020, 9, 1–6. [Google Scholar]
- Fageyimbo, M.S.; Oduniyi, O.; Nwatu, C.J.; Rotimi, K. Anti-inflamatory effect of hydroethanol leaf extract of Mitragyna stipulosa. Univ. Lagos J. Basic Med. Sci. 2017, 5, 6–12. [Google Scholar]
- Panwar, J.; Tarafdar, J.C. Arbuscular mycorrhizal fungal dynamics under Mitragyna parvifolia (Roxb.) Korth. in Thar Desert. Appl. Soil Ecol. 2006, 34, 200–208. [Google Scholar] [CrossRef]
- Pandey, R.; Singh, S.C.; Gupta, M.M. Heteroyohimbinoid type oxindole alkaloids from Mitragyna parvifolia. Phytochemistry 2006, 67, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Khokra, S.L.; Kaushik, P.; Saneja, A.; Arora, D. Anticonvulsant activity of Mitragyna parvifolia leaves extract. Pharmacologyonline 2009, 3, 101–106. [Google Scholar]
- Sahakitpichan, P.; Chadmuk, P.; Disadee, W.; Chimnoi, N.; Ruchirawat, S.; Kanchanapoom, T. New trans- and cis-p-coumaroyl flavonol tetraglycosides from the leaves of Mitragyna rotundifolia. Phytochem. Lett. 2014, 8, 65–68. [Google Scholar] [CrossRef]
- Singh, D.; Müller, C.P.; Vicknasingam, B.K.; Mansor, S.M. Social functioning of Kratom (Mitragyna speciosa) users in Malaysia. J. Psychoact. Drugs 2015, 47, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Saingam, D.; Assanangkornchai, S.; Geater, A.F.; Balthip, Q. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: A qualitative study. Int. J. Drug Policy 2013, 24, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Vicknasingam, B.; Narayanan, S.; Beng, G.T.; Mansor, S.M. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int. J. Drug Policy 2010, 21, 283–288. [Google Scholar] [CrossRef]
- Assanangkornchai, S.; Muekthong, A.; Sam-Angsri, N.; Pattanasattayawong, U. The use of Mitragynine speciosa (‘Krathom’), an addictive plant, in Thailand. Subst. Use Misuse 2007, 42, 2145–2157. [Google Scholar] [CrossRef]
- Ahmad, K.; Aziz, Z. Mitragyna speciosa use in the northern states of Malaysia: A cross-sectional study. J. Ethnopharmacol. 2012, 141, 446–450. [Google Scholar] [CrossRef]
- Compton, D.M.; Garcia, C.; Kamaratos, A.V.; Johnson, B.G.; Wedge, T. An examination of the consequences of chronic exposure to Mitragyna speciosa during adolescence on learning and memory in adulthood. J. Phytopharm. 2014, 3, 300–309. [Google Scholar]
- Raini, M. Kratom (Mitragyna speciosa Korth): Manfaat, Efek Samping dan Legalitas. MPK 2017, 27, 175–184. [Google Scholar] [CrossRef]
- Luliana, S.; Islamy, M.R. Antinociceptive activity of dichlorolethane fraction of kratom leaves (Mitragyna speciosa Korth.) by oral route in male Swiss mice. Pharm. Sci. Res. 2018, 5, 58–64. [Google Scholar]
- Ya, K.; Methaneethorn, J.; Tran, Q.B.; Trakulsrichai, S.; Wananukul, W.; Lohitnavy, M. Development of a physiologically based pharmacokinetic model of mitragynine, psychoactive alkaloid in kratom (Mitragyna speciosa Korth.), in rats and humans. J. Psycoactive Drugs 2021, 53, 127–139. [Google Scholar] [CrossRef]
- Matsumoto, K.; Mizowaki, M.; Suchitra, T.; Murakmi, Y.; Takayama, H.; Sakai, S.; Aimi, N.; Watanabe, H. Antinociceptive action of mitragynine in mice: Evidence for the involvement of supraspinal opioid receptors. Life Sci. 1996, 59, 1149–1155. [Google Scholar] [CrossRef]
- Spetea, M.; Schmidhammer, H. Unveiling 7-hydroxymitragynine as the key active metabolite of mitragynine and the promise for creating novel pain relievers. ACS Cent. Sci. 2019, 5, 936–938. [Google Scholar] [CrossRef] [Green Version]
- Shellard, E.J. Ethnopharmacology of kratom and the mitragyna alkaloids. J. Ethnopharmacol. 1989, 25, 123–124. [Google Scholar] [CrossRef]
- Zarembo, J.E.; Douglas, B.; Valenta, J.; Weisbach, J.A. Metabolites of mitragynine. J. Pharm. Sci. 1974, 63, 1407–1415. [Google Scholar] [CrossRef]
- Asase, A.; Kokubun, T.; Grayer, R.J.; Kite, G.; Simmonds, M.S.J.; Oteng-Yeboah, A.A.; Odamtten, G.T. Chemical constituents and antimicrobial activity of medicinal plants from Ghana: Cassia sieberina, Haematostaphis barteri, Mitragyna inermis, and Pseudocedrela kotschyi. Phyther. Res. 2008, 22, 1013–1016. [Google Scholar] [CrossRef]
- Takayama, H.; Kurihara, M.; Kitajima, M.; Said, I.M.; Aimi, N. New indole alkaloids from the leaves of malaysian: Mitragyna speciosa. Tetrahedron 1998, 54, 8433–8440. [Google Scholar] [CrossRef]
- Takayama, H.; Kurihara, M.; Kitajima, M.; Said, I.M.; Aimi, N. Structure elucidation and chiral-total synthesis of a new indole alkaloid, (-)-9-methoxymitralactonine, isolated from Mitragyna speciosa in Malaysia. Tetrahedron 2000, 56, 3145–3151. [Google Scholar] [CrossRef]
- Phongprueksapattana, S.; Putalun, W.; Keawprabud, N.; Wungsintaweekul, J. Mitragyna speciosa: Hairy root culture for triterpenoid production and high yield of mitragynine by generated plants. Z. Natutforsch. CAJ Biosci. 2008, 63, 691–698. [Google Scholar] [CrossRef]
- Fatima, N.; Tapondjou, L.A.; Lontsi, D.; Sondengam, B.L.; Atta-Ur-Rahman; Choudhary, I.M. Quinovic acid glycosides from Mitragyna stipulosa: First examples of natural inhibitors of snake venom phosphidiesterase I. Nat. Prod. Lett. 2002, 16, 389–393. [Google Scholar] [CrossRef]
- Gogineni, V.; Leon, F.; Avery, B.A.; MacCurdy, C.; Cutler, S.J. Phytochemistry of Mitragyna speciosa. In Kratom and Other Mitragynines: The Chemistry and Pharmacology of Opioids from a Non-Opium Source; Routledge: Abingdon-on-Thames, UK, 2015; p. 77. [Google Scholar]
- Hendrickson, J.B.; Sims, J.J. Mitragyna alkaloids—The structure of stipulatine. Tetrahedron Lett. 1963, 14, 929–935. [Google Scholar]
- Shellard, E.J.; Sarpong, K. The alkaloidal pattern in the leaves, stem-bark and root-bark of Mitragyna species from Ghana. J. Pharm. Pharmacol. 1970, 22, 34–39. [Google Scholar] [CrossRef]
- Takayama, H.; Ishikawa, H.; Kitajima, M.; Aimi, N.; Baba, M. A new 9-methoxyyohombine-type indole alkaloid from Mitragyna africanus. Chem. Pharm. Bull. 2004, 52, 359–361. [Google Scholar] [CrossRef] [Green Version]
- Fiot, J.; Baghdikian, B.; Boyer, L.; Mahiou, V.; Azas, N.; Gasquet, M.; Timon-David, P.; Balansard, G.; Olivier, E. HPLC quantification of uncarine D and the antiplasmodial activity of alkaloids from leaves of Mitragyna inermis (Willd.) O. Kuntze. Phytochem. Anal. 2005, 16, 30–33. [Google Scholar] [CrossRef]
- Donfack, E.V.; Lenta, B.N.; Longue, M.D.T.; Fongang, Y.F.; Ngouela, S.; Tsamo, E.; Dittrich, B.; Laatscah, H. Naucleactonin d, an indole alkaloid and other chemical constituents from roots and fruits of Mitragyna inermis. Z. Naturforsch. Sect. B J. Chem. Sci. 2012, 67, 1159–1165. [Google Scholar] [CrossRef]
- Houghton, P.J.; Lala, P.K.; Shellard, E.J.; Sarpong, K. The alkaloids of Mitragyna stipulosa (D.C.) O. Kuntze. J. Pharm. Pharmacol. 1976, 28, 664. [Google Scholar] [CrossRef]
- Phillipson, J.D.; Tantivatana, P.; Tarpo, E.; Shellard, E.J. Hirusteine and mitrajavine from Mitragyna hirusta. Phytochemistry 1973, 12, 1507. [Google Scholar] [CrossRef]
- Shellard, E.J.; Beckett, A.H.; Tantivatana, P.; Philipson, J.D.; Lee, C.M. Alkaloids from Mitragyna javanica, Koord. and Valeton and Mitragyna hirusta, Havil. J. Pharm. Pharmacol. 1966, 18, 553–555. [Google Scholar] [CrossRef]
- Shellard, E.J.; Tantivatana, P.; Beckett, A.H. The Mitragyna species of Asia. Part X. The alkaloids of the leaves of Mitragyna hirusta Havil. Planta Med. 1967, 15, 366–370. [Google Scholar] [CrossRef]
- Kitajima, M.; Nakayama, T.; Kogure, N.; Wongseripipatana, S.; Takayama, H. New heteroyohimbine-type oxindole alkaloid from the leaves of Thai Mitragyna hirsuta. J. Nat. Med. 2007, 61, 192–195. [Google Scholar] [CrossRef]
- Badger, G.M.; Cook, J.W.; Ongley, P.A. The chemistry of the Mitragyna genus. Part I. J. Chem. Soc. 1950, 29, 867–873. [Google Scholar] [CrossRef]
- Cao, X.F.; Wang, J.S.; Wang, X.B.; Luo, J.; Wang, H.Y.; Kong, L.Y. Monoterpene indole alkaloids from the stem bark of Mitragyna diversifolia and their acetylcholine esterase inhibitory effects. Phytochemistry 2013, 96, 389–396. [Google Scholar] [CrossRef]
- Cao, X.F.; Wang, J.S.; Wang, P.R.; Kong, L.Y. Triterpenes from the stem bark of Mitragyna diversifolia and their cytotoxic activity. Chin. J. Nat. Med. 2014, 12, 628–631. [Google Scholar] [CrossRef]
- Houghton, P.J.; Shellard, E.J. The Mitragyna species of Asia: Part XXVIII: The alkaloidal pattern in Mitragyna rotundifolia from Burma. Planta Med. 1974, 26, 104–112. [Google Scholar] [CrossRef]
- Shellard, E.J.; Philipson, J.D.; Gupta, D. The Mitragyna species of Asia. Part XIII. The alkaloids of the leaves of Mitragyna parvifolia (Roxb.) Korth. Obtained from India. Planta Med. 1968, 16, 436–445. [Google Scholar] [CrossRef]
- Barger, G.; Dyer, E.; Sargent, L.J. The alkaloids of Mitragyna rotundifolia. L. J. Org. Chem. 1939, 4, 418–427. [Google Scholar] [CrossRef]
- Kang, W.Y.; Zhang, B.R.; Xu, Q.T.; Li, L.; Hao, X.J. Study on the chemical constituents of Mitragyna rotundifolia. J. Chin. Med. Mater. 2006, 29, 557–560. [Google Scholar]
- Kang, W.; Hao, X. Triterpenoid saponins from Mitragyna rotundifolia. Biochem. Syst. Ecol. 2006, 34, 585–587. [Google Scholar] [CrossRef]
- Kang, W.Y.; Li, C.F.; Liu, Y.X. Antioxidant phenolic compounds and flavonoids of Mitragyna rotundifolia (Roxb.) Kuntze in vitro. Med. Chem. Res. 2010, 19, 1222–1232. [Google Scholar] [CrossRef]
- Houghton, P.J.; Latiff, A.; Said, I.M. Alkaloids from Mitragyna speciosa. Phytochemistry 1991, 30, 347–350. [Google Scholar] [CrossRef]
- Kikura-Hanajiri, R.; Kawamura, M.; Maruyama, T.; Kitajima, M.; Takayama, H.; Goda, Y. Simultaneous analysis of mitragynine, 7-hydroxymitragynine, and other alkaloids in the psychotropic plant “kratom” (Mitragyna speciosa) by LC-ESI-MS. Forensic Toxicol. 2009, 27, 64–74. [Google Scholar] [CrossRef]
- Houghton, P.J.; Said, I.M. 3-Dehydromitragynine: An alkaloid from Mitragyna speciosa. Phytochemistry 1986, 25, 2910–2912. [Google Scholar] [CrossRef]
- Hinou, J.; Harvala, C. Polyphenolic compounds from the leaves of Mitragyna speciosa. Fitoterapia 1988, 59, 156. [Google Scholar]
- Ponglux, D.; Wongseripipatana, S.; Takayama, H.; Kikuchi, M.; Kurihara, M.; Kitajima, M.; Aimi, N.; Sakai, S. A new indole alkaloid, 7 alphahydroxy-7h-mitragynine, from Mitragyna speciosa in Thailand. Planta Med. 1994, 60, 580–581. [Google Scholar] [CrossRef]
- Phillip, A.A.; Wissembach, D.K.; Weber, A.A.; Zapp, J.; Maurer, H.H. Phase I and II metabolites of speciogynine, a diastereomer of the main Kratom alkaloid mitragynine, identified in rat and human urine by liquid chromatography coupled to low- and high-resolution linear ion trap mass spectrometry. J. Mass Spectrom. 2010, 45, 1344–1357. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Ramanathan, S.; Murugaiyah, V.; Hamdan, M.R.; Said, M.I.; Lai, C.S.; Mansor, S.M. A simple HPLC-DAD method for the detection and quantification of psychotropic mitragynine in Mitragyna speciosa (ketum) and its products for the application in forensic investigation. Forensic Sci. 2013, 226, 183–187. [Google Scholar] [CrossRef]
- Ali, Z.; Demiray, H.; Khan, I.A. Isolation, characterization, and NMR spectroscopic data of indole and oxindole alkaloids from Mitragyna speciosa. Tetrahedron Lett. 2014, 55, 369–372. [Google Scholar] [CrossRef]
- Lesiak, A.D.; Cody, R.B.; Dane, A.J.; Musah, R.A. Rapid detection by direct analysis in real time-mass spectrometry (DART-MS) of psychoactive plant drugs of abuse: The case of Mitragyna speciosa aka Kratom. Forensic Sci. Int. 2014, 242, 210–218. [Google Scholar] [CrossRef]
- Limsuwanchote, S.; Wungsintaweekul, J.; Keawpradub, N.; Putalun, W.; Morimoto, S.; Tanaka, H. Development of indirect competitive ELISA for quantification of mitragynine in Kratom (Mitragyna speciosa (Roxb.) Korth.). Forensic Sci. Int. 2014, 244, 70–77. [Google Scholar] [CrossRef]
- Wang, M.; Carrell, E.J.; Ali, Z.; Avula, B.; Avonto, B.; Parcher, J.F.; Khan, I.A. Comparison of three chromatographic techniques for the detection of mitragynine and other indole and oxindole alkaloids in Mitragyna speciosa (kratom) plants. J. Sep. Sci. 2014, 37, 1411–1418. [Google Scholar] [CrossRef]
- Avula, B.; Sagi, S.; Wang, Y.-H.; Wang, M.; Ali, Z.; Smillie, T.J. Identification and characterization of indole and oxindole alkaloids from leaves of Mitragyna speciosa Korth using liquid chromatography—Accurate QToF mass spectrometry. J. AOAC Int. 2015, 98, 13–21. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Yu, B.Y.; Yang, X.W.; Zhang, J. Triterpenoid saponins from bark Mitragyna inernis. J. Chin. Mater. Med. 2002, 27, 274–277. [Google Scholar]
- Prozialeck, W.C.; Jivan, J.K.; Andurkar, S.V. Pharmacology of Kratom: An emerging botanical agent with stimulant, analgesic and opioid-like effects. J. Am. Osteopath. Assoc. 2012, 112, 792–799. [Google Scholar]
- Rosenbaum, C.D.; Carreiro, S.P.; Babu, K.M. Here today, gone tomorrow and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J. Med. Toxicol. 2012, 8, 15–32. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Mizowaki, M.; Suchitra, T.; Murkami, Y.; Takayama, H.; Aimi, N.; Watanabe, H. Central antinociceptive effects of mitragynine in mice: Contribution of descending noradrenergic and serotonergic systems. Eur. J. Pharmacol. 1996, 317, 75–81. [Google Scholar] [CrossRef]
- Thongpradichote, S.; Matsumoto, K.; Tohda, M.; Takayama, H.; Aimi, N.; Sakai, S.; Watanabe, H. Identification of opioid receptor subtypes in antinociceptive actions of supraspinally-administered mitragynine in mice. Life Sci. 1998, 62, 1371–1378. [Google Scholar] [CrossRef]
- Giovanitti, J.A.; Thoms, S.M.; Ceawford, J.J. Alpha-2 adrenergic receptor agonists: A review of current clinical applications. Anesth. Prog. 2015, 62, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Utar, Z.; Majid, M.I.A.; Adenan, M.I.; Jamil, M.F.A.; Lan, T.M. Mitragynine inhibits the COX-2 mRNA expression and prostaglandin E 2 production induced by lipopolysaccharide in RAW264.7 macrophage cells. J. Ethnopharmacol. 2011, 136, 75–82. [Google Scholar] [CrossRef]
- Matsumoto, K.; Horie, S.; Takayama, H.; Ishikawa, H.; Aimi, N.; Ponglux, D.; Murayama, T.; Watanabe, K. Antinociception, tolerance and withdrawal symptoms induced by 7-hydroxymitragynine, an alkaloid from the Thai medicinal herb Mitragyna speciosa. Life Sci. 2005, 78, 2–7. [Google Scholar] [CrossRef]
- Neerman, M.F.; Frost, R.E.; Deking, J. A drug fatality involving kratom. J. Forensic Sci. 2013, 58, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Kawamura, M.; Kikura-Hanajiri, R.; Takayama, H.; Goda, Y. The botanical origin of kratom (Mitragyna speciosa; Rubiaceae) available as abused drugs in the Japanese markets. J. Nat. Med. 2009, 63, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.M.; Yeakel, J.K.; Logan, B.K. Identification of mitragynine and O-desmethyltramadol in Kratom and legal high products sold online. Drug Test. Anal. 2014, 6, 959–963. [Google Scholar] [CrossRef]
- Kong, W.M.; Chik, Z.; Ramachandran, M.; Subramaniam, U.; Aziddin, R.E.R.; Mohamed, Z. Evaluation of the effects of Mitragyna speciosa alkaloid extract on cytochrome P450 enzymes using a high throughput assay. Molecules 2011, 16, 7344–7356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundmann, O. Patterns of Kratom use and health impact in the US—Results from an online survey. Drug Alcohol Depend. 2017, 176, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Coe, M.A.; Pillitteri, J.L.; Sembower, M.A.; Gerlach, K.K.; Henningfield, J.E. Kratom as a substitute for opioids: Results from an online survey. Drug Alcohol Depend. 2019, 202, 24–32. [Google Scholar] [CrossRef]
- Bath, R.; Bucholz, T.; Buroz, A.F.; Singh, D.; Smith, K.E.; Veltri, C.A.; Grundmann, O. Self-reported health diagnoses and demographic correlates with Kratom use: Results from an Online Survey. J. Addict. Med. 2020, 13, 244–252. [Google Scholar] [CrossRef]
- Feng, L.Y.; Battulga, A.; Han, E.; Chung, H.; Li, J.H. New psychoactive substances of natural origin: A brief review. J. Food Drug Anal. 2017, 25, 461–471. [Google Scholar] [CrossRef]
- Kapp, F.G.; Maurer, H.H.; Auwärter, V.; Winkelmann, M.; Hermanns-Clausen, M. Intrahepatic cholestasis following abuse of powdered Kratom (Mitragyna speciosa). J. Med. Toxicol. 2011, 7, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Fluyau, D.; Revadigar, N. Biochemical benefits, diagnosis, and clinical risks evaluation of kratom. Front. Psychiatry 2017, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Roche, K.M.; Hart, K.; Sangalli, B.; Lefberg, J.; Bayer, M. Kratom: A case of a legal high. Clin. Toxicol. 2008, 46, 598. [Google Scholar]
- Nelsen, J.L.; Lapoint, J.; Hodgman, M.J.; Aldous, K.M. Seizure and coma following Kratom (Mitragynina speciosa Korth) exposure. J. Med. Toxicol. 2010, 6, 424–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheleg, S.V.; Collins, G.B. A coincidence of addiction to ‘kratom’ and Severe primary hypothyroidism. J. Addict. Med. 2011, 5, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Azizi, J.; Ismail, S.; Mordi, M.N.; Ramanathan, S.; Said, M.I.M.; Mansor, S.M. In vitro and in vivo effects of three different mitragyna speciosa korth leaf extracts on phase II drug metabolizing enzymes-glutathione transferases (GSTs). Molecules 2010, 15, 432–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janchawee, B.; Keawprabud, N.; Chittrakarn, S.; Prasettho, S.; Wararatananurak, P.; Sawangjaroen, K. A high-performance liquid chromatographic method for determination of mitragynine in serum and its application to a pharmacokinetic study in rats. Biomed. Chromatogr. 2007, 21, 176–183. [Google Scholar] [CrossRef]
- Harizal, S.N.; Mansor, S.M.; Hasnan, J.; Tharakan, J.K.J.; Abdullah, J. Acute toxicity study of the standardized methanolic extract of Mitragyna speciosa Korth in Rodent. J. Ethnopharmacol. 2010, 131, 404–409. [Google Scholar] [CrossRef]
- Ilmie, M.U.; Jaafar, H.; Mansor, S.M.; Abdullah, J.M. Subchronic toxicity study of standardized methanolic extract of Mitragyna speciosa Korth in sprague-dawley rats. Front. Neurosci. 2015, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Boyer, E.W.; Babu, K.M.; Adkins, J.E.; McCurdy, C.R.; Halpern, J.H. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa Korth). Addiction 2008, 103, 1048–1050. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, M.F.I.L.B.; Singh, D.; Narayanan, S.; Rahim, A.A.; Vicknasingam, B. Socio-demographic characteristics, kratom use and quality of life (QoL) of regular kratom (Mitragyna speciosa Korth.) users. Malays. J. Med. Health Sci. 2019, 15, 4–9. [Google Scholar]
- Aldyab, M.; Ells, P.F.; Bui, R.; Chapman, T.D.; Lee, H. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: A case report and review of literature. Gastroenterol. Res. 2019, 12, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Sablaban, I.M.; Gautam, M. The diagnosis of severe obsessions in the setting of kratom withdrawal and treatment with lorazepam: Case report. J. Addict. Dis. 2020, 39, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Müller, C.P.; Vicknasingam, B.K. Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014, 139, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Saref, A.; Suraya, S.; Singh, D.; Grundmann, O.; Narayanan, S.; Swogger, M.T.; Prozialeck, W.C.; Boyer, E.; Chear, N.J.Y.; Balasingam, V. Self-reported prevalence and severity of opioid and kratom (Mitragyna speciosa Korth.) side effects. J. Ethnopharmacol. 2019, 238, 111876. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.L.; Chakraborty, S.; Eans, S.O.; Cirino, T.J.; Stacy, H.M.; Simons, C.A.; Uprety, R.; Majumdar, S.; McLaughlin, J.P. Kratom alkaloids, natural and semi-synthetic, show less physical dependence and ameliorate opioid withdrawal. Cell. Mol. Neurobiol. 2021, 41, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; See, P.C.; Sreenivasan, S.; Mansor, S.M.; Müller, C.P.; Hassan, Z. Mitragynine attenuates morphine withdrawal effects in rats—A comparison with methadone and buprenorphine. Front. Psychiatry 2020, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Harun, N.; Johari, I.S.; Mansor, S.M.; Shoaib, M. Assessing physiological dependence and withdrawal potential of mitragynine using schedule-controlled behaviour in rats. Psychopharmacology 2020, 237, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.M.; McCurdy, C.R.; Boyer, E.W. Opioid receptors and legal highs: Salvia divinorum and Kratom. Clin Toxicol. 2008, 46, 146–152. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Kratom Drug Profile. 2021. Available online: https://www.emcdda.europa.eu/publications/drug-profiles/kratom_en (accessed on 18 January 2021).
- Bergen-Cico, D.; MacClurg, K. Kratom (Mitragyna speciosa) Use, Addiction Potential, and Legal Status; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 3. [Google Scholar]
- Aziz, Z. Kratom: The epidemiology use and abuse, addicition potential, and legal status. In Kratom and Other Mitragynines: The Chemistry and Pharmacology of Opioids from a Non-Opium Source; Raffa, R.B., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2015; pp. 309–318. [Google Scholar]
- Hillebrand, J.; Olszewski, D.; Sedefov, R. Legal highs on the internet. Subst. Use Misuse 2010, 45, 330–340. [Google Scholar] [CrossRef]
- Ancuceanu, R.V.; Dinu, M.; Anghel, I.; Rebegea, O.C.; Olaru, O.T.; Popescu, D.; Popescu, G. Recent prohibition of certain psycoactive “Ethnobotanicals” in Romania. Farmacia 2010, 58, 121–127. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. Annual Report 2010: The State of the Drugs Problem in Europe; Publication Office of the European Union: Luxembourg, 2012. [Google Scholar]
- Thailand Kratom Act 2486 of 1943. Available online: https://www.conventuslaw.com/report/thailand-decriminalizes-kratom-in-amended/ (accessed on 24 October 2021).
- The Thai Royal Government. Kratom Legality in Thailand will Go into Effect 24 August 2021. 2021. Available online: https://www.kratomscience.com/2021/06/02/kratom-legality-in-thailand-will-go-into-effect-august-24-2021/ (accessed on 12 October 2021).
- Indiana Senate Bill No. 305. Schedule I Controlled Substance of Controlled Substance Look Alike. SB 305 LS 6843/DI 106. 24 January 2014. Available online: http://iga.in.gov/legislative/2021/bills/senate/3 (accessed on 12 October 2021).
- Louisiana Senate Bill No 130, Act No 355. R.S. 40:989.3 §989.3. Unlawful Distribution of Products Containing Mitragyna speciosa to Minors. 31 May 2012. Available online: https://legis.la.gov/legis/ViewDocument.aspx?d=1235683 (accessed on 12 October 2021).
- Tennessee House Bill No 12. Section 39-17-452(a)(2). Illegal Drugs and Analogues of Drugs Section 6 Amended to Include Mitragynine and Hydroxymitragynine. 1 July 2013. Available online: http://www.capitol.tn.gov/bills/108/Bill/HB0012.PDF (accessed on 12 October 2021).
- Drug Enforcement Administration. Drug and Chemical Evaluation. 2009. Available online: http://www.deadiversion.usdoj.gov/drug_%0Achem_info/kratom.pdf. (accessed on 10 August 2021).
- Drug Enforcement Administration. Herbal drug update: Kratom. Microgram Bull. 2005, 38, 114–115. [Google Scholar]
- Russian Federation. The Government of the Russion Federation Decision of 6 October 2011, No. 822. Moscow (Translation of Russian Documents Provided by Alexei Chingin). Available online: http://government.ru/en/docs/. (accessed on 24 October 2021).
- Malaysia Poisons Act of 1952 Section 30(3). Available online: https://www.pharmacy.gov.my/v2/sites/default/files/document-upload/poisons-act-1952-act-366.pdf. (accessed on 24 October 2021).
- Surat Edaran Badan Pengawas Obat dan Makanan Republik Indonesia. No. HK. 04.4.42.421.16.1740. 2016. Available online: https://asrot.pom.go.id/img/Peraturan/Surat%20Edaran%20EPA%20DHA.pdf. (accessed on 24 October 2021).
- Peter, H. Kratom Could Be Illegal before It Gets a Change Solve the Opioid Crisis. 2018. Available online: https://www.inverse.com/article/45966-kratom-what-is-it-and-why-its-controversial (accessed on 12 March 2021).
- Schmidt, M.M.; Sharma, A.; Schifano, F.; Feinmann, C. “Legal highs” on the net—Evaluation of UK-based Websites, products and product information. Forensic Sci. 2011, 206, 92–97. [Google Scholar] [CrossRef] [PubMed]
- PT. Triton Nusantara Tangguh. Harga Kratom. 2020. Available online: https://misterexportir.com/harga-kratom/ (accessed on 26 July 2021).
- Ramanathan, S.; McCurdy, C.R. Kratom (Mitragyna speciosa): Worldwide issues. Curr. Opin. Psychiatry 2020, 33, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Prevete, E.; Kuypers, K.P.C.; Theunissen, E.L.; Corazza, O.; Bersani, G.; Ramaekers, J.G. A systematic review of (pre)clinical studies on the therapeutic potential and safety profile of kratom in humans. Hum. Psychopharmacol. Clin. Exp. 2021, 37, e2805. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.F.I.L.B.; Singh, D. Assessment of cardiovascular functioning among regular kratom (Mitragyna speciosa Korth) users: A case series. Front. Pharmacol. 2021, 12, 2099. [Google Scholar]
- Abdullah, M.F.I.L.B.; Yuvashnee, N.; Singh, D. Effect of regular kratom (Mitragyna speciosa Korth.) use on quality of life of people who use kratom. Subst. Abus. 2021, 42, 444–449. [Google Scholar]
- Ishak, S.S.O.A.; Putra, N.; Salsabila, S.; Al Muqarrabun, L.M.R. Mitragynine: A review of its extraction, identification, and purification methods. Curr. Res. Biosci. Biotechnol. 2021, 3, 165–171. [Google Scholar]
- Herman; Ibrahim, A.; Rahayu, B.P.; Arifuddin, M.; Nur, Y.; Prabowo, W.C.; Maryono; Ambarwati, N.S.S.; Rijai, L.; Ahmad, I. Single factor effect of natural deep eutectic solvent citric acid-glucose based microwave-assisted extraction on total polyphenols content from Mitragyna speciosa Korth. Havil leaves. Pharmacogn. J. 2021, 13, 1109–1115. [Google Scholar] [CrossRef]
- Ahmad, I.; Prabowo, W.C.; Nur, Y.; Irawan, L.; Yusniah, A.; Rahayu, B.P.; Hidayati, R.; Nurlinda, H. Enhanced extraction of total polyphenols content from Mitragyna speciosa (Korth.) Havil leaves using the natural deep eutectic solvent-based microwave-assisted extraction method. In Proceedings of the 1st International Conference on Health (ICOH 2019), Jakarta, Indonesia, 14–15 October 2020; pp. 72–77. [Google Scholar]
- Zakaria, F.; Tan, J.; Mohd Faudzi, S.M.; Rahman, M.B.A.; Ashari, S.E. Ultrasound-assisted extraction conditions optimisation using response surface methodology from Mitragyna speciosa (Korth.) Havil leaves. Ultrason Sonochem. 2021, 81, 105851. [Google Scholar] [CrossRef]
| No. | Species | Synonyms | Botanical Origin | References |
|---|---|---|---|---|
| 1 | M. ledermannii (K. Krause) Risdale | M. ciliate Aubrev. & Pellegr.: Fleroya ledermannii (K. Krause) Risdale Y.F.Deng.; and Hallea ciliate (Aubrev. & Pellegr.) J.F.Leroy | The species spreads from eastern Liberia to the Central African Republic, the south of Gabon, Congo, and Angola | [48,49,50] |
| 2 | M. rubrostipulata (K. Schum) Havil. | F. rubrostipulata (K. Schum) Y. F. Dengs; H. rubrostipulata (K. Schum.) J.F.Leroy; and Adina rubrostipulata K. Schum. | The species spreads across various regions in Africa, including the Democratic Republic of Congo, Ethiopia, Tanzania, Malawi, and Mozambique | [44,47,49,50] |
| 3 | M. inermis (Willd.) Kuntze | M. africanum (Willd.) Hook; Nauclea africana Willd.; N. africana var. luzoniensis DC.; N. platanocarpus Hook.f.; N. inermis (Willd.) Baill; Cephalanthus africanus Rchb.; Platanocarpum africanum (Willd.) Korth; Uncaria inermis Willd.; and Adina inermis (Willd.) Roberty | The species spreads across eastern Mauritania to Sudan | [44,47,49,50] |
| 4 | M. stipulosa (DC.) Kuntze | M. chevalieri K. Krause.; M. macrophylla Hiern.; F. stipulosa (DC.) Y.F. Deng.; N. stipulosa DC.; N. bracteosa Welw.; Adina stipulosa (DC.) Roberty; Mamboga stipulosa (DC.); and H. stipulosa (DC.) J.F. Leroy | The species spreads from eastern Senegal to Uganda and southern Senegal to Zambia and Angola | [44,49,50] |
| 5 | M. hirusta Havil. | M. Africana (Willd.) Korth.; Platanocarpum Africana (Willd.) Hook.; Cephalanthus africanus Rchb.; N. africana Willd.; and Paradina hirusta (Havil.) Pit | The species is found in the Asian region, mainly in Thailand, Vietnam, Laos, China, and Cambodia | [43,44,49,50] |
| 6 | M. diversifolia (Wall. Ex G.Don) Havil. | M. javanica Koord; Stephegyne parvifolia Vidal; S. tubulosa Fern.; N. diversifolia Wall. Ex. G. Don; N. adina Blanco; and Mamboga capitata Blanco | This species spreads across Asia, i.e., Indonesia, Malaysia, the Philippines, Thailand, Vietnam, Cambodia, Laos, and China | [43,44,49,50] |
| 7 | M. parvifolia (Roxb.) Korth. | Stephegyne parvifolia (Roxb.) Kuntze; N. parvifolia Roxb.; and N. parvifolia Willd | The species has been found in Asia, especially in Myanmar, Sri Lanka, India, and Bangladesh | [43,49,50] |
| 8 | M. rotundifolia (Roxb.) Kuntze | M. brunonis (Wall. Ex. G. Don) Craib.; N. rotundifolia Roxb.; N. brunonis Wall, Ex. G. Don.; and Bancalus rotundifolius (Roxb.) Kuntze | This species has been found in Asia, mainly in the regions of Thailand, Myanmar, Laos, China, India, and Bangladesh | [43,44,49,50] |
| 9 | M. tubulosa (Arn.) Kuntze | N. tubulosa Arn | The species is endemic to Asia (mainly India) and has spread to Kerala, Tamil, Nadu, and Sri Lanka | [43,49,50,51] |
| 10 | M. speciosa (Korth.) Havil | N. luzoniensis Blanco, N. korthalsii Steud, N. speciosa (Korth.), and Stephegyne speciosa Korth | The species is endemic to southeastern Asia and is scattered across various regions of Myanmar, Vietnam, Thailand, Malaysia, Indonesia, and Papua New Guinea | [40,49,50,52] |
| No | Species | Indole and Oxindole Alkaloids | Other Compounds | References |
|---|---|---|---|---|
| 1 | M. ledermannii (K. Krause) Risdale | Mitraciliatine, rhynchophylline, rhynchociline, ciliaphylline, rotundifoline, isorotundifoline | - | [2,50] |
| 2 | M. rubrostipulata (K. Schum) Havil. | Hirsuteine, mitraphylline, isomitraphylline, isorotundifoline, rotundifoline N- oxide, isorhynchophylline, rhynchophylline N-oxide, rhynchophylline, rotundifoline, | - | [50,86] |
| 3 | M. inermis (Willd.) Kuntze | Uncarine D (speciophylline), rhynchophylline, isorhynchophylline, rotundifoline, isorotundifoline | Quercetin, dihydrodehydrodiconiferyl alcohol, isolariciresinol, isolariciresinol-3α-O-β-D-glucopyranoside, ursolic acid, oleanoic acid, betulinic acid, barbinervic acid, quinovic acid and its derivates, inermiside I, inermiside II, | [2,50,87,88,89,90] |
| 4 | M. stipulosa (DC.) Kuntze | Mitraphylline, rhyncophylline, isorhynchophylline, rotundifoline, isorotundifoline | Ursolic acid, quinovic acid and its derivates, sitoseterol, stigmasterol, daucosterol | [2,50,84,91] |
| 5 | M. hirusta Havil. | Mitraciliatine, mitraphylline, isomitraphylline, isomitraphylline N-oxide, rhynchophylline, isorhynchophylline, isopteropodine, isomitraphyllinol, hirsuteine, mitrajavine, uncarine D (speciophylline), rhynchophylline, isorhynchophylline, rotundifoline, isorotundifoline | - | [50,92,93,94,95] |
| 6 | M. diversifolia (Wall. Ex G.Don) Havil. | 7- hydroxy-isopaynantheine, 3-dehydro-paynantheine, 3-isopaynantheine-N(4)- oxide, mitrafoline, mitradiversifoline, specionoxeine-N(4)-oxide, specionoxeine-N(4)-oxide | 3α, 6β, 19α-trihydroxy-urs-12-en-28-oic acid, 3β, 6β, 19α- trihydroxy-urs-12-en-28-oic acid; 3-oxo-6β-19α-dihydroxy-urs-12-en-28-oic acid; 3β, 6β, 19α-trihydroxy-urs-12-en-24, 28-dioic acid 24-methyl ester; 3β, 6β, 19α, 24-tetrahydroxy-urs-12-en-28-oic acid; rotundic acid; 23-nor-24-exomethylene- 3β, 6β, 19α-trihydroxy-urs-12-en-28-oic acid; pololic acid | [50,96,97,98] |
| 7 | M. parvifolia (Roxb.) Korth. | Dihydrocorynantheol, dihydrocorynantheol N-oxide, akuammigine, akuammigine N-oxide, 3-isoajmalicine, mitraphylline, isomitraphylline, rhynchophylline, isorhynchophylline, rotundifoline, isorotundifoline, speciophylline N-oxide, uncarine F, uncarine F N-oxide, pteropodine, isopteropodine, uncarine D (speciophylline), 16,17-dihydro-17β-hydroxy isomitraphylline, 16,17-dihydro- 17β-hydroxy mitraphylline | - | [50,64,99,100] |
| 8 | M. rotundifolia (Roxb.) Kuntze | mitraphylline, isomitraphylline, rhynchophylline, isorhynchophylline, isorhynchophylline N-oxide, rotundifoline | 3,4-dihydroxybenzoic acid, cathecin, caffeic acid, epicathecin, kaempferol, 4′-O-methyl-gallocatechin, 4-hydroxy-3-methoxybenzoic acid, 3-hydroxy-4-methyloxybenzoic acid, cincholic acid, quinovic acid and its derivates | [50,99,101,102,103,104] |
| 9 | M. tubulosa (Arn.) Kuntze | Mitraciliatine, rhynchociline, ciliaphylline, rotundifoline, isorotundifoline, rhynchophylline, isorhyncophylline, mitraphylline, isomitraphylline, ciliaphylline N-oxide | - | [2,50,91] |
| 10 | M. speciosa (Korth.) Havil | mitragynine, 7-hydroxymitragynine, paynantheine, mitralactonal, mitragynaline, speciociliatine, speciogynine, mitrasulgynine, 3,4,5,6-tetradehydromitragynine, mitragynaline, mitragynalinic acid, corynantheidinaline, corynantheidinalinc acid, 3-dehydromitragynine, 9-methoxymitralactonine, 3-isopaynantheine, ajmalicine, isocorynantheidine, mitragynine pseudoindoxyl, mitraphylline, mitragynine oxindole A, mitragynine oxindole B, corynoxine, corynoxine B, mitraciliatine, 7β-hydroxy-7H-mitraciliatine, isomitraphylline, rhynchophylline, rhyncocilline, cilaphylline, isospeciofoleine, isospeciofoline, isorotundifoline | Apigenin, apigenin 7-glycosides, quercetin, quecitrin, rutin isoquercitrin, hyperoside, quercetin-3-galactoside-7-rhamnoside, kaempferol, kaempferol 3-glucoside, epicatecin, caffeic acid, chlorogenic acid, 1-O-feruloyl-β-D-glucopyranoside, benzyl-β-D-glucopyranoside, quinovic acid and its derivates, monoterpenes 3-oxo-α-ionyl-O-β-Dglucopyranoside, roseoside, secoiridoid, vogeloside, epigeloside | [3,23,50,71,72,105,106,107,108,109,110,111,112,113,114,115,116] |
| Country | Status | Details |
|---|---|---|
| Austria [157] | Legal | |
| Belgium [157] | Legal | |
| Hungary [157] | Legal | Not approved for human consumption, but available as incense in head shops |
| The Netherlands [157] | Legal | Available in head shops |
| United Kingdom [157] | Legal | Sold in head shops (smart shops) |
| Thailand [157,158,159] | Legal | Thailand is considering making M. speciosa legal again to find safer and healthier stimulants to combat Thailand’s high rate of methamphetamine addictions. This plant was formerly listed as a narcotic in Thailand; the change took effect on 24 August 2021. |
| United States | Varying regulation legal or regulated in most states | Banned in the state of Indiana [160]. The state of Louisiana [161] prohibits the distribution of products containing M. speciosa to minors (under age 18). It is controlled and illegal to sell in the state of Tennessee [162]. In 2005, US Drug Enforcement Agency (DEA) listed this as a drug of concern with abuse potential [163,164] starting in 2014. M. speciosa is not a scheduled or restricted drug at the federal level |
| Russia [146,165] | Illegal | Mitragynine (9-methoxy-corynanthidine) and its derivatives are illegal |
| Malaysia [156,166] | Illegal | Controlled under narcotic law |
| Myanmar [157] | Illegal | Controlled under narcotic law |
| South Korea [156] | Illegal | |
| Indonesia [167] | Illegal | M. speciosa plants and their processed products, including active chemical compounds, are included in Narcotics Group I, and are stipulated under the Regulation of the Minister of Health, with a maximum transition period of five years, since 2020. M. speciosa, containing the alkaloid mitragynine at high doses, can have a sedative effect. It is classified as a narcotic and is included in the list of ingredients that are prohibited for use in dietary supplements and traditional medicines. |
| Burma [156] | Controlled | |
| Denmark [157] | Controlled | |
| Finland [157] | Controlled | Requires a prescription. Shipments can be seized at the border |
| Germany [157] | Controlled | Controlled as an approved pharmaceutical drug. |
| Latvia [157] | Controlled | |
| Lithuania [157] | Controlled | |
| Poland [157] | Controlled | |
| Sweden [157] | Controlled | |
| Vietnam [156] | Controlled | |
| Romania [156,157] | Controlled, illegal | |
| New Zealand [156,157] | Controlled, restricted | M. speciosa and mitragynine are controlled under Schedule 1 of the Medicines Amendment Regulations 2009 (SR 2009/212 prescription, restricted, and pharmacy-only medicines). It is not legal to sell M. speciosa without a license, although it is not illegal to possess it. |
| Australia [156,157] | Restricted | Both mitragynine, one of the active chemicals in M. speciosa, and M. speciosa were placed in Schedule 9 of the Australian Standard for the Uniform Scheduling of Drugs and Poisons (SUSDP) in 2005. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, I.; Prabowo, W.C.; Arifuddin, M.; Fadraersada, J.; Indriyanti, N.; Herman, H.; Purwoko, R.Y.; Nainu, F.; Rahmadi, A.; Paramita, S.; et al. Mitragyna Species as Pharmacological Agents: From Abuse to Promising Pharmaceutical Products. Life 2022, 12, 193. https://doi.org/10.3390/life12020193
Ahmad I, Prabowo WC, Arifuddin M, Fadraersada J, Indriyanti N, Herman H, Purwoko RY, Nainu F, Rahmadi A, Paramita S, et al. Mitragyna Species as Pharmacological Agents: From Abuse to Promising Pharmaceutical Products. Life. 2022; 12(2):193. https://doi.org/10.3390/life12020193
Chicago/Turabian StyleAhmad, Islamudin, Wisnu Cahyo Prabowo, Muhammad Arifuddin, Jaka Fadraersada, Niken Indriyanti, Herman Herman, Reza Yuridian Purwoko, Firzan Nainu, Anton Rahmadi, Swandari Paramita, and et al. 2022. "Mitragyna Species as Pharmacological Agents: From Abuse to Promising Pharmaceutical Products" Life 12, no. 2: 193. https://doi.org/10.3390/life12020193
APA StyleAhmad, I., Prabowo, W. C., Arifuddin, M., Fadraersada, J., Indriyanti, N., Herman, H., Purwoko, R. Y., Nainu, F., Rahmadi, A., Paramita, S., Kuncoro, H., Mita, N., Narsa, A. C., Prasetya, F., Ibrahim, A., Rijai, L., Alam, G., Mun’im, A., & Dej-adisai, S. (2022). Mitragyna Species as Pharmacological Agents: From Abuse to Promising Pharmaceutical Products. Life, 12(2), 193. https://doi.org/10.3390/life12020193











