1. Introduction
Heart failure (HF) is a complex clinical syndrome with symptoms and signs that result from any structural or functional impairment of ventricular filling or ejection of blood. Its prevalence is rapidly growing and is becoming a serious public health issue, a true “epidemic” [
1]. Patients with previously diagnosed HF are at greater risk for subsequent morbidity and mortality when hospitalized for an acute myocardial infarction (AMI) in comparison with those who do not have this clinical syndrome [
2,
3]. Outcomes in HF patients presenting with AMI are improved by a strategy of early revascularization [
4], but emergency CABGs are associated with significantly higher adverse outcomes [
5]. In recent years, the proportion of emergent CABG in STEMI compared to NSTEMI hospitalization has decreased, but these patients have experienced a rise in noncardiac acute organ injury and cardiogenic shock [
6]. The purpose of our study was to describe the time trend of the incidence of emergent CABG in patients with and without HF, the clinical characteristics, outcomes, and the risk factors for the mortality of surgical revascularization in the short and medium term.
2. Patients and Methods
2.1. Study Population
This was a single-center retrospective observational study of patients who underwent isolated emergency CABG from January 2009 to January 2020. The data were extracted from the Institutional Database and Reimbursement Registry, regarding patients with AMI as the primary diagnosis (International Classification of Diseases 9.0 Clinical Modification [ICD-9CM] 410.x and ICD-10CM I22.x-22.x). Patients with concomitant valvular disease warranting surgery were excluded as well as patients below 18 years of age. The emergent status of the operation was defined according to the STS criteria: patients who had ongoing, refractory, unrelenting cardiac compromise, with or without hemodynamic instability, who were not responsive to any form of therapy except cardiac surgery [
7]. The population was divided into two groups, patients without pre-existing heart failure diagnosis (No HF) and patients with pre-existing heart failure diagnosis (HF). The definition of HF followed the most recent ESC 2021 guidelines [
8].
2.2. Clinical Outcomes
Clinical outcomes included major events, such as 30-day death, cerebrovascular events, new-onset acute renal failure, new-onset atrial fibrillation, bleeding requiring surgical re-exploration, and also the main postoperative features of surgical patients.
2.3. Data Collection and
Followup
Data were acquired anonymously from the institutional database. Due to the retrospective nature of the study, the IRB waived the informed consent.
2.4. Statistical Analysis
Data are presented as the mean ± standard deviation for normal or the median for nonnormal distribution. Continuous variables were tested with the Kolmogorov–Smirnov test for normality. Categorical variables are given as frequencies and percentages. The Wilcoxon rank sum test, Pearson’s chi-square test, and Fisher’s exact test were used to compare the categorical variables between patients with heart failure and those without heart failure. To reduce the treatment selection bias and potential confounding factors and to adjust for significant differences in patient characteristics, the propensity score-matching was performed.
The propensity scores were estimated using a multivariate logistic regression model for receiving emergent CABG in patients with and without heart failure. All baseline characteristics were entered to calculate the propensity score. A local optimal algorithm with the caliper method was used for the development of the propensity score-matched pairs without replacement (1:1 match), (area under the curve of 0.78). A matching caliper of 0.2 standard deviations of the logit of the estimated propensity score was enforced to ensure that matches of poor fit were excluded. The matching procedure was performed by using the R MatchIt package (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria). After the propensity score-matching, the covariates were compared as described before.
Univariate logistic regression analysis was performed to assess the association between the patient baseline characteristics and postoperative features with in-hospital death. All variables with p < 0.2 entered the multivariable logistic regression analysis to identify an association with in-hospital death.
Survival analysis was conducted using the Kaplan–Meier method, and comparison by the presence of heart failure (No HF vs. HF) was tested using the log-rank test.
Statistical significance was assumed when the null hypothesis could be rejected at p < 0.05. All P values reflect the results of two-sided tests. Statistical analyses were conducted using R (version 3.6.2).
4. Discussion
Ischemic coronary artery disease (CAD) remains the leading cause of mortality globally due to sudden death and heart failure (HF). In recent years, the number of patients undergoing coronary angiography increased substantially per year driven as indicated by HF, more than angina pectoris or STEMI [
9].
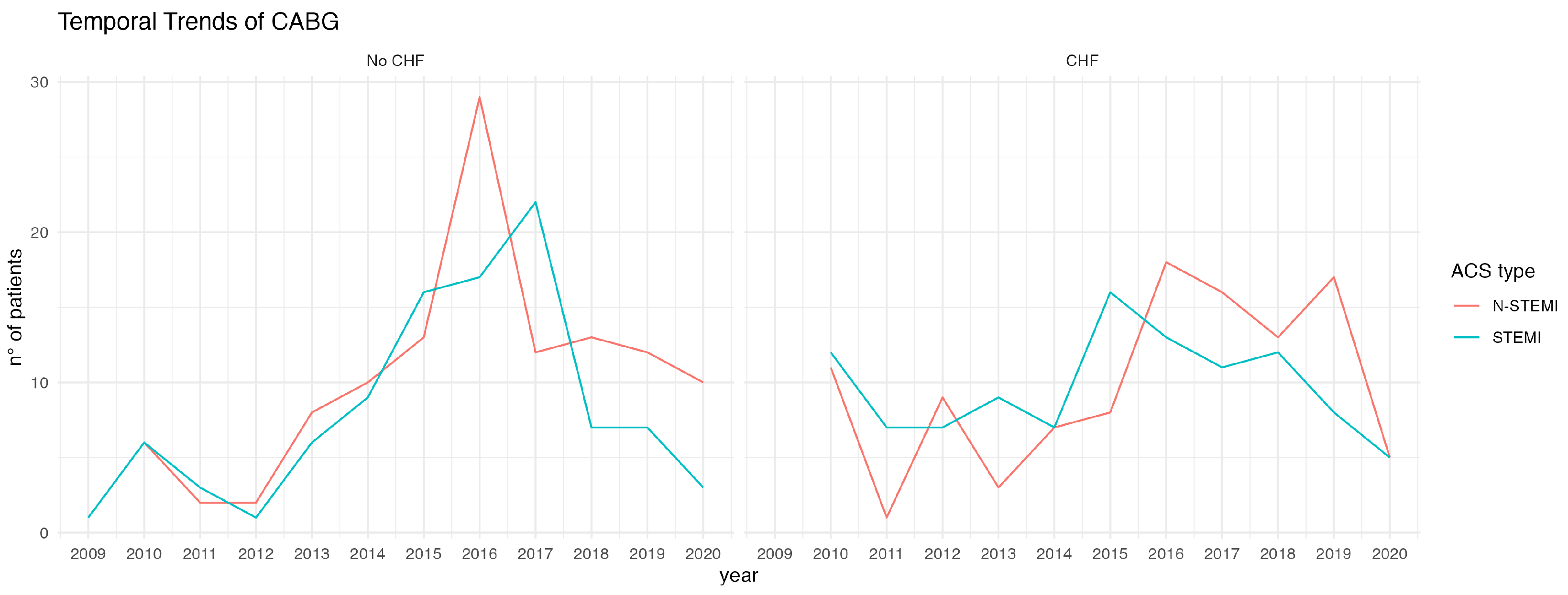
In a recent National Inpatient Sample (NIS) analysis of the time trend and outcome of patients undergoing emergency surgical revascularization, the incidence of chronic heart failure on admission did not change in the three eras examined, ranging from 28.1% to a 30.7% [
6].
The cornerstone of therapy in acute coronary syndromes is early revascularization [
4,
5,
10].
Revascularization with CABG is associated with better long term prognosis, lower incidence of cardiovascular death and myocardial infarction, along with less repeated revascularization, compared to percutaneous interventions (PCI) [
11]. The superiority of CABG over PCI is particularly evident in patients with severely reduced left ventricular function [
12].
In this retrospective work we compared, using propensity-score matching, a sample of patients diagnosed with chronic heart failure compared with a control sample who needed emergent surgical revascularization.
We saw that the effect was a good immediate outcome with low mortality (2.8%) in both groups, which were comparable to each other.
This finding was certainly an improvement over data from studies reporting an in-hospital mortality of between 7.4% and 8.7% [
4,
10] and others reporting a progressive decline in such mortality in more recent years but with an overall figure of 6.6% [
5].
In our study, patients with chronic heart failure had almost twice the incidence of cardiogenic shock; this figure correlated with the observed increased need for mechanical circulatory support (MCS), up to 25%.
This finding correlated with those in the large study by Patlolla et al., where the incidence of cardiogenic shock in patients undergoing emergency CABG has increased over time, from 6.4% to 11.5%, with mechanical circulatory support being used in about 20% of subjects [
6]. Furthermore, therapies to provide hemodynamic support are increasingly being used with success in the context of acute myocardial infarction [
13].
This occurrence identified these patients as an increased high-risk group, as evidenced by the significant association of MCS with in-hospital mortality. The very nature of the acute coronary syndrome, associated in some patients in our study with cardiogenic shock with the need for peri- and postoperative circulatory support, resulted in a higher critical status after surgery; in fact, these patients had a higher incidence of acute renal failure, both from low flow and related to MCS, with longer ICU and hospital stay times. These data were in keeping with other studies in which cardiogenic shock was burdened with high mortality and correlated with the onset of renal failure and atrial fibrillation [
14].
An interesting finding from the analysis at followup is that patients with chronic heart failure had a higher mortality, already evident from the third year onward; this trend continued steadily upward, until it almost doubled at 12 years.
When we considered the type of acute coronary syndrome, we saw a bimodal mortality trend. In the immediate perioperative period, presentation with STEMI had a trend of association with mortality; the achieved nonstatistical significance was probably due to the presence in our center’s territory of a cardiology network for heart attack that leads to early diagnosis and equally rapid revascularization [
15].
This is in contrast to other work that has identified STEMI as a factor associated with in-hospital mortality [
16].
Following an established trend in recent years, NSTEMI are increasing and, given the characteristics of the greater chronic morbidity of these patients, their prognosis is also worse [
3,
4]. In fact, we observed a stable increase in the mortality of such subjects as early as the fifth year post-event to more than double at the end of the followup period considered. This trend is driven by the unfavorable prognosis in the medium term of patients with chronic heart failure and acute coronary syndrome NSTEMI, whose association has a greater negative weight than age itself in the determinism of the death event.
5. Limitations and Future
Directions
The present study had as limitations the retrospective observational nature in which the imbalance of covariates was mitigated by the use of propensity-score matching; this method, although commonly agreed upon and effective, significantly reduced the sample size and inherently resulted in data loss in order to achieve “pseudo-randomization”.
The choice to refer patients for emergency revascularization depends heavily on the Heart Team’s discussion performed and the professionals involved. In future studies, it will be necessary to determine on the basis of these data as well which timing is most appropriate for surgical revascularization in the context of AMI.
The study also considered the period of onset of the Sars-CoV2 pandemic, which drastically reduced the sample size and thus, although this trend was common worldwide, it may have partially affected the results on the time course. With regard to followup, a mortality analysis was conducted based on the census status of the patients considered; other events such as heart failure recurrence as a risk factor for mortality were not considered; this event was beyond the scope of the present study but of high interest, and it will need to be addressed in future studies, also based on the results of the present scientific work.