Computational Model Exploring Characteristic Pattern Regulation in Periventricular Vessels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and In Vivo Experiment
2.2. Immunohistochemistry and Imaging Analysis
2.3. Numerical Simulations
3. Modeling
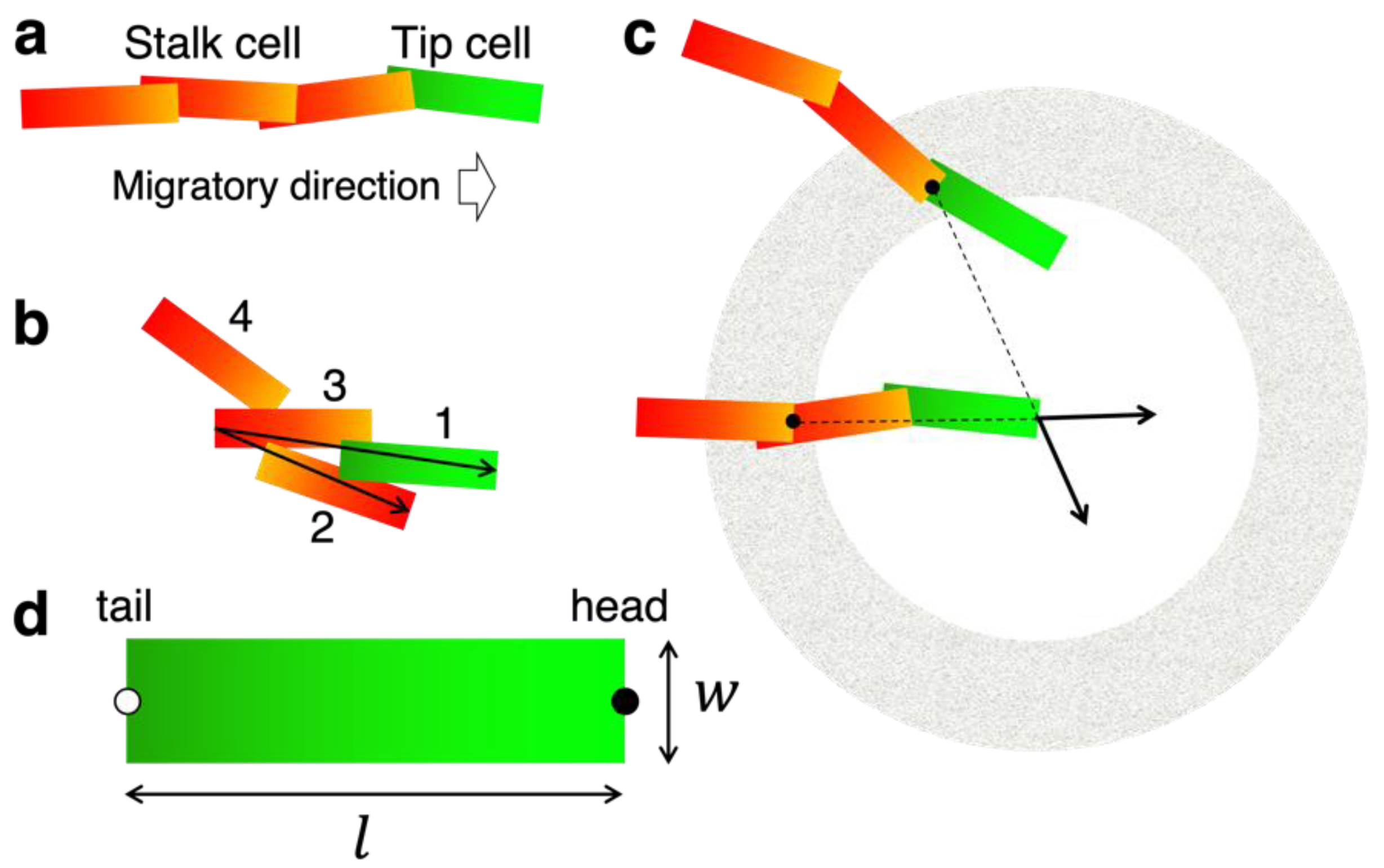
3.1. Concept and Rational of EC Model
- 4.
- Branch formation occurs by chance.
- 5.
- Tip cells may undergo directed vertical migration by chemotaxis;
- 6.
- Tip cells may receive repulsive guidance from other ECs.
3.2. Construction of the EC Model
4. Results
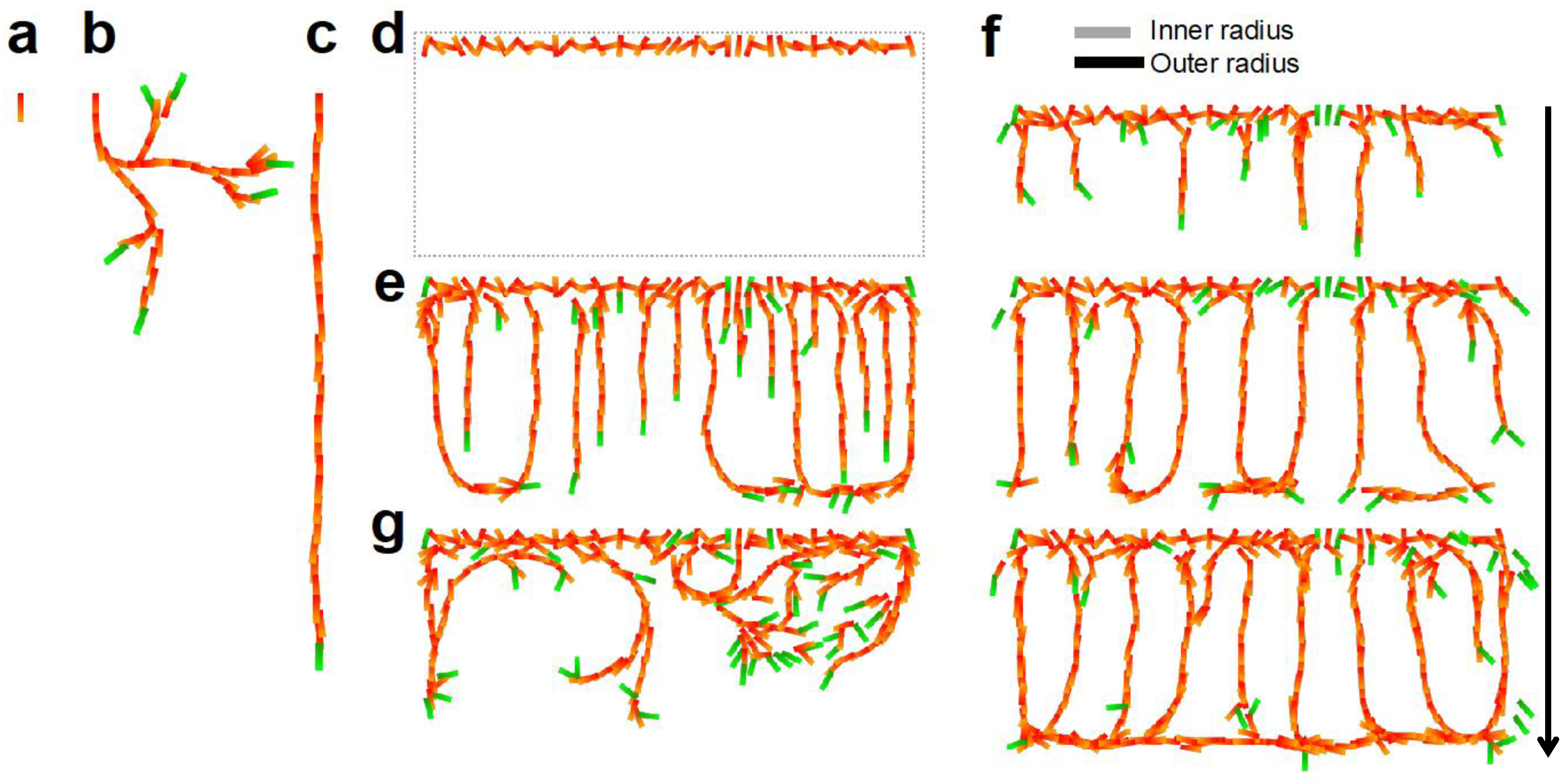
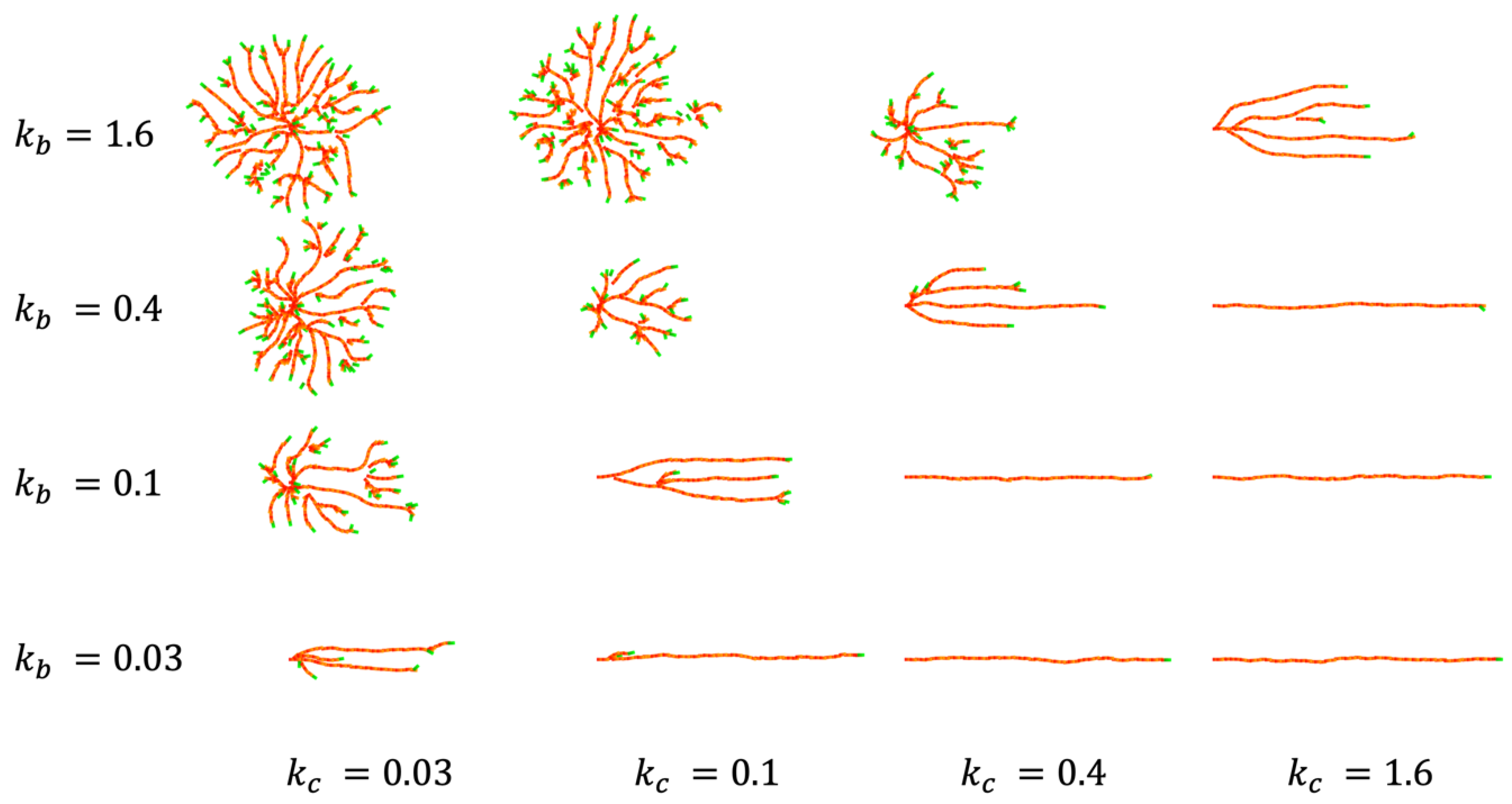
4.1. CP Vessel Pattern
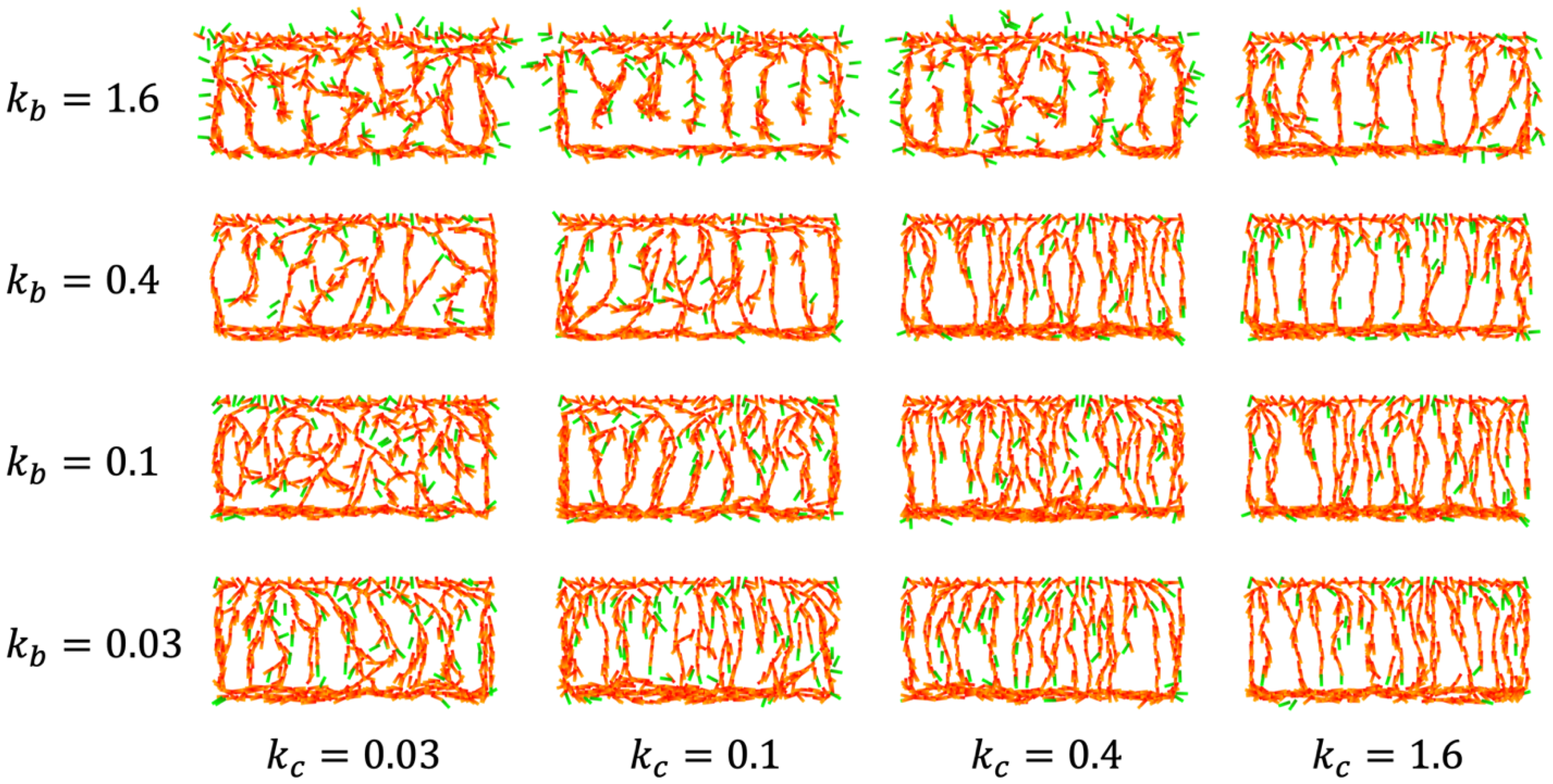
4.2. IZ/SVZ Vessel Patterning
4.3. Effect of Impaired SP Function
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicosia, R.F.; Bonanno, E.; Smith, M. Fibronectin Promotes the Elongation of Microvessels during Angiogenesis In Vitro. J. Cell. Physiol. 1993, 154, 65–661. [Google Scholar] [CrossRef] [PubMed]
- Geudens, I.; Gerhardt, O. Coordinating cell behaviour during blood vessel formation. Development 2011, 138, 4569–4583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsson, L.; Kreuger, J.; Claesson-Welsh, L. Building blood vessels—Stem cell models in vascular biology. J. Cell Biol. 2007, 177, 751–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arima, S.; Nishiyama, K.; Ko, T.; Arima, Y.; Hakozaki, Y.; Sugihara, K.; Koseki, H.; Uchijima, Y.; Kurihara, Y.; Kurihara, H. Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development 2011, 138, 4763–4776. [Google Scholar] [CrossRef] [Green Version]
- Götz, M.; Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 777–788. [Google Scholar] [CrossRef]
- Komabayashi-Suzuki, M.; Yamanishi, E.; Watanabe, C.; Okamura, M.; Tabata, H.; Iwai, R.; Ajioka, I.; Matsushita, J.; Kidoya, H.; Takakura, N.; et al. Spatiotemporally Dependent Vascularization Is Differently Utilized among Neural Progenitor Subtypes during Neocortical Development. Cell Rep. 2019, 29, 1113–1129.e5. [Google Scholar] [CrossRef] [Green Version]
- Melani, M.; Weinstein, B.M. Common factors regulating patterning of the nervous and vascular systems. Annu. Rev. Cell Dev. Biol. 2010, 26, 639–665. [Google Scholar] [CrossRef] [Green Version]
- Wälchli, T.; Wacker, A.; Frei, K.; Regli, L.; Schwab, M.E.; Hoerstrup, S.P.; Gerhardt, H.; Engelhardt, B. Wiring the Vascular Network with Neural Cues: A CNS Perspective. Neuron 2015, 87, 271–296. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, S.; Hampel, F.; Hata, K.; Del Toro, D.; Schwark, M.; Kvachnina, E.; Bastmeyer, M.; Yamashita, T.; Tarabykin, V.; Klein, R.; et al. FLRT2 and FLRT3 act as repulsive guidance cues for Unc5-positive neurons. EMBO J. 2011, 30, 2920–2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, T.J.; Finkelstein, E.B.; Cox, C.M. The role of FGF and VEGF in angioblast induction and migration during vascular development. Dev. Dyn. 2001, 220, 1–17. [Google Scholar] [CrossRef]
- Anderson, A.R.A.; Chaplain, M.A.J. Continuous and discrete mathematical models of tumor-induced angiogenesis. Bull. Math. Biol. 1998, 60, 857–899. [Google Scholar] [CrossRef] [PubMed]
- Köhn-Luque, A.; De Back, W.; Yamaguchi, Y.; Yoshimura, K.; Herrero, M.A.; Miura, T. Dynamics of VEGF matrix-retention in vascular network patterning. Phys. Biol. 2013, 10, 066007. [Google Scholar] [CrossRef] [PubMed]
- Travasso, R.D.M.; Poiré, E.C.; Castro, M.; Rodrguez-Manzaneque, J.C.; Hernández-Machado, A. Tumor angiogenesis and vascular patterning: A mathematical model. PLoS ONE 2011, 6, e19989. [Google Scholar] [CrossRef]
- Merks, R.M.H.; Glazier, J.A. Dynamic mechanisms of blood vessel growth. Nonlinearity 2006, 19, C1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, K.; Azami, T.; Otsu, A.; Takase, H.; Ishitobi, H.; Tanaka, J.; Miwa, Y.; Takahashi, S.; Ema, M. Study of normal and pathological blood vessel morphogenesis in Flt1-tdsRed BAC Tg mice. Genesis 2012, 50, 561–571. [Google Scholar] [CrossRef]
- Ohtaka-Maruyama, C.; Okamoto, M.; Endo, K.; Oshima, M.; Kaneko, N.; Yura, K.; Okado, H.; Miyata, T.; Maeda, N. Synaptic transmission from subplate neurons controls radial migration of neocortical neurons. Science 2018, 360, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Marin-Padilla, M. Early vascularization of the embryonic cerebral cortex: Golgi and electron microscopic studies. J. Comp. Neurol. 1985, 241, 237–249. [Google Scholar] [CrossRef]
- Eilken, H.M.; Adams, R.H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010, 22, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Ruhrberg, C.; Gerhardt, H.; Golding, M.; Watson, R.; Ioannidou, S.; Fujisawa, H.; Betsholtz, C.; Shima, D.T. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002, 16, 2684–2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takubo, N.; Yura, F.; Naemura, K.; Yoshida, R.; Tokunaga, T.; Tokihiro, T.; Kurihara, H. Cohesive and anisotropic vascular endothelial cell motility driving angiogenic morphogenesis. Sci. Rep. 2019, 9, 9304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wälchli, T.; Mateos, J.M.; Weinman, O.; Babic, D.; Regli, L.; Hoerstrup, S.P.; Gerhardt, H.; Schwab, M.E.; Vogel, J. Quantitative assessment of angiogenesis, perfused blood vessels and endothelial tip cells in the postnatal mouse brain. Nat. Protoc. 2015, 10, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Herbert, S.P.; Stainier, D.Y.R. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 551–564. [Google Scholar] [CrossRef] [Green Version]
- Jakobsson, L.; Franco, C.A.; Bentley, K.; Collins, R.T.; Ponsioen, B.; Aspalter, I.M.; Rosewell, I.; Busse, M.; Thurston, G.; Medvinsky, A.; et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010, 12, 943–953. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Aguilar, E.; Friedlander, M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3500–3510. [Google Scholar]
- Eichmann, A.; Makinen, T.; Alitalo, K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005, 19, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Le Noble, F.; Yuan, L.; Jiang, Q.; De Lafarge, B.; Sugiyama, D.; Bréant, C.; Claes, F.; De Smet, F.; Thomas, J.L.; et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature 2004, 432, 179–186. [Google Scholar] [CrossRef]
- Nesmith, J.E.; Chappell, J.C.; Cluceru, J.G.; Bautch, V.L. Blood vessel anastomosis is spatially regulated by Flt1 during angiogenesis. Development 2017, 144, 869–889. [Google Scholar] [CrossRef] [Green Version]
- Blinder, Y.J.; Freiman, A.; Raindel, N.; Mooney, D.J.; Levenberg, S. Vasculogenic dynamics in 3D engineered tissue constructs. Sci. Rep. 2015, 5, 17840. [Google Scholar] [CrossRef] [Green Version]
- Lenard, A.; Ellertsdottir, E.; Herwig, L.; Krudewig, A.; Sauteur, L.; Belting, H.G.; Affolter, M. In vivo analysis reveals a highly stereotypic morphogenetic pathway of vascular anastomosis. Dev. Cell 2013, 25, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.B.; Darden, J.; Suarez-Martinez, A.D.; Zhao, H.; Hendricks, A.; Hartland, C.; Chong, D.; Kushner, E.J.; Murfee, W.L.; Chappell, J.C. Pericyte migration and proliferation are tightly synchronized to endothelial cell sprouting dynamics. Integr. Biol. 2021, 13, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, A.; Long, J.E.; Crandall, J.E.; Rubenstein, J.L.R.; Bhide, P.G. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat. Neurosci. 2008, 11, 429–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekine, K.; Kubo, K.I.; Nakajima, K. How does Reelin control neuronal migration and layer formation in the developing mammalian neocortex? Neurosci. Res. 2014, 86, 50–58. [Google Scholar] [CrossRef]
- Podjaski, C.; Alvarez, J.I.; Bourbonniere, L.; Larouche, S.; Terouz, S.; Bin, J.M.; Lécuyer, M.A.; Saint-Laurent, O.; Larochelle, C.; Darlington, P.J.; et al. Netrin 1 regulates blood-brain barrier function and neuroinflammation. Brain 2015, 138, 1598–1612. [Google Scholar] [CrossRef] [Green Version]
- Claro, V.; Ferro, A. Netrin-1: Focus on its role in cardiovascular physiology and atherosclerosis. JRSM Cardiovasc. Dis. 2020, 9, 204800402095957. [Google Scholar] [CrossRef]
- Nacht, M.; St. Martin, T.B.; Byrne, A.; Klinger, K.W.; Teicher, B.A.; Madden, S.L.; Jiang, Y. Netrin-4 regulates angiogenic responses and tumor cell growth. Exp. Cell Res. 2009, 315, 784–794. [Google Scholar] [CrossRef]
- Serini, G.; Valdembri, D.; Zanivan, S.; Morterra, G.; Burkhardt, C.; Caccavari, F.; Zammataro, L.; Primo, L.; Tamagnone, L.; Logank, M.; et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 2003, 424, 391–397. [Google Scholar] [CrossRef]
- Mada, J.; Tokihiro, T. Pattern formation of vascular network in a mathematical model of angiogenesis. Jpn. J. Ind. Appl. Math. 2022, 39, 351–384. [Google Scholar] [CrossRef]
- Blanco, R.; Gerhardt, H. VEGF and Notch in Tip and Stalk Cell Selection. Cold Spring Harb. Perspect. Med. 2013, 3, a006569. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takigawa-Imamura, H.; Hirano, S.; Watanabe, C.; Ohtaka-Maruyama, C.; Ema, M.; Mizutani, K.-i. Computational Model Exploring Characteristic Pattern Regulation in Periventricular Vessels. Life 2022, 12, 2069. https://doi.org/10.3390/life12122069
Takigawa-Imamura H, Hirano S, Watanabe C, Ohtaka-Maruyama C, Ema M, Mizutani K-i. Computational Model Exploring Characteristic Pattern Regulation in Periventricular Vessels. Life. 2022; 12(12):2069. https://doi.org/10.3390/life12122069
Chicago/Turabian StyleTakigawa-Imamura, Hisako, Saito Hirano, Chisato Watanabe, Chiaki Ohtaka-Maruyama, Masatsugu Ema, and Ken-ichi Mizutani. 2022. "Computational Model Exploring Characteristic Pattern Regulation in Periventricular Vessels" Life 12, no. 12: 2069. https://doi.org/10.3390/life12122069
APA StyleTakigawa-Imamura, H., Hirano, S., Watanabe, C., Ohtaka-Maruyama, C., Ema, M., & Mizutani, K.-i. (2022). Computational Model Exploring Characteristic Pattern Regulation in Periventricular Vessels. Life, 12(12), 2069. https://doi.org/10.3390/life12122069







