Potential Role of Tumor-Derived Exosomes in Non-Small-Cell Lung Cancer in the Era of Immunotherapy
Abstract
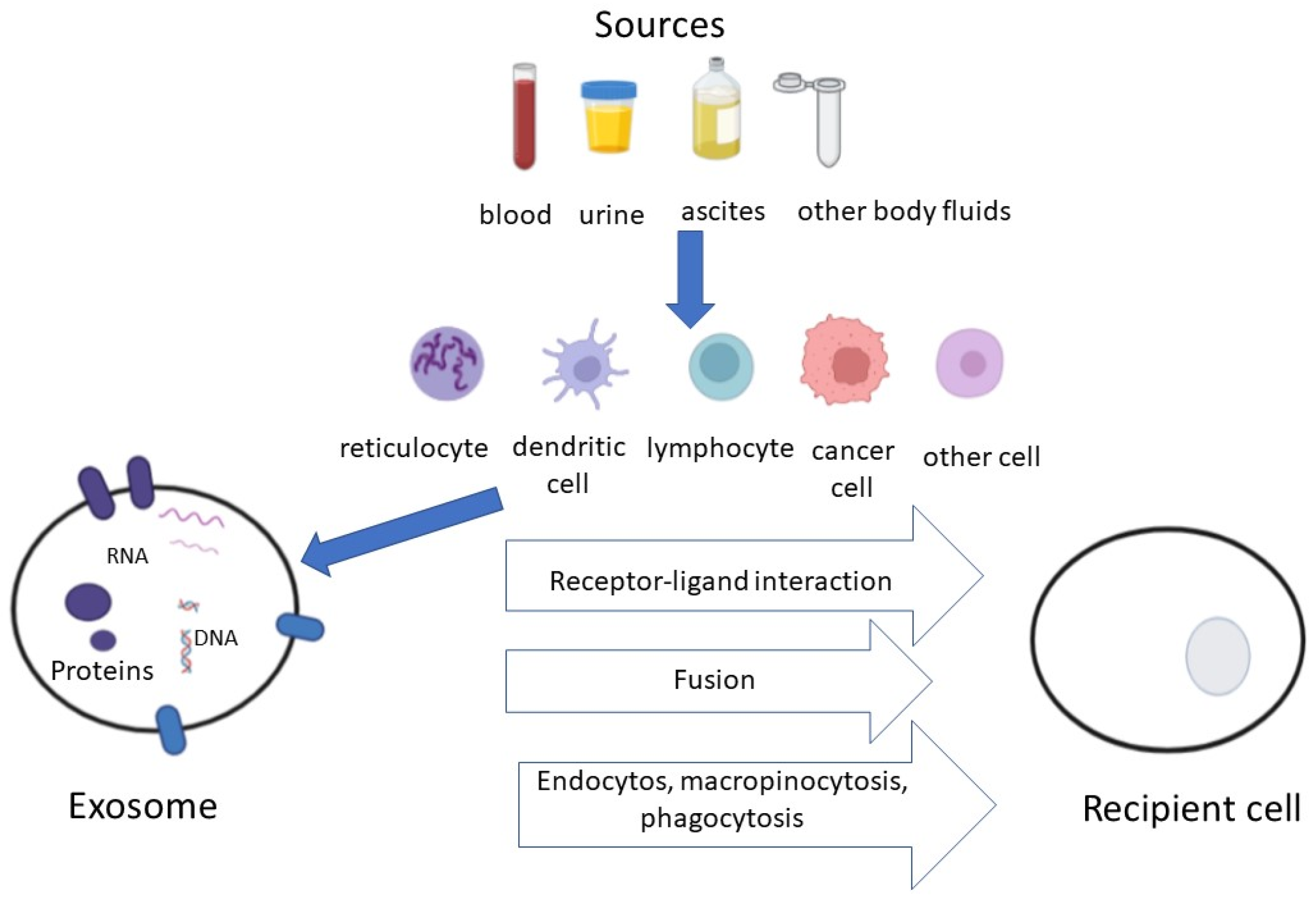
:1. Introduction
2. Exosomes and Tumor-Derived Exosomes
3. Methods for the Isolation of Exosomes
4. Tumor-Derived Exosomes/Micro-RNAS and Immunotherapy: Data from the Literature
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck, M.; Remon, J.; Hellmann, M.D. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced squamous cell non small cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Tartarone, A.; Roviello, G.; Lerose, R.; Roudi, R.; Aieta, M.; Zoppoli, P. Anti-PD-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: A meta-analysis. Future Oncol. 2019, 15, 2423–2433. [Google Scholar] [CrossRef]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M. The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: Formation of exosomes as a mechanism for shedding membrane proteins. Biochem. Cell Biol. 1992, 70, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Rogers, K. “Exosome”. Encyclopedia Britannica. 8 September 2020. Available online: https://www.britannica.com/science/exosome (accessed on 20 September 2022).
- Zheng, H.; Zhan, Y.; Liu, S.; Lu, J.; Luo, J.; Feng, J.; Fan, S. The roles of tumor-derived exosomes in non-small cell lung cancer and their clinical implications. J. Exp. Clin. Cancer Res. 2018, 37, 226. [Google Scholar] [CrossRef] [PubMed]
- Laurenzana, I.; Lamorte, D.; Trino, S.; De Luca, L.; Ambrosino, C.; Zoppoli, P.; Ruggieri, V.; Del Vecchio, L.; Musto, P.; Caivano, A.; et al. Extracellular vesicles: A new prospective in crosstalk between microenvironment and stem cells in hematological malignancies. Stem Cells Int. 2018, 27, 9863194. [Google Scholar] [CrossRef] [PubMed]
- Del Re, M.; van Schaik, R.H.N.; Fogli, S.; Mathijssen, R.H.J.; Cucchiara, F.; Capuano, A.; Scavone, C.; Jenster, G.W.; Danesi, R. Blood-based PD-L1 analysis in tumor-derived extracellular vesicles: Applications for optimal use of anti-PD-1/PD-L1 axis inhibitors. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188463. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Amreddy, N.; Razaq, M.; Towner, R.; Zhao, Y.D.; Ahmed, R.A.; Munshi, A.; Ramesh, R. Exosomes as theranostics for lung cancer. Adv. Cancer Res. 2018, 139, 1–33. [Google Scholar]
- Cossu, A.M.; Scrima, M.; Lombardi, A.; Grimaldi, A.; Russo, M.; Ottaiano, A.; Caraglia, M.; Bocchetti, M. Future directions and management of liquid biopsy in non-small cell lung cancer. Explor. Target. Antitumor Ther. 2020, 1, 239–252. [Google Scholar] [CrossRef]
- Rasihashemi, S.Z.; Rezazadeh Gavgani, E.; Majidazar, R.; Seraji, P.; Oladghaffari, M.; Kazemi, T.; Lotfinejad, P. Tumor-derived exosomal PD-L1 in progression of cancer and immunotherapy. J. Cell. Physiol. 2022, 237, 1648–1660. [Google Scholar] [CrossRef]
- van Doormaal, F.F.; Kleinjan, A.; Di Nisio, M.; Büller, H.R.; Nieuwland, R. Cell-derived microvesicles and cancer. Neth. J. Med. 2009, 67, 266–273. [Google Scholar]
- Xu, K.; Zhang, C.; Du, T.; Gabriel, A.N.A.; Wang, X.; Li, X.; Sun, L.; Wang, N.; Jiang, X.; Zhang, Y. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed. Pharmacother. 2021, 134, 111111. [Google Scholar] [PubMed]
- Taverna, S.; Giallombardo, M.; Gil-Bazo, I.; Carreca, A.P.; Castiglia, M.; Chacártegui, J.; Araujo, A.; Alessandro, R.; Pauwels, P.; Peeters, M.; et al. Exosomes isolation and characterization in serum is feasible in non-small cell lung cancer patients: Critical analysis of evidence and potential role in clinical practice. Oncotarget 2016, 7, 28748–28760. [Google Scholar] [PubMed]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [PubMed] [Green Version]
- Kim, D.H.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Lee, J.E.; Sung, K.J.; Sung, Y.H.; Pack, C.G.; Jung, M.K.; Han, B.; et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [PubMed] [Green Version]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 2019, 177, 414–427. [Google Scholar]
- Gao, J.; Qiu, X.; Li, X.; Fan, H.; Zhang, F.; Lv, T.; Song, Y. Expression profiles and clinical value of plasma exosomal Tim-3 and Galectin-9 in non small cell lung cancer. Biochem. Biophys. Res. Commun. 2018, 498, 409–415. [Google Scholar]
- Fan, Y.; Che, X.; Qu, J.; Hou, K.; Wen, T.; Li, Z.; Li, C.; Wang, S.; Xu, L.; Liu, Y.; et al. Exosomal PD-L1 retains immunosuppressive activity and is associated with gastric cancer prognosis. Ann. Surg. Oncol. 2019, 26, 3745–3755. [Google Scholar]
- Suzuki, H.I.; Katsura, A.; Matsuyama, H.; Miyazono, K. MicroRNA regulons in tumor microenvironment. Oncogene 2015, 34, 3085–3094. [Google Scholar]
- Huang, W.; Yan, Y.; Liu, Y.; Lin, M.; Ma, J.; Zhang, W.; Dai, J.; Li, J.; Guo, Q.; Chen, H.; et al. Exosomes with low miR-34c-3p expression promote invasion and migration of non-small cell lung cancer by upregulating integrin α2β1. Signal Transduct. Target. Ther. 2020, 5, 39. [Google Scholar]
- Liu, Q.; Yu, Z.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Xiang, Y.; Wu, N.; Wu, L.; Bai, L.; et al. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget 2017, 8, 13048–13058. [Google Scholar]
- Cazzoli, R.; Buttitta, F.; Di Nicola, M.; Malatesta, S.; Marchetti, A.; Rom, W.N.; Pass, H.I. microRNAs derived from circulating exosomes as noninvasive biomarkers for screening and diagnosing lung cancer. J. Thorac. Oncol. 2013, 8, 1156–1162. [Google Scholar] [PubMed] [Green Version]
- Marconi, S.; Croce, M.; Chiorino, G.; Rossi, G.; Guana, F.; Profumo, A.; Ostano, P.; Alama, A.; Longo, L.; De Luca, G.; et al. A Circulating risk score, based on combined expression of exo-miR-130a-3p and fibrinopeptide, A.; as predictive biomarker of relapse in resectable Non-Small Cell Lung Cancer patients. Cancers 2022, 14, 3412. [Google Scholar] [PubMed]
- Li, X.; Chen, C.; Wang, Z.; Liu, J.; Sun, W.; Shen, K.; Lv, Y.; Zhu, S.; Zhan, P.; Lv, T.; et al. Elevated exosome-derived miRNAs predict osimertinib resistance in non-small cell lung cancer. Cancer Cell Int. 2021, 21, 428. [Google Scholar] [CrossRef] [PubMed]
- Rasihashemi, S.; Sahrai, H.; Rezazadeh-Gavgani, E.; Yazdani, Y.; Khalaji, A.; Lotfinejad, P. Exosomes carrying immune checkpoints, a promising therapeutic approach in cancer treatment. Med. Oncol. 2022, 39, 183. [Google Scholar] [CrossRef] [PubMed]
- Janse van Rensburg, H.J.; Spiliopoulou, P.; Siu, L.L. Circulating biomarkers for therapeutic monitoring of anti-cancer agents. Oncologist 2022, 27, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cai, H.; Deng, R.; Cheng, J.; Shi, Y. Effects of exosomes on tumor immunomodulation and their potential clinical applications. Int. J. Oncol. 2022, 61, 147. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, H.; Zhang, H.; Han, Y. The role of exosomal PD-L1 in tumor immunotherapy. Transl. Oncol. 2021, 14, 101047. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, M.; Gu, J.; Niu, K.; Zhao, X.; Zheng, L.; Xu, Z.; Yu, Y.; Li, F.; Meng, L.; et al. Novel biomarkers of dynamic blood PD-L1 expression for immune checkpoint inhibitors in advanced non-small-cell lung cancer patients. Front. Immunol. 2021, 12, 665133. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Zhi, C.; Liang, W.; Wang, X.; Chen, X.; Lv, T.; Shen, Q.; Song, Y.; Lin, D.; et al. Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients. J. Transl. Med. 2019, 17, 355. [Google Scholar] [CrossRef] [Green Version]
- Del Re, M.; Marconcini, R.; Pasquini, G.; Rofi, E.; Vivaldi, C.; Bloise, F.; Restante, G.; Arrigoni, E.; Caparello, C.; Bianco, M.G.; et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br. J. Cancer 2018, 118, 820–824. [Google Scholar] [CrossRef] [Green Version]
- Okuma, Y.; Wakui, H.; Utsumi, H.; Sagawa, Y.; Hosomi, Y.; Kuwano, K.; Homma, S. Soluble programmed cell death ligand 1 as a novel biomarker for nivolumab therapy for non-small-cell lung cancer. Clin. Lung Cancer 2018, 19, 410–417. [Google Scholar] [CrossRef] [PubMed]
- de Miguel-Perez, D.; Russo, A.; Arrieta, O.; Ak, M.; Barron, F.; Gunasekaran, M.; Mamindla, P.; Lara-Mejia, L.; Peterson, C.B.; Er, M.E.; et al. Extracellular vesicle PD-L1 dynamics predict durable response to immune-checkpoint inhibitors and survival in patients with non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2022, 41, 186. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.X.; Yu, R.; Wu, X.; Wu, S.Y.; Pi, C.; Chen, Z.H.; Zhang, X.C.; Gao, C.Y.; Shao, Y.W.; Liu, L.; et al. Correlation of plasma exosomal microRNAs with the efficacy of immunotherapy in EGFR/ALK wild-type advanced non-small cell lung cancer. J. Immunother. Cancer 2020, 8, e000376. [Google Scholar] [PubMed] [Green Version]
- Halvorsen, A.R.; Sandhu, V.; Sprauten, M.; Flote, V.G.; Kure, E.H.; Brustugun, O.T.; Helland, Å. Circulating microRNAs associated with prolonged overall survival in lung cancer patients treated with nivolumab. Acta Oncol. 2018, 57, 1225–1231. [Google Scholar] [CrossRef] [Green Version]
- Boeri, M.; Milione, M.; Proto, C.; Signorelli, D.; Lo Russo, G.; Galeone, C.; Verri, C.; Mensah, M.; Centonze, G.; Martinetti, A.; et al. Circulating miRNAs and PD-L1 tumor expression are associated with survival in advanced NSCLC patients treated with immunotherapy: A prospective study. Clin. Cancer Res. 2019, 25, 2166–2173. [Google Scholar] [CrossRef] [Green Version]
- Pantano, F.; Zalfa, F.; Iuliani, M.; Simonetti, S.; Manca, P.; Napolitano, A.; Tiberi, S.; Russano, M.; Citarella, F.; Foderaro, S.; et al. Large-scale profiling of extracellular vesicles identified miR-625-5p as a novel biomarker of immunotherapy response in advanced non-small-cell lung cancer patients. Cancers 2022, 14, 2435. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, W.; Xu, K.; Zheng, X.; Zhou, Y.; Luo, M.; Yan, C.; Zheng, X.; Jin, E. Blood exosome PD-L1 is associated with PD-L1 expression measured by immunohistochemistry, and lymph node metastasis in lung cancer. Tissue Cell 2022, 79, 101941. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, Y.; Che, X.; Zhang, M.; Li, Z.; Li, C.; Wang, S.; Wen, T.; Hou, K.; Shao, X.; et al. Anti-PD-1 therapy response predicted by the combination of exosomal PD-L1 and CD28. Front. Oncol. 2020, 10, 760. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Serratì, S.; Guida, M.; Di Fonte, R.; De Summa, S.; Strippoli, S.; Iacobazzi, R.M.; Quarta, A.; De Risi, I.; Guida, G.; Paradiso, A.; et al. Circulating extracellular vesicles expressing PD1 and PD-L1 predict response and mediate resistance to checkpoint inhibitors immunotherapy in metastatic melanoma. Mol. Cancer 2022, 21, 20. [Google Scholar] [CrossRef]
- Cordonnier, M.; Nardin, C.; Chanteloup, G.; Derangere, V.; Algros, M.P.; Arnould, L.; Garrido, C.; Aubin, F.; Gobbo, J. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J. Extracell. Vesicles 2020, 9, 1710899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodoraki, M.N.; Yerneni, S.; Gooding, W.E.; Ohr, J.; Clump, D.A.; Bauman, J.E.; Ferris, R.L.; Whiteside, T.L. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology 2019, 8, 1593805. [Google Scholar] [CrossRef] [PubMed]
- Galbo, P.M., Jr.; Ciesielski, M.J.; Figel, S.; Maguire, O.; Qiu, J.; Wiltsie, L.; Minderman, H.; Fenstermaker, R.A. Circulating CD9+/GFAP+/survivin+ exosomes in malignant glioma patients following survivin vaccination. Oncotarget 2017, 8, 114722–114735. [Google Scholar] [CrossRef]
- Rolfo, C. Exosomal Proteins in Lung Cancer: The Last Frontier in Liquid Biopsies. J. Thorac. Oncol. 2016, 11, 1609–1611. [Google Scholar] [CrossRef]
| Effect | Mechanism |
|---|---|
| Improvement of cellular survival | Escape from apoptosis |
| Escape from immune-surveillance | |
| Angiogenesis | Activation of coagulation system |
| Transfer of mRNA coding for growth factors | |
| Metastasis | Transfer of oncogenes |
| Matrix metalloproteinase (MMP) activity |
| Diagnosis [15] |
| Prognosis [28,31,32] |
| Monitoring therapeutic efficacy [36] |
| Drug delivery system [35,37] |
| Vaccine [35,37] |
| Methods | Advantages | Disadvantages |
|---|---|---|
| Ultracentrifugation | The gold standard for protein detection | Time-consuming Exosomes may be damaged |
| Ultrafiltration | Short processing time and easy procedure | Small sample volume limitations Protein contamination |
| Chromatography | Suitable for isolation from complex biofluids | Time-consuming Small sample volume limitations |
| Density gradient centrifugation | High yield | Time-consuming No absolute separation of vesicles subpopulations |
| Immunomagnetic beads | High isolation purity | Only for exosomes with specific markers |
| Size-exclusion chromatography | High isolation purity | Poor selectivity compared to other chromatographic techniques |
| Microfluidic chip | High recovery and purity Short time-consuming | Requires sophisticated technology |
| Microfluidic method combined with immune separation | Simultaneous separation extraction, purification and targeted protein analysis | Only for exosomes with specific markers |
| Author | Ref. | exomiRNAs | TDEs |
|---|---|---|---|
| Huang | [30] | miR-34c-3p | |
| Liu | [31] | miR-23b-3p, miR-10b-5p, | |
| miR-21-5p | |||
| Cazzoli | [32] | miR-378-a, miR-379, etc. | |
| Marconi | [33] | miR-130a-3p | |
| (in association with fibrinopeptide A) | |||
| Li | [34] | miR-184, miR-3913-5p | |
| Yang | [39] | PD-L1 mRNA, exoPD-L1 | |
| Li | [40] | exoPD-L1 | |
| Del Re | [41] | PD-L1 mRNA | |
| de Miguel-Perez | [43] | exoPD-L1 | |
| Peng | [44] | has-miR-320b/-320c/-320d, | |
| has-miR-125b-5p | |||
| Halvorsen | [45] | miR-215-5p, miR-411-3p, miR-493-5p, | |
| miR-494-3p, miR-495-3p, etc. | |||
| Pantano | [47] | miR-625-5p | |
| Zhang Z | [48] | exoPD-L1 | |
| Zhang C | [49] | exoPD-L1 | |
| (in association with CD28) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tartarone, A.; Lerose, R.; Tartarone, M.; Aieta, M. Potential Role of Tumor-Derived Exosomes in Non-Small-Cell Lung Cancer in the Era of Immunotherapy. Life 2022, 12, 2104. https://doi.org/10.3390/life12122104
Tartarone A, Lerose R, Tartarone M, Aieta M. Potential Role of Tumor-Derived Exosomes in Non-Small-Cell Lung Cancer in the Era of Immunotherapy. Life. 2022; 12(12):2104. https://doi.org/10.3390/life12122104
Chicago/Turabian StyleTartarone, Alfredo, Rosa Lerose, Marina Tartarone, and Michele Aieta. 2022. "Potential Role of Tumor-Derived Exosomes in Non-Small-Cell Lung Cancer in the Era of Immunotherapy" Life 12, no. 12: 2104. https://doi.org/10.3390/life12122104
APA StyleTartarone, A., Lerose, R., Tartarone, M., & Aieta, M. (2022). Potential Role of Tumor-Derived Exosomes in Non-Small-Cell Lung Cancer in the Era of Immunotherapy. Life, 12(12), 2104. https://doi.org/10.3390/life12122104





