1. Introduction
The Eurasian Griffon vulture (
Gyps fulvus fulvus Hablizl, 1783) belongs to the subfamily
Aegypiinae or
Gypiinae, a group of Old World vultures that is listed as critically endangered on the International Union for Conservation of Nature’s Red List of Threatened Species (IUCN Red list) [
1] and is an object of several international conservation conventions and directives (reviewed in [
2,
3]). Of 16 Old World vulture species, 11 are classified as “Globally Threatened”, with 8 “Critically Endangered” and 3 “Endangered” species. The causes of the worldwide decline of vulture populations are mainly due to the negative anthropogenic influence, which includes habitat destruction and degradation, human disturbance of nesting sites and changes in livestock management, as well as the disappearance of nomadic grazing on natural pastures, electrocution, windmills, poaching, use of non-steroidal anti-inflammatory drugs and poisoning [
4]. Many conservative strategies around the globe are successfully implemented, especially in Europe [
5,
6,
7,
8,
9], but the coordination and implementation of the global action plan still remains a necessity [
3,
4].
Vultures play a key role in maintaining the harmonious processes and functionality of ecosystems. As obligate scavengers, their main role is the decomposition of organic matter in nature, via efficient carcass removal that reduces the rates of transmission of infectious diseases. The Griffon vulture itself is an economically and ecologically important species that provides several ecosystem services in the form of animal carcass removal, which is beneficial from both the sanitary and economic perspective [
10,
11,
12]. It is estimated that the Spanish vulture population, where the Griffon vulture is the dominant species, on average removes between 134 and 201 tons of bones and between 5551 and 8326 tons of meat each year, which allows for a minimum economic savings of approximately USD1.19 million to USD1.94 million [
10]. Apart from this crucial ecosystem service that the vultures provide, the Griffon vulture is recognized as an important species that can improve the income of the region through ecotourism since the nature reserves that host this magnificent bird attract a lot of bird watchers [
13,
14,
15]. The recognized role of the Griffon vulture in nature, as well as its economic value, makes this species important for protection and conservation efforts. Due to the all above mentioned reasons, as well as species recent predicaments, it is necessary to properly evaluate the existing genetic diversity of different Griffon vulture populations in order to design proper conservation strategies [
16].
The Eurasian Griffon vulture has a large area of distribution, ranging from Kazakhstan and Nepal to Southern Europe and the Maghreb countries [
17] and is the most widespread species of
Gyps genus. Spain holds the largest breeding Griffon vulture population in the world. Estimates are that around 75% (about 26,000 couples) of the world’s total Griffon vulture population and more than 95% of European populations are situated in this country [
18,
19,
20]. In the Balkan Peninsula, there are an estimated number of 550 couples, with the exclusion of the population from Crete [
21]. The latest census estimate of the Griffon vulture population from Serbia for the year 2021 is 290 couples, which accounts for more than half of the couples estimated for the entire Balkan Peninsula [
22]. The Griffon vulture population of the Balkan Peninsula consists of several subpopulations (addressed as populations further in the text) including those from Serbia, Croatia, Bulgaria, Greece and North Macedonia (reviewed in [
3]), while in Europe, several isolated island populations in Sardinia, Cyprus and Crete exist (reviewed in [
3]). According to the IUCN criterion, this species is currently classified as “Least Concern” due to its large population in Spain. However, bearing in mind that this species is now considered extinct from most parts of North Africa and Apennines [
23] and several parts of its historical range in southeast Europe (Albania and Romania), protection and conservation of its remaining natural breeding colonies remain a necessity. Furthermore, the species is classified as a sensitive and dependent species and as such it is protected by various laws, directives and conventions. Genetic diversity of the Griffon vulture populations across its distribution range was evaluated in several studies and it is described on both biparentally inherited autosomal loci and its microsatellites [
2,
5,
24] and uniparentally inherited mitochondrial DNA (mtDNA) [
16,
25,
26,
27,
28,
29]. The study [
5], based on the variability of microsatellites, demonstrated a need for proper evaluation of genetic variability in native populations used for restocking and post-released populations in order to maintain the genetic diversity of reintroduced populations and preserve their stability. Further studies based on microsatellite variability demonstrated the existence of two distinct genetic clusters [
2]. The first one is characteristic for populations from the Balkan Peninsula and another one for the population of the Iberian Peninsula, while the population from the Middle East showed the admixture of both genetic clusters. These new data are of great importance for future reintroduction projects [
2], foremost because the study included the populations from the Balkan Peninsula for the first time. An earlier study demonstrated no genetic differentiation among the Griffon vulture populations from Spain, Israel and Cyprus, but it did not include specimens from the Balkan Peninsula populations [
24]. The same study showed the lack of genetic diversity in the population of Indian vulture (
Gyps indicus), which called for the proper genetic evaluation of birds that will be used for future restocking programs [
24], further stressing the need for an accurate genetic characterization of the populations of conservation interest.
Considering mtDNA, the majority of the available data for different Griffon vulture populations are based on the analysis of
Cytb [
16,
25,
26,
29] and D-loop [
27,
28]. In addition to the available data the first complete mitogenome was reported in [
28], which allowed for a more accurate reconstruction of the evolutionary history of Griffon vulture, especially with regard to molecular dating.
Cytb data were mostly used to determine the phylogeny of different vulture species, including the members of the
Gyps genus [
16,
26,
29], while only one study used
Cytb for both phylogenetic and phylogeographic analysis [
25]. A highly variable and selectively neutral D-loop region of mtDNA was used recently in studies whose goal was to determine the population genetic history of the isolated island Griffon vulture populations from Sardinia, Crete and Cyprus [
27,
28]. While substantially adding to the body of knowledge about the phylogeny of different vulture species, most of these studies, with the exception of [
27,
28], lack the population part of studying genetic diversity in Griffon vulture, which is of crucial importance for proper design of conservation, restocking and reintroduction strategies as well as establishing the appropriate scale and units for conservation management.
Properly designed conservation strategies recognize the need to analyze information from both uniparentally and biparentally inherited genetic markers [
30,
31,
32]. Unlike genetic markers from autosomal loci, uniparental markers such as mtDNA and sex chromosomes (Y in mammals and W in birds) have a four times smaller effective population size [
33,
34], which makes them more vulnerable to genetic drift and rapid demographic change [
35]. These characteristics of mtDNA are important when the conservation efforts are made for the populations that became highly divergent due to the long-term isolation [
36] or have experienced a serious bottleneck event that can create enough genetic differences for evolutionary significant units (ESU) to be recognized [
37]. This is the case with the Balkans’ Griffon vulture population from Serbia [
2]. Analysis of mtDNA variability can also provide an insight into the reproductive behavior of the organisms, i.e., the identification of strong natal philopatric behavior [
32,
38], which is essential information for creating a proper conservation strategy. The mitochondrial
Cytb gene is widely used in studies considering the genetic diversity of birds [
39,
40] for deciphering their evolutionary histories [
41,
42] and species identification [
43,
44]. As a genetic marker,
Cytb has several valuable properties that can be used for inferring different evolutionary and population processes.
Cytb gene sequence is characterized by both slowly and rapidly evolving codon positions as well as conserved and variable regions, which makes it ideal for deciphering diversity and systematics questions starting from detailed phylogeny [
45,
46,
47,
48,
49,
50] to the population and recent divergence levels [
51,
52,
53,
54].
Cytb gene variability can be used to identify the signs of adaptive evolution [
55] as well as local declines in diversity, which is indicative of selective sweeps [
56,
57].
The majority of data available for the Griffon vulture and its sister species are based on the variability of Cytb and new data about Cytb variability in the Serbian Griffon vulture population will further improve our understanding of the population structure and local genetic variability of this significant species. In addition, Cytb can be used to decipher if specific adaptations to different climate conditions have emerged in the Griffon vulture populations inhabiting various geographical regions. With the detailed analysis performed in this study, we aim to further determine the status of the Griffon vulture population from Serbia, and its perspective as a source population for further reintroduction efforts in the Balkan Peninsula.
4. Discussion
Most of the species classified as endangered are facing extinction at a rapid rate today. Species that are of economic importance have a chance to be preserved, and the Griffon vulture is one of those species. Species belonging to the
Gyps genus are particularly endangered in Asia and Africa, where their extinction poses a threat to the functioning of the grassland communities and migratory ruminants. Evaluating genetic diversity and differentiation of threatened wildlife populations is essential for determining conservation units and developing appropriate conservation and management strategies. Although the Griffon vulture as a species is not itself threatened, some of its populations are under threat of extinction from their natural habitats or have already disappeared. Human-induced declines in numbers and habitat occupancy can lead to the extinction of populations of this species throughout its native range of distribution. Many conservation efforts have been devised for the protection and reintroduction of the endangered Griffon vulture species of the Balkan Peninsula. Based on previous work on genetic variability and population health, the Serbian Griffon vulture population has the potential to be an important source for reintroduction efforts in other continental parts of southeast Europe [
2,
6]. In this paper, we present the results of the analysis of genetic variability of the most commonly used mtDNA marker in birds,
Cytb, with the aim to further assess the status of the Serbian Griffon vulture population and its perspectives for conservation efforts in the Balkan Peninsula.
A total of 16
Cytb haplotypes defined by 19 polymorphic sites were detected in contemporary Griffon vulture populations and museum samples. The most common 14560C haplotype is present in all analyzed populations with the exception of Turkey and Sardinia, which are represented with only two and one bird, respectively. Analysis of museum samples collected in Serbia showed that the more recent samples, collected between 1980 and 1998, have the most common haplotype prevalent in the contemporary Serbian Griffon population. However, the oldest successfully amplified sample from 1920 has a distinct haplotype never before identified in any analyzed Griffon vulture populations. Loss of genetic diversity and elevated inbreeding levels are expected in populations that experienced serious bottleneck events, such as those that happened with the Serbian Griffon vulture population from 1950 to 1995 [
6].
Cytb genetic diversity of the contemporary Serbian population with three haplotypes is a strong indication that this population successfully recovered from its previous predicament and is now a strong and stable population. Although it may seem low, the observed haplotype diversity is in concordance with previously published studies regarding mtDNA variability of the entire
Gyps genus [
16,
26] and overall higher values of genetic diversity level in
G. fulvus compared to species with similar biology and ecological niche
Gypaetus barbatus [
73] and
Neophron percnopterus [
74]. The same conclusion can be inferred with our analysis of haplotype diversity of all publicly available
Cytb sequences within the
Gyps genus. The greatest number of haplotypes was detected in the
Gyps vulture, followed by
G. africanus and
G. bengalensis. This is not a surprising result bearing in mind that
G. vulture has the largest area of distribution of all analyzed species [
25]. It is interesting to note that the population from South Asia, with only four individuals represented in the sample, has the highest values of genetic diversity represented by haplotype diversity and that the Spanish population, with more than 75% of total Griffon vulture individuals, although high showed lower values for this parameter. However, as expected, overall, the Spanish population harbors the highest genetic diversity among all analyzed populations.
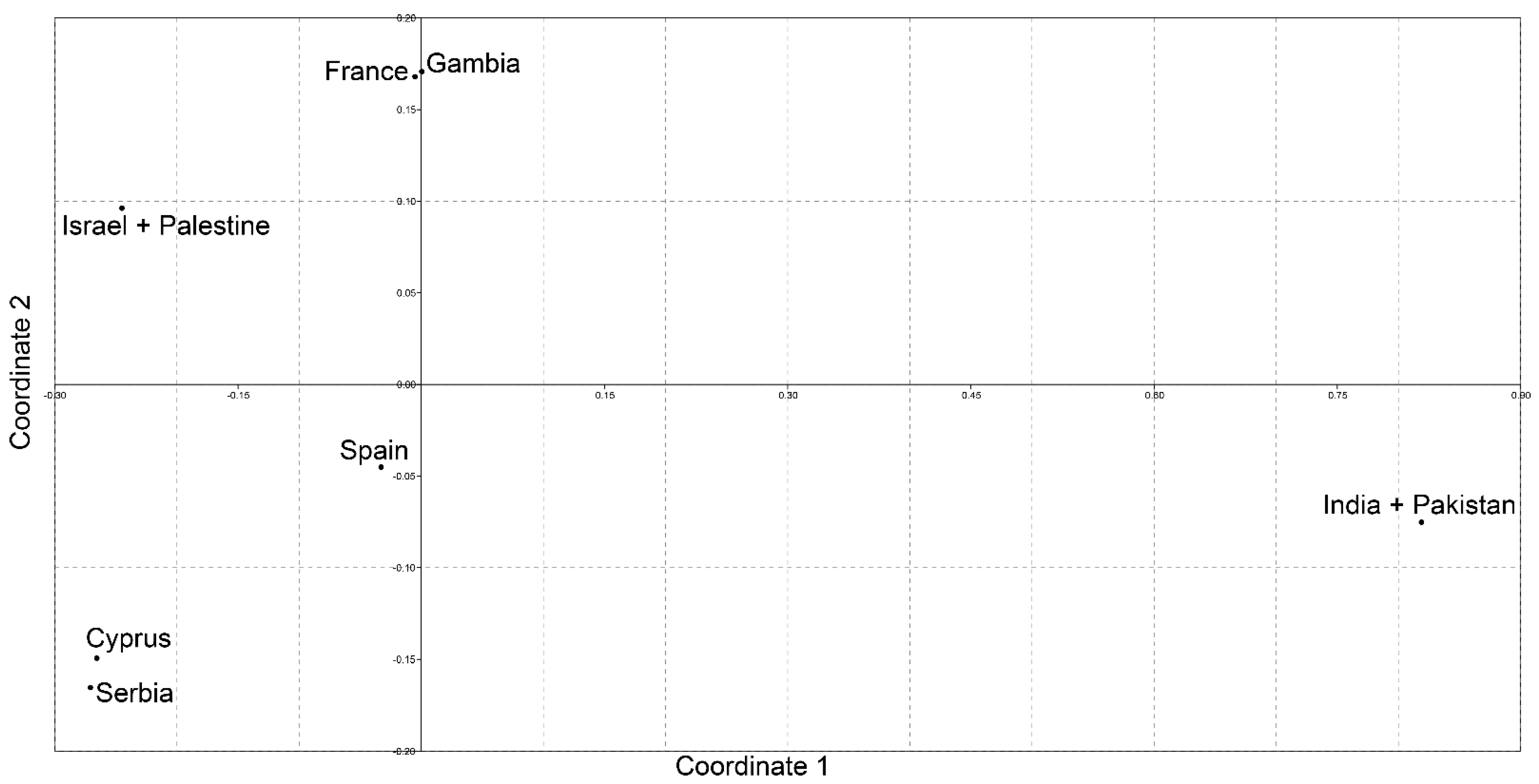
Closer analysis of haplotype distribution within specific populations, with one prevalent and few private haplotypes, is a strong indication of discrete genetic segregation between populations and is further corroborated by analysis of molecular variances. A significant percentage of genetic variance can be attributed to the variation among populations and the position of populations on the MDS plot shows clear differentiation among them as four distinct clusters (southeast Europe with Cyprus, southwest Europe with West Africa, the Middle East and South Asia) can be observed. In addition, when populations with the highest numbers of sequenced individuals, i.e., Serbian and Spanish, were compared, a significantly high 10% of the genetic variance could be explained by the variation among populations. The observed high and statistically significant FST value further confirms a pronounced genetic differentiation between these two populations.
The presented results are in concordance with previously reported genetic differentiation based on microsatellite variability between these two populations [
2]. It is interesting to note that a similar pattern of genetic differentiation between Iberian and Balkan populations was observed in Eurasian black vulture, where
Cytb analysis revealed the existence of two distinct evolutionary lineages corresponding to breeding populations in Spain and the Balkans [
75]. Considering the fact that, in general, raptor birds have a low level of genetic diversity [
76,
77,
78,
79,
80,
81,
82] and that in species with high dispersal capabilities, low values of genetic structuring is expected [
83,
84], such clear differentiation between the two largest remaining European Griffon vulture populations may seem surprising. Genetic distinctiveness between these populations may be contributed to several factors and probably arise foremost due to the social monogamy and pronounced philopatric behavior of this species and the specific environmental setting characteristic for these two geographic regions that are reflected in slightly different physiology and reproductive phenology. Griffon vulture individuals show high nest fidelity and strong adult natal philopatry, which is presumed to give an advantage in the intra-sexual competition for territories [
85,
86]. This behavior favors colony formation and reduction in dispersion rate and may have several consequences for the levels of genetic variability in any given population. Strong adult natal philopatry may increase the inbreeding level and lead to the reduction of population genetic variability but, on the other hand, it is considered as a factor that stimulates a rise in frequencies of genetic variants that are better adapted to the specific habitat requirements and lead to the population-specific genetic variability, such as that previously detected in different geographical regions [
2,
27,
28].
The establishment of population-specific genetic variants may be further propelled by social monogamous behavior and multigenerational natal philopatry leading to genetic differentiation between populations, as already shown for the island populations of Crete and Sardinia [
27,
28]. Although our selection analysis did not reveal the presence of variants favored by selection, and all mutations were defined as neutral, the same analysis showed that the
Cytb gene is under strong purifying selection, which eliminates all substitutions that could have a negative effect on phenotype, as shown in the case of low-altitude deer mice [
87] and four different
Gerbillus species [
55]. The second reason behind genetic differentiation between populations may be specific morphological and reproductive differences in different climates. For example, the Serbian Griffon vulture population is the only continental population in Europe and individuals, on average, have greater body mass and later hatching time than individuals from Spain. Adaptation to harsher continental climate is the most likely explanation of larger body size [
88,
89] and delay in hatching time is in correlation with the delayed pasturing season in continental compared to Mediterranean climate [
3,
90,
91]. In addition, Griffon vultures marked in the nests in Serbia have been recorded in large numbers during migrations in the Middle East. The absence of migration of young birds from Serbia across the Alps confirms the isolation of the Western (Iberian) from Eastern (Balkan) European populations of the Griffon vultures [
92].
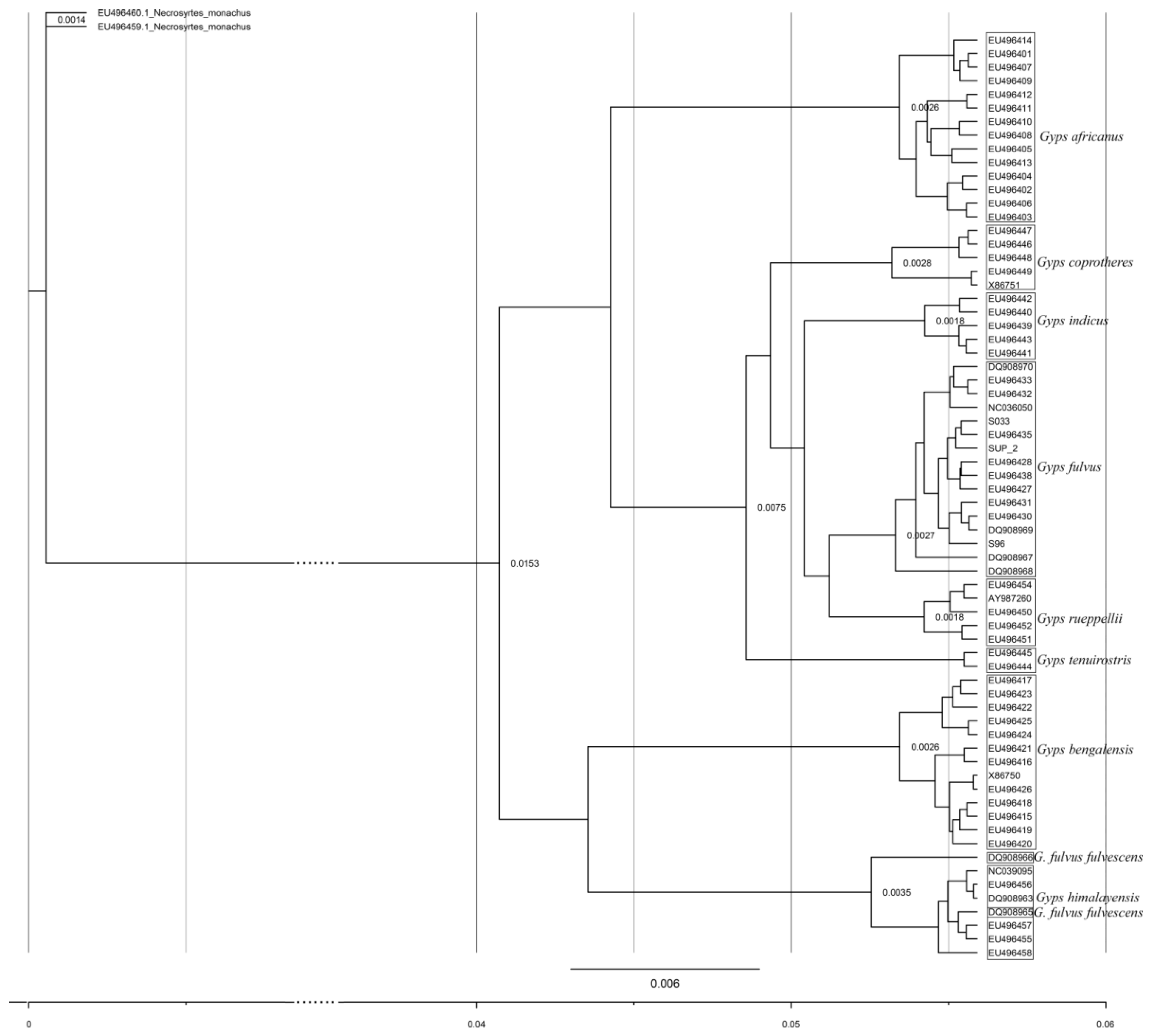
The
Gyps genus phylogenetic tree of
Cytb lineages based on Bayesian tree analysis is supported by high posterior probability for each node and in concordance with previously published results based on different mtDNA genes [
16,
25]. Both the haplotype network and phylogeny confirm genealogical relationships between different mtDNA lineages of the same species and between sister species of
Gyps genus. Haplotype networks also reflect the demographic history of the species indicating a rapid diversification [
16], and it can be observed that the species that underwent pronounced demographic decline exhibit lower numbers of haplotypes, as can be seen for Indian vulture and Slender-billed vulture [
25]. The origin of the Griffon vulture species is dated to about 2–3 million years ago (Mya) and proposed times of differentiation between private mtDNA lineages obtained from our analysis could be traced to around 358–644 kya.
Population genetic variability of any given species is shaped by its distant and recent history, demography and biogeography. In highly philopatric, gregarious, monogamous species, such as the Griffon vulture, behavior may be a factor that also shapes genetic variability. As already demonstrated in almost all analyzed vulture species, human-induced demographic declines significantly influence standing genetic variation reflected in the loss of diversity, inbreeding depression and presence of population-specific genetic variants [
93]. All these must be taken into account when defining the appropriate scale and subunits for conservation management as well as making restocking and population restoration strategies. Large-scale genetic analyses of different markers as well as in situ evaluation of overall general population health must be taken into consideration when conservation strategies are made.
European Griffon vulture populations are the subject of several active restocking programs with variable success (reviewed in [
3]). Successful reintroduction in its historic habitats in France encouraged many other conservation and reintroduction initiatives worldwide. The French Griffon vulture population is stable and thriving, and genetically similar to the Spanish population from which most individuals used for restocking descend [
5]. However, an example from Sardinia, where the successful reintroduction of individuals of Spanish descent was performed, showed significant change in native genetic diversity due to the import of foreign haplotypes and elicits extreme caution in the selection of source population and individual birds for restocking purposes [
3,
28]. Finally, failed attempts of repopulation in Bulgaria with individuals from Spain demonstrate that adaptation to territory and environmental conditions such as climate is an important factor that must be taken into consideration when reintroduction efforts are made [
3].