Anaplasma phagocytophilum Transmission Activates Immune Pathways While Repressing Wound Healing in the Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anaplasma Phagocytophilum Culture
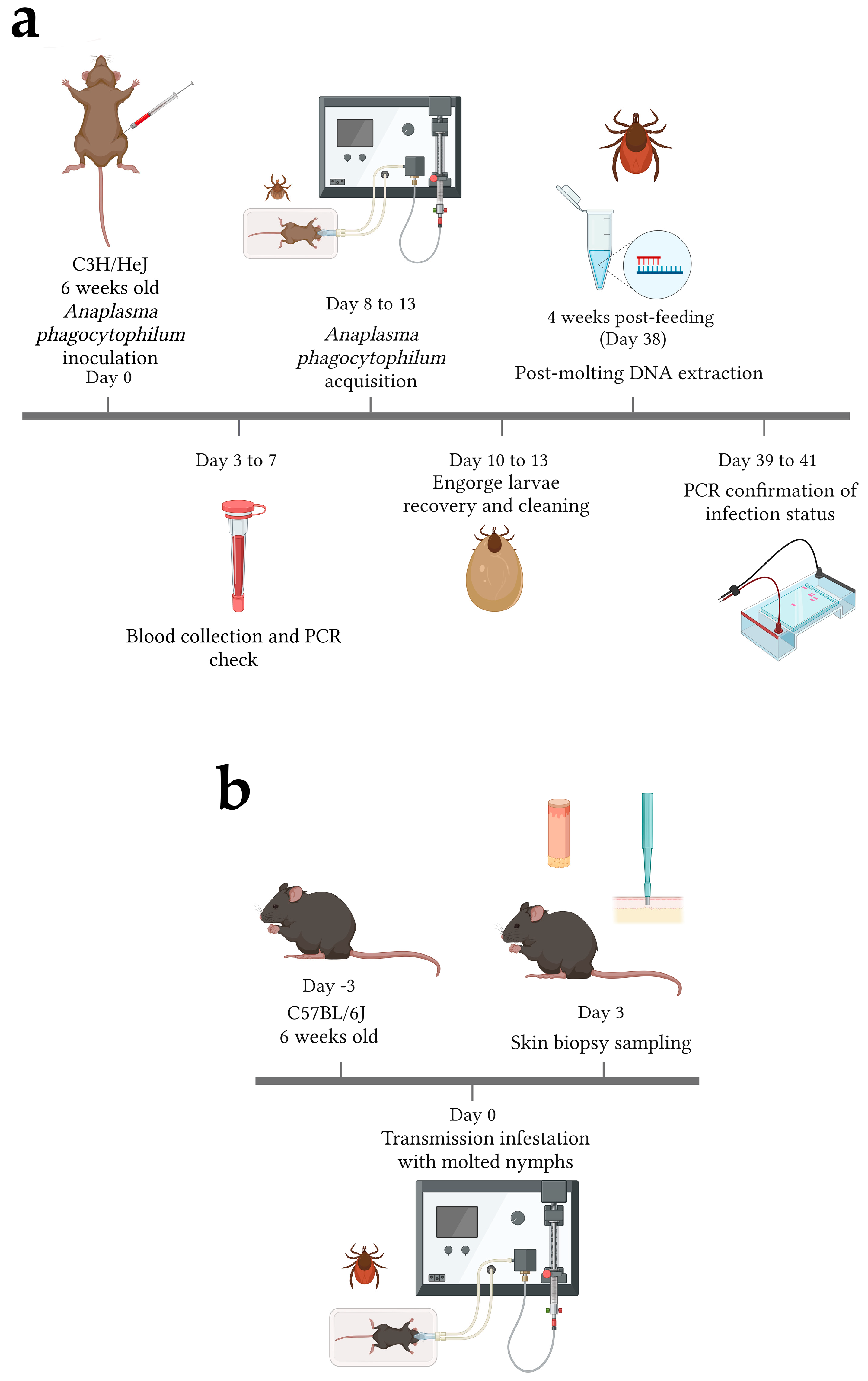
2.2. Mice Infections
2.3. Tick Infestations
2.4. RNA-Seq and Pathway Analysis of Skin Biopsies
2.5. qRT-PCR of Skin Biopsies
3. Results
3.1. Tick Feeding Induces the Expression of Neutrophil Chemotaxis and Inflammatory Responses in the Skin
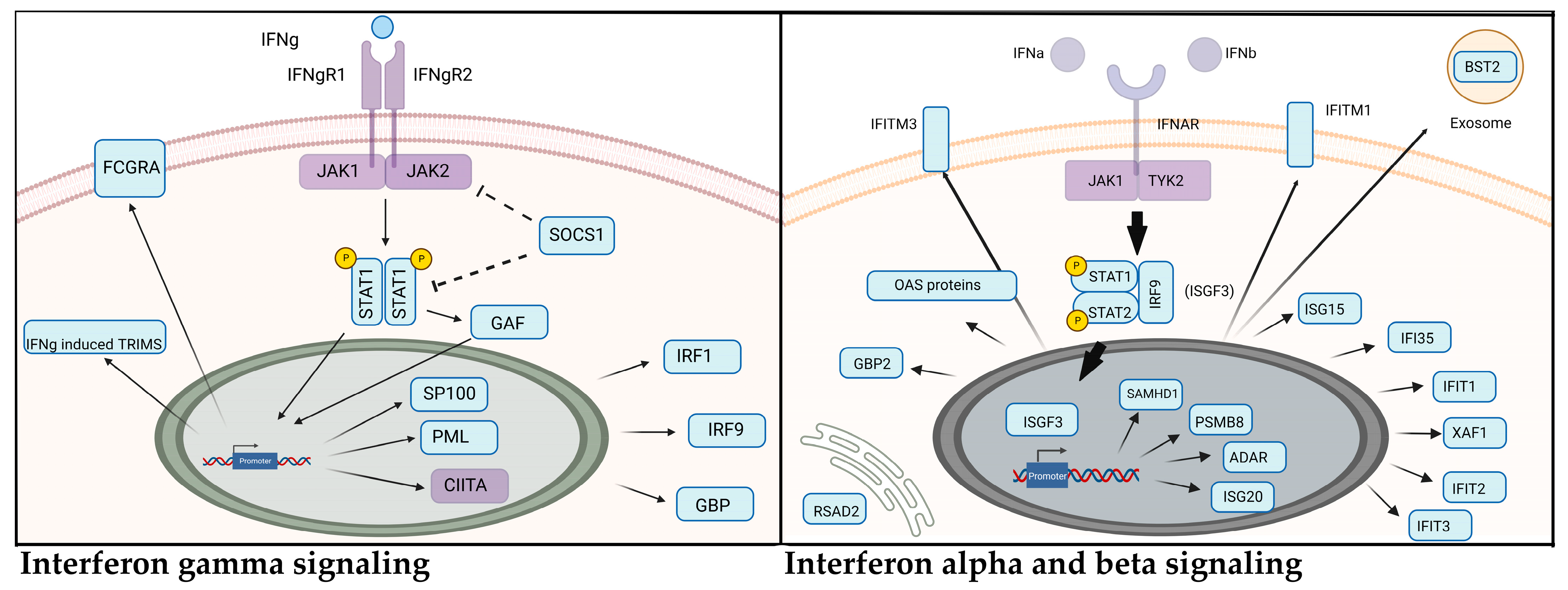
3.2. Anaplasma Phagocytophilum Transmission Induces the Upregulation of Interferon Signaling Genes
3.3. Differentially Expressed Genes (DEGs) Stimulated during Tick Feeding and A. Phagocytophilum Transmission
3.4. Confirmation of Th1 Cytokines Upregulation and Downregulation of ECM Genes by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisen, R.J.; Eisen, L. The Blacklegged Tick, Ixodes scapularis: An increasing public health concern. Trends Parasitol. 2018, 34, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Dumler, J.S.; Chen, S.M.; Eckman, M.R.; Van Etta, L.L.; Walker, D.H. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 1994, 272, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.J.; Brady, G.; Ackman, D.M.; Wong, S.J.; Jacquette, G.; Lloyd, E.E.; Birkhead, G.S. Human granulocytic ehrlichiosis in New York. Arch. Intern. Med. 1998, 158, 769–773. [Google Scholar] [CrossRef]
- CDC. Epidemiology and Statistics. Available online: https://www.cdc.gov/anaplasmosis/stats/index.html (accessed on 28 February 2022).
- Bakken, J.S.; Dumler, J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. N. Am. 2015, 29, 341–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, N.; McBride, J.W. Tick-borne emerging infections: Ehrlichiosis and Anaplasmosis. Clin. Lab. Med. 2017, 37, 317–340. [Google Scholar] [CrossRef]
- Otten, C.; Brilli, M.; Vollmer, W.; Viollier, P.H.; Salje, J. Peptidoglycan in obligate intracellular bacteria. Mol. Microbiol. 2018, 107, 142–163. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Rikihisa, Y. Expression of interleukin-1beta, tumor necrosis factor alpha, and interleukin-6 in human peripheral blood leukocytes exposed to human granulocytic ehrlichiosis agent or recombinant major surface protein P44. Infect. Immun. 2000, 68, 3394–3402. [Google Scholar] [CrossRef] [Green Version]
- Dumler, J.S.; Barat, N.C.; Barat, C.E.; Bakken, J.S. Human granulocytic anaplasmosis and macrophage activation. Clin. Infect. Dis. 2007, 45, 199–204. [Google Scholar] [CrossRef]
- Akkoyunlu, M.; Fikrig, E. Gamma interferon dominates the murine cytokine response to the agent of human granulocytic ehrlichiosis and helps to control the degree of early rickettsemia. Infect. Immun. 2000, 68, 1827–1833. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.E.; Caspersen, K.; Dumler, J.S. Immunopathology and ehrlichial propagation are regulated by interferon-gamma and interleukin-10 in a murine model of human granulocytic ehrlichiosis. Am. J. Pathol. 2001, 158, 1881–1888. [Google Scholar] [CrossRef]
- Wang, T.; Akkoyunlu, M.; Banerjee, R.; Fikrig, E. Interferon-gamma deficiency reveals that 129Sv mice are inherently more susceptible to Anaplasma phagocytophilum than C57BL/6 mice. FEMS Immunol. Med. Microbiol. 2004, 42, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gussmann, K.; Kirschnek, S.; von Loewenich, F.D. Interferon-γ-dependent control of Anaplasma phagocytophilum by murine neutrophil granulocytes. Parasit. Vectors 2017, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Dumler, J.S. Anaplasma phagocytophilum, interferon gamma production and Stat1 signaling. Microbiol. Immunol. 2013, 57, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.S.; Scorpio, D.G.; Dumler, J.S. Stat1 negatively regulates immune-mediated injury with Anaplasma phagocytophilum infection. J. Immunol. 2014, 193, 5088–5098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granquist, E.G.; Aleksandersen, M.; Bergström, K.; Dumler, S.J.; Torsteinbø, W.O.; Stuen, S. A morphological and molecular study of Anaplasma phagocytophilum transmission events at the time of Ixodes ricinus tick bite. Acta Vet. Scand. 2010, 52, 43. [Google Scholar] [CrossRef] [Green Version]
- Reppert, E.; Galindo, R.C.; Ayllón, N.; Breshears, M.A.; Kocan, K.M.; Blouin, E.F.; de la Fuente, J. Studies of Anaplasma phagocytophilum in sheep experimentally infected with the human NY-18 isolate: Characterization of tick feeding sites. Ticks Tick Borne Dis. 2014, 5, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Oliva Chávez, A.S.; Fairman, J.W.; Felsheim, R.F.; Nelson, C.M.; Herron, M.J.; Higgins, L.; Burkhardt, N.Y.; Oliver, J.D.; Markowski, T.W.; Kurtti, T.J.; et al. An O-Methyltransferase is required for infection of tick cells by Anaplasma phagocytophilum. PLoS Pathog. 2015, 11, e1005248. [Google Scholar] [CrossRef] [Green Version]
- Blas-Machado, U.; de la Fuente, J.; Blouin, E.F.; Almazán, C.; Kocan, K.M.; Mysore, J.V. Experimental infection of C3H/HeJ mice with the NY18 isolate of Anaplasma phagocytophilum. Vet. Pathol. 2007, 44, 64–73. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suganami, T.; Mieda, T.; Itoh, M.; Shimoda, Y.; Kamei, Y.; Ogawa, Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem. Biophys. Res. Commun. 2007, 354, 45–49. [Google Scholar] [CrossRef]
- Gosemann, J.H.; van Griensven, M.; Barkhausen, T.; Kobbe, P.; Thobe, B.M.; Haasper, C.; Pape, H.C.; Krettek, C.; Hildebrand, F.; Frink, M. TLR4 influences the humoral and cellular immune response during polymicrobial sepsis. Injury 2010, 41, 1060–1067. [Google Scholar] [CrossRef]
- McClure Carroll, E.E.; Wang, X.; Shaw, D.K.; O’Neal, A.J.; Oliva Chavez, A.S.; Brown, L.J.; Boradia, V.M.; Hammond, H.L.; Pedra, J.H.F. p47 licenses activation of the immune deficiency pathway in the tick Ixodes scapularis. Proc. Natl. Acad. Sci. USA 2019, 116, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Shaw, D.K.; Hammond, H.L.; Sutterwala, F.S.; Rayamajhi, M.; Shirey, K.A.; Perkins, D.J.; Bonventre, J.V.; Velayutham, T.S.; Evans, S.M.; et al. The Prostaglandin E2-EP3 receptor axis regulates Anaplasma phagocytophilum-mediated NLRC4 inflammasome activation. PLoS Pathog. 2016, 12, e1005803. [Google Scholar] [CrossRef] [Green Version]
- Oliva Chávez, A.S.; Wang, X.; Marnin, L.; Archer, N.K.; Hammond, H.L.; Carroll, E.E.M.; Shaw, D.K.; Tully, B.G.; Buskirk, A.D.; Ford, S.L.; et al. Tick extracellular vesicles enable arthropod feeding and promote distinct outcomes of bacterial infection. Nat. Commun. 2021, 12, 3696. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Graham, C.B.; Maes, S.E.; Hojgaard, A.; Fleshman, A.; Boegler, K.A.; Delory, M.J.; Slater, K.S.; Karpathy, S.E.; Bjork, J.K.; et al. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick Borne Dis. 2018, 9, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Mastronunzio, J.E.; Kurscheid, S.; Fikrig, E. Postgenomic analyses reveal development of infectious Anaplasma phagocytophilum during transmission from ticks to mice. J. Bacteriol. 2012, 194, 2238–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodzic, E.; Fish, D.; Maretzki, C.M.; De Silva, A.M.; Feng, S.; Barthold, S.W. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J. Clin. Microbiol. 1998, 36, 3574–3578. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Nakano, Y.; Doi, H.; Nakamizo, S.; Nakajima, S.; Matsumoto, R.; Farkas, T.; Wong, P.M.; Narang, V.; Moreno Traspas, R.; et al. C10orf99/GPR15L regulates proinflammatory response of keratinocytes and barrier formation of the skin. Front. Immunol. 2022, 13, 825032. [Google Scholar] [CrossRef] [PubMed]
- Briant, L.; Desprès, P.; Choumet, V.; Missé, D. Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology 2014, 464–465, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Passelli, K.; Billion, O.; Tacchini-Cottier, F. The impact of neutrophil recruitment to the skin on the pathology induced by Leishmania infection. Front. Immunol. 2021, 12, 649348. [Google Scholar] [CrossRef] [PubMed]
- Prates, D.B.; Araújo-Santos, T.; Luz, N.F.; Andrade, B.B.; França-Costa, J.; Afonso, L.; Clarêncio, J.; Miranda, J.C.; Bozza, P.T.; Dosreis, G.A.; et al. Lutzomyia longipalpis saliva drives apoptosis and enhances parasite burden in neutrophils. J. Leukoc. Biol. 2011, 90, 575–582. [Google Scholar] [CrossRef]
- Schmid, M.A.; Glasner, D.R.; Shah, S.; Michlmayr, D.; Kramer, L.D.; Harris, E. Mosquito saliva increases endothelial permeability in the skin, immune cell migration, and Dengue pathogenesis during Antibody-Dependent Enhancement. PLoS Pathog. 2016, 12, e1005676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, C.R.; Santos, C.D.S.; Prates, D.B.; Dos Santos, R.T.; Araújo-Santos, T.; de Souza-Neto, S.M.; Borges, V.M.; Barral-Netto, M.; Brodskyn, C.I. Lutzomyia longipalpis Saliva Drives Interleukin-17-Induced Neutrophil Recruitment Favoring Leishmania infantum Infection. Front. Microbiol. 2018, 9, 881. [Google Scholar] [CrossRef]
- Hastings, A.K.; Uraki, R.; Gaitsch, H.; Dhaliwal, K.; Stanley, S.; Sproch, H.; Williamson, E.; MacNeil, T.; Marin-Lopez, A.; Hwang, J.; et al. Aedes aegypti NeSt1 protein enhances Zika virus pathogenesis by activating neutrophils. J. Virol. 2019, 93, e00395-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikel, S. Ticks and tick-borne pathogens at the cutaneous interface: Host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front. Microbiol. 2013, 4, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banajee, K.H.; Verhoeve, V.I.; Harris, E.K.; Macaluso, K.R. Effect of Amblyomma maculatum (Acari: Ixodidae) saliva on the acute cutaneous immune response to Rickettsia parkeri infection in a murine model. J. Med. Entomol. 2016, 53, 1252–1260. [Google Scholar] [CrossRef] [Green Version]
- Banajee, K.H.; Embers, M.E.; Langohr, I.M.; Doyle, L.A.; Hasenkampf, N.R.; Macaluso, K.R. Amblyomma maculatum feeding augments Rickettsia parkeri infection in a Rhesus Macaque model: A pilot study. PLoS ONE 2015, 10, e0135175. [Google Scholar] [CrossRef]
- Heinze, D.M.; Wikel, S.K.; Thangamani, S.; Alarcon-Chaidez, F.J. Transcriptional profiling of the murine cutaneous response during initial and subsequent infestations with Ixodes scapularis nymphs. Parasit. Vectors 2012, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Prete, A.; Martínez-Muñoz, L.; Mazzon, C.; Toffali, L.; Sozio, F.; Za, L.; Bosisio, D.; Gazzurelli, L.; Salvi, V.; Tiberio, L.; et al. The atypical receptor CCRL2 is required for CXCR2-dependent neutrophil recruitment and tissue damage. Blood 2017, 130, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Capucetti, A.; Albano, F.; Bonecchi, R. Multiple roles for chemokines in neutrophil biology. Front. Immunol. 2020, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Tatchell, R.J.; Moorhouse, D.E. Neutrophils: Their role in the formation of a tick feeding lesion. Science 1970, 167, 1002–1003. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Hile, G.A.; Gudjonsson, J.E.; Kahlenberg, J.M. The influence of interferon on healthy and diseased skin. Cytokine 2020, 132, 154605. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.P.; Engström, P.; Tran, C.J.; Langohr, I.M.; Glasner, D.R.; Espinosa, D.A.; Harris, E.; Welch, M.D. Interferon receptor-deficient mice are susceptible to eschar-associated rickettsiosis. eLife 2021, 10, e67029. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.P.; Engström, P.; Chavez, R.A.; Fonbuena, J.A.; Vance, R.E.; Welch, M.D. Inflammasome-mediated antagonism of type I interferon enhances Rickettsia pathogenesis. Nat. Microbiol. 2020, 5, 688–696. [Google Scholar] [CrossRef]
- Singh, B.; Fleury, C.; Jalalvand, F.; Riesbeck, K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol. Rev. 2012, 36, 1122–1180. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.S.; Grab, D.J.; Dumler, J.S. Anaplasma phagocytophilum infection induces protracted neutrophil degranulation. Infect. Immun. 2004, 72, 3680–3683. [Google Scholar] [CrossRef] [Green Version]
- Grab, D.J.; Nyarko, E.; Barat, N.C.; Nikolskaia, O.V.; Dumler, J.S. Anaplasma phagocytophilum-Borrelia burgdorferi coinfection enhances chemokine, cytokine, and matrix metalloprotease expression by human brain microvascular endothelial cells. Clin. Vaccine Immunol. 2007, 14, 1420–1424. [Google Scholar] [CrossRef]




| Primer Name | Primer Sequence | Ta * | Product Size (bp) | Reference |
|---|---|---|---|---|
| Mactin F | 5′-ACGCAGAGGGAAATCGTCCGTGAC-3′ | 60 °C | 101 | [24] |
| Mactin R | 5′-ACGCGGGAGGAAGAGGATGCGGCAGTG-3′ | 60 °C | ||
| Actin Is F | 5′-GGTCATCACAATCGGCAA-3′ | 54 °C | 108 | [25] |
| Actin Is R | 5′-ATGGAGTTGTACGTGGTCTC-3′ | 54 °C | ||
| P44 F | 5′-ATGGAAGGTAGTGTTGGTTATGGTATT-3′ | 56 °C | 77 | [26] |
| P44 R | 5′-TTGGTCTTGAAGCGCTCGTA-3′ | 56 °C | ||
| 16s rRNA F | 5′-GGTGAGTAATGCATAGGAATC-3′ | 53 °C | 108 | [27] |
| 16s rRNA R | 5′-GCTCATCTAATAGCGATAAATC-3′ | 53 °C | ||
| rpoB F | 5′-CTTTATCCTGCTTTAGAACAACATC-3′ | 52 °C | 286 | [18] |
| rpoB R | 5′-GGTCCGTATGGTCTGGTTACT-3′ | 52 °C | ||
| Ifng F | 5′-AGCGTCATTGAATCACACCT-3′ | 54 °C | 196 | This study |
| Ifng R | 5′-ATCAGCAGCGACTCCTTTTC-3′ | 54 °C | ||
| IL1βF | 5′-CCTGTGTAATGAAAGACGGC-3′ | 54 °C | 216 | This study |
| IL1βR | 5′-TGTCCTGACCACTGTTGTTT-3′ | 54 °C | ||
| Irf1 F | 5′-ATAACTCCAGCACTGTCACC-3′ | 54 °C | 177 | This study |
| IrF1 R | 5′-AAGGTCTTCGGCTATCTTCC-3′ | 54 °C | ||
| Stat2 F | 5′- TGGGACTTCGGCTTCTTGAC-3′ | 57 °C | 247 | This study |
| Stat2 R | 5′- TCTTGGGATTTGGGCTGAGC-3′ | 57 °C | ||
| S100a8 F | 5′-CACCATGCCCTCTACAAGAA-3′ | 54 °C | 161 | This study |
| S100a8 R | 5′-CCCACTTTTATCACCATCGC-3′ | 54 °C | ||
| Acan F | 5′-CAGATGGCACCCTCCGATAC-3′ | 57 °C | 151 | This study |
| Acan R | 5′-GACACACCTCGGAAGCAGAA-3′ | 57 °C | ||
| Matn3 F | 5′-GAGGGTGGCTGTGGTGAACT-3′ | 59 °C | 160 | This study |
| Matn3 R | 5′-GGCTTCCTCCATCGCTGTCT-3′ | 59 °C |
| Comparison | Upregulated Genes | Downregulated Genes | Total Significantly DEGs |
|---|---|---|---|
| Intact skin v uninfected tick bite sites | 797 | 416 | 1213 |
| Intact skin v Anaplasma-infected tick bite | 1417 | 1142 | 2559 |
| Uninfected tick bite sites v Anaplasma-infected tick bite | 476 | 146 | 622 |
| Pathway Identifier | Pathway Name | Number Entities Found | Number Entities Total | Entities p-Value | Entities FDR |
|---|---|---|---|---|---|
| R-HSA-909733 | Interferon alpha/beta signaling | 50 | 188 | 1.11 × 10−16 | 1.52 × 10−14 |
| R-HSA-6783783 | Interleukin-10 signaling | 38 | 86 | 1.11 × 10−16 | 1.52 × 10−14 |
| R-HSA-913531 | Interferon signaling | 81 | 394 | 1.11 × 10−16 | 1.52 × 10−14 |
| R-HSA-1280215 | Cytokine signaling in immune system | 149 | 1092 | 1.11 × 10−16 | 1.52 × 10−14 |
| R-HSA-168256 | Immune system | 213 | 2684 | 1.11 × 10−16 | 1.52 × 10−14 |
| R-HSA-449147 | Signaling by interleukins | 70 | 643 | 4.66 × 10−15 | 5.32 × 10−13 |
| R-HSA-877300 | Interferon gamma signaling | 41 | 250 | 1.18 × 10−14 | 1.15 × 10−12 |
| R-HSA-380108 | Chemokine receptors bind chemokines | 17 | 57 | 1.40 × 10−10 | 1.21 × 10−8 |
| R-HSA-6785807 | Interleukin-4 and interleukin-13 signaling | 26 | 211 | 2.48 × 10−7 | 1.88 × 10−5 |
| R-HSA-1169410 | Antiviral mechanism by IFN-stimulated genes | 14 | 94 | 1.97 × 10−5 | 0.001342146 |
| R-HSA-375276 | Peptide ligand-binding receptors | 20 | 203 | 1.29 × 10−4 | 0.007973888 |
| R-HSA-1169408 | ISG15 antiviral mechanism | 11 | 83 | 3.99 × 10−4 | 0.022716167 |
| R-HSA-9705462 | Inactivation of CSF3 (G-CSF) signaling | 6 | 27 | 6.44 × 10−4 | 0.03349729 |
| Pathway Identifier | Pathway Name | Number Entities Found | Number Entities Total | Entities p-Value | Entities FDR |
|---|---|---|---|---|---|
| R-HSA-1474244 | Extracellular matrix organization | 29 | 329 | 1.11 × 10−16 | 3.26 × 10−14 |
| R-HSA-2022090 | Assembly of collagen fibrils and other multimeric structures | 13 | 67 | 7.42 × 10−13 | 5.98 × 10−11 |
| R-HSA-1474290 | Collagen formation | 15 | 104 | 8.05 × 10−13 | 5.98 × 10−11 |
| R-HSA-1474228 | Degradation of the extracellular matrix | 17 | 148 | 8.20 × 10−13 | 5.98 × 10−11 |
| R-HSA-8948216 | Collagen chain trimerization | 11 | 44 | 3.13 × 10−12 | 1.72 × 10−10 |
| R-HSA-1650814 | Collagen biosynthesis and modifying enzymes | 13 | 76 | 3.52 × 10−12 | 1.72 × 10−10 |
| R-HSA-3000178 | 13 | 79 | 5.66 × 10−12 | 2.38 × 10−10 | |
| R-HSA-216083 | Integrin cell surface interactions | 11 | 86 | 3.39 × 10−9 | 1.22 × 10−7 |
| R-HSA-1442490 | Collagen degradation | 10 | 69 | 5.56 × 10−9 | 1.78 × 10−7 |
| R-HSA-419037 | NCAM1 interactions | 6 | 44 | 9.59 × 10−6 | 2.78 × 10−4 |
| R-HSA-1566948 | Elastic fiber formation | 6 | 46 | 1.23 × 10−5 | 3.20 × 10−4 |
| R-HSA-8874081 | MET activates PTK2 signaling | 5 | 32 | 2.86 × 10−5 | 6.86 × 10−4 |
| R-HSA-3000171 | Non-integrin membrane-ECM interactions | 6 | 61 | 5.86 × 10−5 | 0.001289879 |
| R-HSA-375165 | NCAM signaling for neurite outgrowth | 6 | 70 | 1.24 × 10−4 | 0.002108039 |
| R-HSA-186797 | Signaling by PDGF | 6 | 70 | 1.24 × 10−4 | 0.002108039 |
| R-HSA-3656244 | Defective B4GALT1 causes B4GALT1-CDG (CDG-2d) | 3 | 9 | 1.38 × 10−4 | 0.002108039 |
| R-HSA-3656225 | Defective CHST6 causes MCDC1 | 3 | 9 | 1.38 × 10−4 | 0.002108039 |
| R-HSA-3656243 | Defective ST3GAL3 causes MCT12 and EIEE15 | 3 | 9 | 1.38 × 10−4 | 0.002108039 |
| R-HSA-8875878 | MET promotes cell motility | 5 | 45 | 1.41 × 10−4 | 0.002108039 |
| R-HSA-2022854 | Keratan sulfate biosynthesis | 4 | 37 | 7.38 × 10−4 | 0.010327613 |
| R-HSA-2129379 | Molecules associated with elastic fibers | 4 | 38 | 8.14 × 10−4 | 0.011395335 |
| R-HSA-2022857 | Keratan sulfate degradation | 3 | 22 | 0.001819501 | 0.023653508 |
| R-HSA-2243919 | Crosslinking of collagen fibrils | 3 | 24 | 0.002325531 | 0.027906369 |
| R-HSA-1638074 | Keratan sulfate/keratin metabolism | 4 | 52 | 0.002542129 | 0.029455221 |
| R-HSA-399710 | Activation of AMPA receptors | 2 | 7 | 0.002677747 | 0.029455221 |
| R-HSA-6806834 | Signaling by MET | 5 | 88 | 0.002781469 | 0.03059616 |
| R-HSA-1369062 | ABC transporters in lipid homeostasis | 3 | 29 | 0.00394569 | 0.039456898 |
| R-HSA-8951671 | RUNX3 regulates YAP1-mediated transcription | 2 | 9 | 0.004364679 | 0.043646794 |
| Pathway Identifier | Pathway Name | Number Entities Found | Number Entities Total | Entities p-Value | Entities FDR |
|---|---|---|---|---|---|
| R-HSA-6783783 | Interleukin-10 signaling | 36 | 86 | 7.12 × 10−14 | 1.17 × 10−10 |
| R-HSA-6798695 | Neutrophil degranulation | 61 | 480 | 4.41 × 10−13 | 3.62 × 10−10 |
| R-HSA-380108 | Chemokine receptors bind chemokines | 24 | 57 | 7.27 × 10−11 | 3.97 × 10−8 |
| R-HSA-6785807 | Interleukin-4 and interleukin-13 signaling | 42 | 211 | 2.18 × 10−8 | 8.94 × 10−6 |
| R-HSA-2500257 | Resolution of sister chromatid cohesion | 28 | 134 | 4.57 × 10−7 | 1.50 × 10−4 |
| R-HSA-2467813 | Separation of sister chromatids | 27 | 195 | 1.10 × 10−6 | 3.00 × 10−4 |
| R-HSA-141424 | Amplification of signal from the kinetochores | 22 | 94 | 7.82 × 10−6 | 0.001602622 |
| R-HSA-141444 | Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal | 22 | 94 | 7.82 × 10−6 | 0.001602622 |
| R-HSA-5663220 | RHO GTPases activate formins | 24 | 149 | 1.20 × 10−5 | 0.002177163 |
| R-HSA-68877 | Mitotic prometaphase | 31 | 211 | 1.79 × 10−5 | 0.002933421 |
| R-HSA-9648025 | EML4 and NUDC in mitotic spindle formation | 24 | 121 | 3.02 × 10−5 | 0.004498095 |
| R-HSA-69618 | Mitotic spindle checkpoint | 22 | 111 | 1.34 × 10−4 | 0.018203955 |
| Pathway Identifier | Pathway Name | Number Entities Found | Number Entities Total | Entities p-Value | Entities FDR |
|---|---|---|---|---|---|
| R-HSA-400253 | Circadian clock | 9 | 105 | 0.001723 | 0.60096047 |
| R-HSA-5682910 | LGI-ADAM interactions | 3 | 14 | 0.005775 | 0.60096047 |
| R-HSA-3000480 | Scavenging by Class A receptors | 5 | 49 | 0.008965 | 0.60096047 |
| R-HSA-8874081 | MET activates PTK2 signaling | 4 | 32 | 0.009601 | 0.60096047 |
| R-HSA-3000178 | ECM proteoglycans | 6 | 79 | 0.016775 | 0.60096047 |
| R-HSA-2022870 | Chondroitin sulfate biosynthesis | 3 | 25 | 0.026832 | 0.60096047 |
| R-HSA-1482922 | Acyl chain remodeling of PI | 3 | 25 | 0.026832 | 0.60096047 |
| R-HSA-391903 | Eicosanoid ligand-binding receptors | 3 | 25 | 0.026832 | 0.60096047 |
| R-HSA-8949275 | RUNX3 regulates immune response and cell migration | 2 | 10 | 0.02741 | 0.60096047 |
| R-HSA-8875878 | MET promotes cell motility | 4 | 45 | 0.029135 | 0.60096047 |
| R-HSA-1482925 | Acyl chain remodeling of PG | 3 | 26 | 0.029633 | 0.60096047 |
| R-HSA-1442490 | Collagen degradation | 5 | 69 | 0.033148 | 0.60096047 |
| R-HSA-430116 | GP1b-IX-V activation signaling | 2 | 12 | 0.038188 | 0.60096047 |
| R-HSA-3000170 | Syndecan interactions | 3 | 29 | 0.038924 | 0.60096047 |
| R-HSA-1482801 | Acyl chain remodeling of PS | 3 | 31 | 0.045843 | 0.60096047 |
| R-HSA-6785807 | Interleukin-4 and interleukin-13 signaling | 10 | 211 | 0.046493 | 0.60096047 |
| R-HSA-1650814 | Collagen biosynthesis and modifying enzymes | 5 | 76 | 0.046724 | 0.60096047 |
| R-HSA-391908 | Prostanoid ligand receptors | 2 | 14 | 0.050296 | 0.60096047 |
| R-HSA-2214320 | Anchoring fibril formation | 2 | 15 | 0.0568 | 0.60096047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Underwood, J.; Harvey, C.; Lohstroh, E.; Pierce, B.; Chambers, C.; Guzman Valencia, S.; Oliva Chávez, A.S. Anaplasma phagocytophilum Transmission Activates Immune Pathways While Repressing Wound Healing in the Skin. Life 2022, 12, 1965. https://doi.org/10.3390/life12121965
Underwood J, Harvey C, Lohstroh E, Pierce B, Chambers C, Guzman Valencia S, Oliva Chávez AS. Anaplasma phagocytophilum Transmission Activates Immune Pathways While Repressing Wound Healing in the Skin. Life. 2022; 12(12):1965. https://doi.org/10.3390/life12121965
Chicago/Turabian StyleUnderwood, Jacob, Cristina Harvey, Elizabeth Lohstroh, Branden Pierce, Cross Chambers, Stephanie Guzman Valencia, and Adela S. Oliva Chávez. 2022. "Anaplasma phagocytophilum Transmission Activates Immune Pathways While Repressing Wound Healing in the Skin" Life 12, no. 12: 1965. https://doi.org/10.3390/life12121965
APA StyleUnderwood, J., Harvey, C., Lohstroh, E., Pierce, B., Chambers, C., Guzman Valencia, S., & Oliva Chávez, A. S. (2022). Anaplasma phagocytophilum Transmission Activates Immune Pathways While Repressing Wound Healing in the Skin. Life, 12(12), 1965. https://doi.org/10.3390/life12121965







