Metformin Acutely Mitigates Oxidative Stress in Human Atrial Tissue: A Pilot Study in Overweight Non-Diabetic Cardiac Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Organ Culture
2.2. Oxidative Stress Assessment with the Ferrous Oxidation-Xylenol Orange (FOX) Assay
2.3. Oxidative Stress Assessment Using DHE in Confocal Microscopy
2.4. Statistics
3. Results
3.1. Metformin in Acute Administration Mitigates Oxidative Stress in Human Atrial Tissue
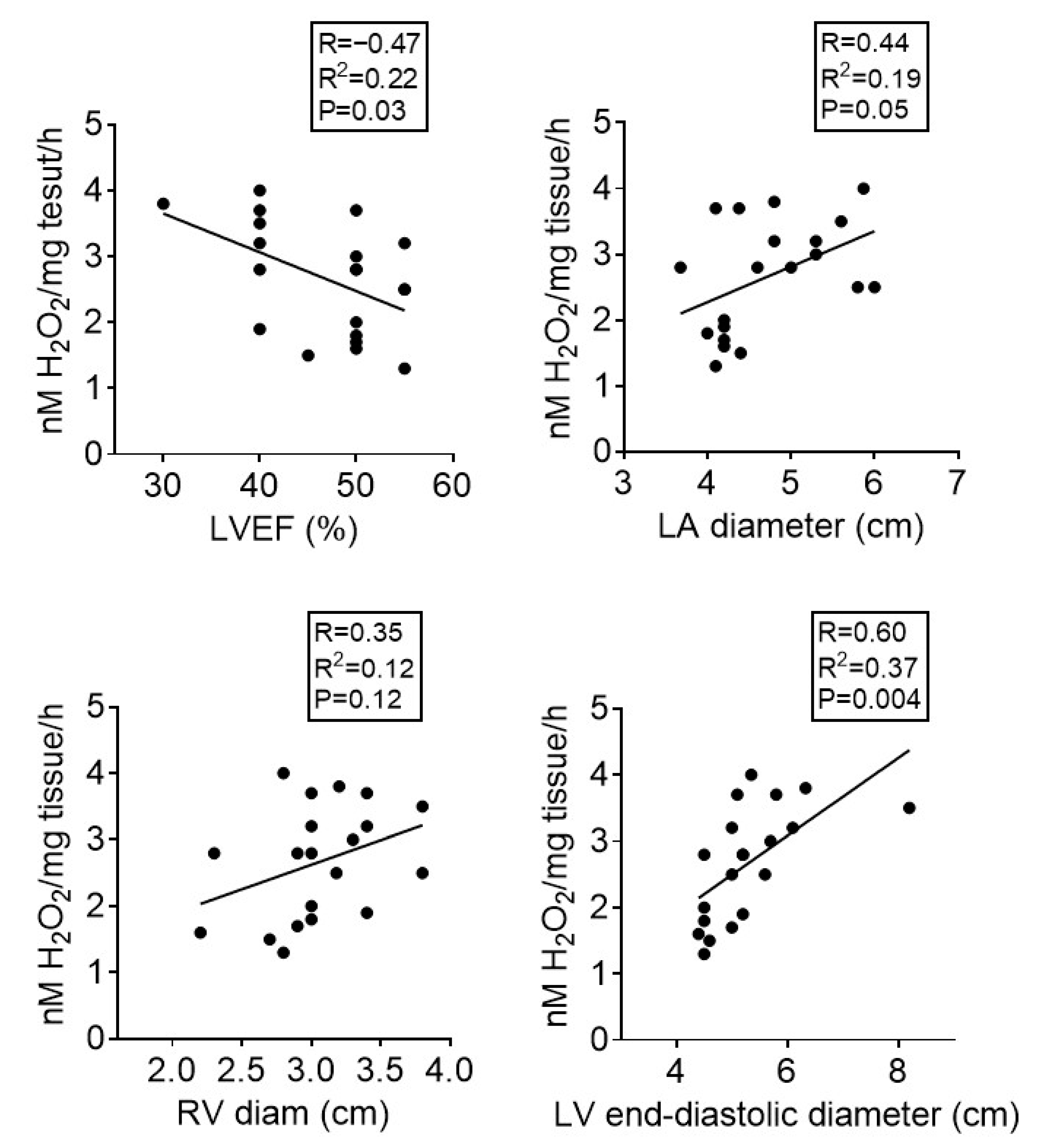
3.2. Atrial Oxidative Stress Correlates with Echocardiographic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Wenclewska, S.; Śliwińska, A. Metabolic Action of Metformin. Pharmaceuticals 2022, 15, 810. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Peng, M.; Tang, X.; Xu, X.; Wu, Y.; Chen, A.F.; Yang, X. Protective effects of metformin in various cardiovascular diseases: Clinical evidence and AMPK-dependent mechanisms. J. Cell. Mol. Med. 2022, 26, 4886–4903. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Hollenberg, M.D.; Ding, H.; Triggle, C.R. A Critical Review of the Evidence that Metformin Is a Putative Anti-Aging Drug that Enhances Healthspan and Extends Lifespan. Front. Endocrinol. 2021, 12, 718942. [Google Scholar] [CrossRef] [PubMed]
- Masarwa, R.; Brunetti, V.C.; Aloe, S.; Henderson, M.; Platt, R.W.; Filion, K.B. Efficacy and Safety of Metformin for Obesity: A Systematic Review. Pediatrics 2021, 147, e20201610. [Google Scholar] [CrossRef]
- Astiz, S.; Gonzalez-Bulnes, A.; Astiz, I.; Barbero, A.; Pesantez-Pacheco, J.L.; Garcia-Contreras, C.; Vazquez-Gomez, M.; Heras-Molina, A. Metformin Alleviates Obesity and Systemic Oxidative Stress in Obese Young Swine. Pharmaceuticals 2020, 13, 142. [Google Scholar] [CrossRef]
- Schubert, M.; Hansen, S.; Leefmann, J.; Guan, K. Repurposing Antidiabetic Drugs for Cardiovascular Disease. Front. Physiol. 2020, 11, 568632. [Google Scholar] [CrossRef]
- El Messaoudi, S.; Nederlof, R.; Zuurbier, C.J.; van Swieten, H.A.; Pickkers, P.; Noyez, L.; Dieker, H.J.; Coenen, M.J.; Donders, A.R.; Vos, A.; et al. Effect of metformin pretreatment on myocardial injury during coronary artery bypass surgery in patients without diabetes (MetCAB): A double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 615–623. [Google Scholar] [CrossRef]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef]
- Sumandea, M.P.; Steinberg, S.F. Redox signaling and cardiac sarcomeres. J. Biol. Chem. 2011, 286, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Elimban, V.; Bartekova, M.; Adameova, A. Involvement of Oxidative Stress in the Development of Subcellular Defects and Heart Disease. Biomedicines 2022, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Gori, T.; Keaney, J.F., Jr.; Maack, C.; Daiber, A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart J. 2015, 36, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Rosca, M.G.; Hoppel, C.L. Mitochondrial dysfunction in heart failure. Heart Fail. Rev. 2013, 18, 607–622. [Google Scholar] [CrossRef]
- Drzewoski, J.; Hanefeld, M. The Current and Potential Therapeutic Use of Metformin—The Good Old Drug. Pharmaceuticals 2021, 14, 122. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- Panfoli, I.; Puddu, A.; Bertola, N.; Ravera, S.; Maggi, D. The Hormetic Effect of Metformin: “Less Is More”? Int. J. Mol. Sci. 2021, 22, 6297. [Google Scholar] [CrossRef]
- Ionică, L.N.; Gaiță, L.; Bînă, A.M.; Soșdean, R.; Lighezan, R.; Sima, A.; Malița, D.; Crețu, O.M.; Burlacu, O.; Muntean, D.M.; et al. Metformin alleviates monoamine oxidase-related vascular oxidative stress and endothelial dysfunction in rats with diet-induced obesity. Mol. Cell. Biochem. 2021, 476, 4019–4029. [Google Scholar] [CrossRef]
- Sturza, A.; Leisegang, M.S.; Babelova, A.; Schroder, K.; Benkhoff, S.; Loot, A.E.; Fleming, I.; Schulz, R.; Muntean, D.M.; Brandes, R.P. Monoamine oxidases are mediators of endothelial dysfunction in the mouse aorta. Hypertension 2013, 62, 140–146. [Google Scholar] [CrossRef]
- Merce, A.P.; Ionică, L.N.; Bînă, A.M.; Popescu, S.; Lighezan, R.; Petrescu, L.; Borza, C.; Sturza, A.; Muntean, D.M.; Creţu, O.M. Monoamine oxidase is a source of cardiac oxidative stress in obese rats: The beneficial role of metformin. Mol. Cell. Biochem. 2022, 20, 1–9. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Mohammed, I.; Bshesh, K.; Marei, I.; Ye, K.; Ding, H.; MacDonald, R.; Hollenberg, M.D.; Hill, M.A. Metformin: Is it a drug for all reasons and diseases? Metabolism 2022, 133, 155223. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef]
- Top, W.M.C.; Kooy, A.; Stehouwer, C.D.A. Metformin: A Narrative Review of Its Potential Benefits for Cardiovascular Disease, Cancer and Dementia. Pharmaceuticals 2022, 15, 312. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, K.; Krako Jakovljevic, N.; Isakovic, A.M.; Ivanovic, T.; Markovic, I.; Lalic, N.M. Therapeutic vs. Suprapharmacological Metformin Concentrations: Different Effects on Energy Metabolism and Mitochondrial Function in Skeletal Muscle Cells in vitro. Front. Pharmacol. 2022, 13, 930308. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, N.; Hua, Y.; Wang, B.; Ling, L.; Ferro, A.; Xu, B. Metformin inhibits angiotensin II-induced differentiation of cardiac fibroblasts into myofibroblasts. PLoS ONE 2013, 8, e72120. [Google Scholar] [CrossRef] [PubMed]
- Zemgulyte, G.; Tanaka, S.; Hide, I.; Sakai, N.; Pampuscenko, K.; Borutaite, V.; Rastenyte, D. Evaluation of the Effectiveness of Post-Stroke Metformin Treatment Using Permanent Middle Cerebral Artery Occlusion in Rats. Pharmaceuticals 2021, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Zhang, W.; Zheng, G.; Zhang, Z.; Yin, J.; He, Z. Metformin reverses multidrug resistance and epithelial-mesenchymal transition (EMT) via activating AMP-activated protein kinase (AMPK) in human breast cancer cells. Mol. Cell. Biochem. 2014, 386, 63–71. [Google Scholar] [CrossRef]
- Ravera, S.; Cossu, V.; Tappino, B.; Nicchia, E.; Dufour, C.; Cavani, S.; Sciutto, A.; Bolognesi, C.; Columbaro, M.; Degan, P.; et al. Concentration-dependent metabolic effects of metformin in healthy and Fanconi anemia lymphoblast cells. J. Cell. Physiol. 2018, 233, 1736–1751. [Google Scholar] [CrossRef]
- Cozma, G.V.; Apostu, A.; Macasoi, I.; Dehelean, C.A.; Cretu, O.M.; Dinu, S.; Gaiță, D.; Manea, A. In Vitro and In Ovo Evaluation of the Potential Hepatoprotective Effect of Metformin. Medicina 2022, 58, 705. [Google Scholar] [CrossRef]
- Thomas, I.; Gregg, B. Metformin; a review of its history and future: From lilac to longevity. Pediatr. Diabetes 2017, 18, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Batandier, C.; Guigas, B.; Detaille, D.; El-Mir, M.Y.; Fontaine, E.; Rigoulet, M.; Leverve, X.M. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J. Bioenerg. Biomembr. 2006, 38, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front. Endocrinol. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Vial, G.; Detaille, D.; Guigas, B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Butrico, G.M.; Kalpage, H.A.; Goedeke, L.; Hubbard, B.T.; Vatner, D.F.; Gaspar, R.C.; Zhang, X.M.; Cline, G.W.; Nakahara, K.; et al. Metformin, phenformin, and galegine inhibit complex IV activity and reduce glycerol-derived gluconeogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2122287119. [Google Scholar] [CrossRef]
- Leverve, X.M.; Guigas, B.; Detaille, D.; Batandier, C.; Koceir, E.A.; Chauvin, C.; Fontaine, E.; Wiernsperger, N.F. Mitochondrial metabolism and type-2 diabetes: A specific target of metformin. Diabetes Metab. 2003, 29, 6s88–6s94. [Google Scholar] [CrossRef]
- Hu, M.; Ye, P.; Liao, H.; Chen, M.; Yang, F. Metformin Protects H9C2 Cardiomyocytes from High-Glucose and Hypoxia/Reoxygenation Injury via Inhibition of Reactive Oxygen Species Generation and Inflammatory Responses: Role of AMPK and JNK. J. Diabetes Res. 2016, 2016, 2961954. [Google Scholar] [CrossRef]
- Cheng, G.; Li, L. High-glucose-induced apoptosis, ROS production and pro-inflammatory response in cardiomyocytes is attenuated by metformin treatment via PP2A activation. J. Biosci. 2020, 45, 1–11. [Google Scholar] [CrossRef]
- Liu, X.D.; Li, Y.G.; Wang, G.Y.; Bi, Y.G.; Zhao, Y.; Yan, M.L.; Liu, X.; Wei, M.; Wan, L.L.; Zhang, Q.Y. Metformin protects high glucose-cultured cardiomyocytes from oxidative stress by promoting NDUFA13 expression and mitochondrial biogenesis via the AMPK signaling pathway. Mol. Med. Rep. 2020, 22, 5262–5270. [Google Scholar] [CrossRef]
- Kukidome, D.; Nishikawa, T.; Sonoda, K.; Imoto, K.; Fujisawa, K.; Yano, M.; Motoshima, H.; Taguchi, T.; Matsumura, T.; Araki, E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 2006, 55, 120–127. [Google Scholar] [CrossRef]
- Daubert, M.A.; Yow, E.; Dunn, G.; Marchev, S.; Barnhart, H.; Douglas, P.S.; O’Connor, C.; Goldstein, S.; Udelson, J.E.; Sabbah, H.N. Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circ. Heart Fail. 2017, 10, e004389. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Rawish, E.; Nording, H.M.; Langer, H.F. Inflammation in Metabolic and Cardiovascular Disorders-Role of Oxidative Stress. Life 2021, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.; Le Jemtel, T.H. Therapeutic Stalemate in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2021, 10, e021120. [Google Scholar] [CrossRef]
- Franssen, C.; Chen, S.; Hamdani, N.; Paulus, W.J. From comorbidities to heart failure with preserved ejection fraction: A story of oxidative stress. Heart 2016, 102, 320–330. [Google Scholar] [CrossRef]
- Yan, Y.; Li, S.; Guo, Y.; Fernandez, C.; Bazzano, L.; He, J.; Mi, J.; Chen, W. Life-Course Cumulative Burden of Body Mass Index and Blood Pressure on Progression of Left Ventricular Mass and Geometry in Midlife: The Bogalusa Heart Study. Circ. Res. 2020, 126, 633–643. [Google Scholar] [CrossRef]
- Canton, M.; Menazza, S.; Sheeran, F.L.; Polverino de Laureto, P.; Di Lisa, F.; Pepe, S. Oxidation of myofibrillar proteins in human heart failure. J. Am. Coll. Cardiol. 2011, 57, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Sheeran, F.L.; Pepe, S. Posttranslational modifications and dysfunction of mitochondrial enzymes in human heart failure. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E449–E460. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Koniari, I.; Velissaris, D.; Kounis, N.G.; Koufou, E.; Artopoulou, E.; de Gregorio, C.; Mplani, V.; Paraskevas, T.; Tsigkas, G.; Hung, M.Y.; et al. Anti-Diabetic Therapy, Heart Failure and Oxidative Stress: An Update. J. Clin. Med. 2022, 11, 4660. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Marfella, R.; Sardu, C.; et al. Effects of Metformin in Heart Failure: From Pathophysiological Rationale to Clinical Evidence. Biomolecules 2021, 11, 1834. [Google Scholar] [CrossRef]
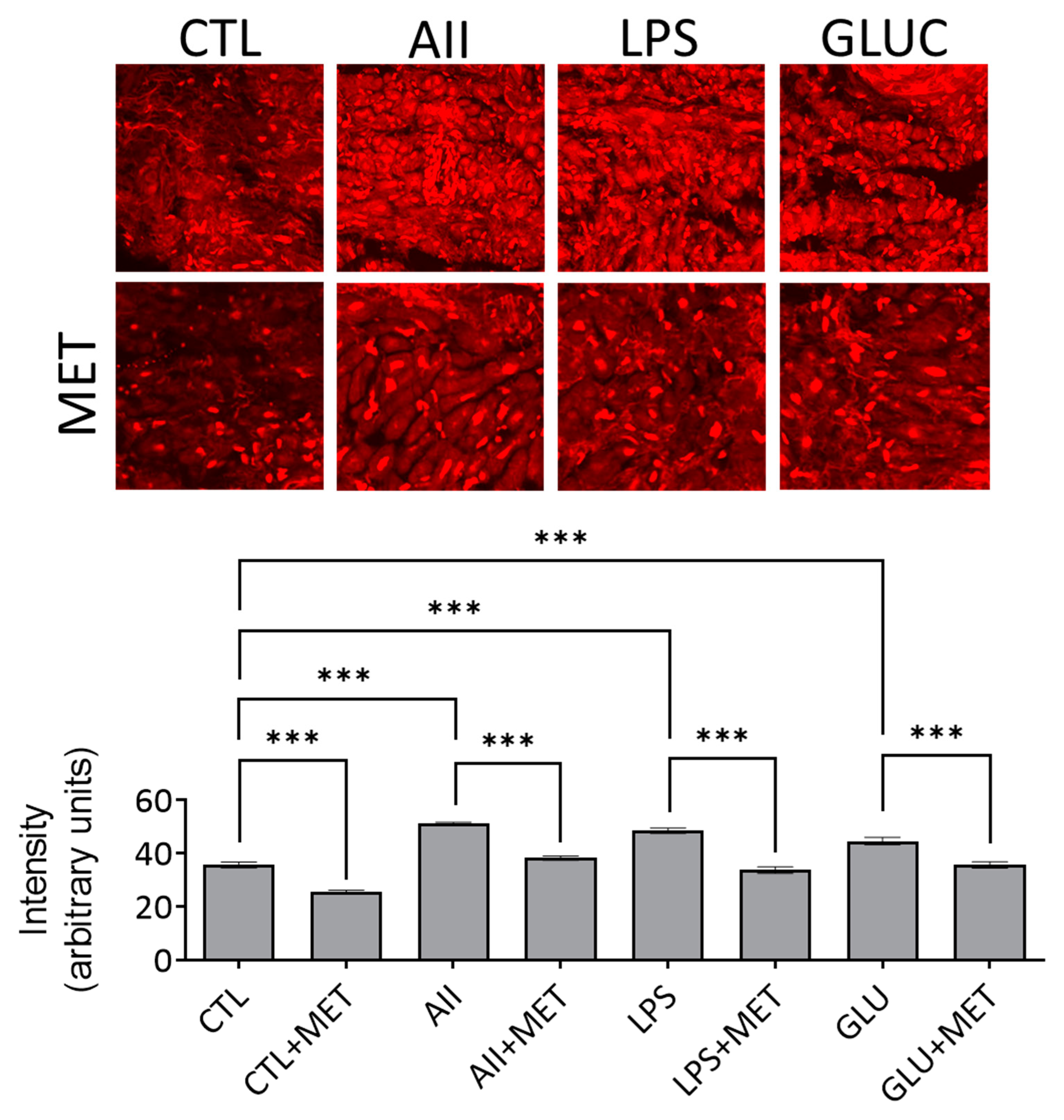

| Parameter | Value |
|---|---|
| Age (years) | 65.35 ± 1.7 |
| Sex (M/F) | 14/6 |
| BMI (kg/m2) | 27.21 ± 0.96 |
| Systolic Blood Pressure (mmHg) | 128.85 ± 3 |
| Diastolic Blood Pressure (mmHg) | 76.1 ± 1.9 |
| Heart Rate (b/min) | 67.55 ± 2.02 |
| Erythrocyte Sedimentation Rate (mm/h) | 23.65 ± 4.56 |
| Red Blood Count (mil./mm3) | 4.55 ± 0.14 |
| PCV (%) | 41.63 ± 1.31 |
| Hemoglobin (g/dL) | 13.84 ± 0.42 |
| White Blood Count (×103/mm3) | 7.66 ± 0.39 |
| Platelets (×103/mm3) | 223.2 ± 12.18 |
| Creatinine (mg/dL) | 1.03 ± 0.07 |
| Uric Acid (mg/dL) | 7.73 ± 1.38 |
| Total Cholesterol (mg/dL) | 176.7 ± 12.85 |
| HDL-Cholesterol (mg/dL) | 41 ± 4.59 |
| LDL-Cholesterol (mg/dL) | 99.55 ± 16.19 |
| Triglycerides | 185 ± 53.57 |
| FPG (mg/dL) | 103.8 ± 4.29 |
| ALAT (U/L) | 23.95 ± 3.29 |
| ASAT (U/L) | 21.7 ± 1.73 |
| Na+ (mmol/L) | 140.9 ± 0.91 |
| K+ (mmol/L) | 4.18 ± 0.07 |
| LA diameter (cm) | 4.75 ± 0.16 |
| LV end-diastolic diameter (cm) | 5.28 ± 0.19 |
| RV diameter (cm) | 3.05 ± 0.09 |
| LVEF (%) | 47.10 ± 1.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lascu, A.; Ionică, L.-N.; Merce, A.-P.; Dănilă, M.-D.; Petrescu, L.; Sturza, A.; Muntean, D.-M.; Streian, C.G. Metformin Acutely Mitigates Oxidative Stress in Human Atrial Tissue: A Pilot Study in Overweight Non-Diabetic Cardiac Patients. Life 2022, 12, 2058. https://doi.org/10.3390/life12122058
Lascu A, Ionică L-N, Merce A-P, Dănilă M-D, Petrescu L, Sturza A, Muntean D-M, Streian CG. Metformin Acutely Mitigates Oxidative Stress in Human Atrial Tissue: A Pilot Study in Overweight Non-Diabetic Cardiac Patients. Life. 2022; 12(12):2058. https://doi.org/10.3390/life12122058
Chicago/Turabian StyleLascu, Ana, Loredana-Nicoleta Ionică, Adrian-Petru Merce, Maria-Daniela Dănilă, Lucian Petrescu, Adrian Sturza, Danina-Mirela Muntean, and Caius Glad Streian. 2022. "Metformin Acutely Mitigates Oxidative Stress in Human Atrial Tissue: A Pilot Study in Overweight Non-Diabetic Cardiac Patients" Life 12, no. 12: 2058. https://doi.org/10.3390/life12122058
APA StyleLascu, A., Ionică, L.-N., Merce, A.-P., Dănilă, M.-D., Petrescu, L., Sturza, A., Muntean, D.-M., & Streian, C. G. (2022). Metformin Acutely Mitigates Oxidative Stress in Human Atrial Tissue: A Pilot Study in Overweight Non-Diabetic Cardiac Patients. Life, 12(12), 2058. https://doi.org/10.3390/life12122058








