Foliar Application of Spermidine Alleviates Waterlogging-Induced Damages to Maize Seedlings by Enhancing Antioxidative Capacity, Modulating Polyamines and Ethylene Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatment
2.2. Measurement of Chlorophyll Content and Other Growth Attributes
2.3. Measurement of Reactive Oxygen Species (ROS) and Malondialdehyde (MDA) Contents
2.4. Protein Extraction and Estimation of Antioxidant Enzyme Activities
2.5. Estimation of Non-Enzymatic Antioxidants
2.6. Determination of Polyamines
2.7. Measurement of Polyamines Metabolic Enzymes Activities
2.8. Quantification of Ethylene
2.9. Extraction of RNA and Quantitative Real-Time PCR Analyses
2.10. Statistical Analyses
3. Results
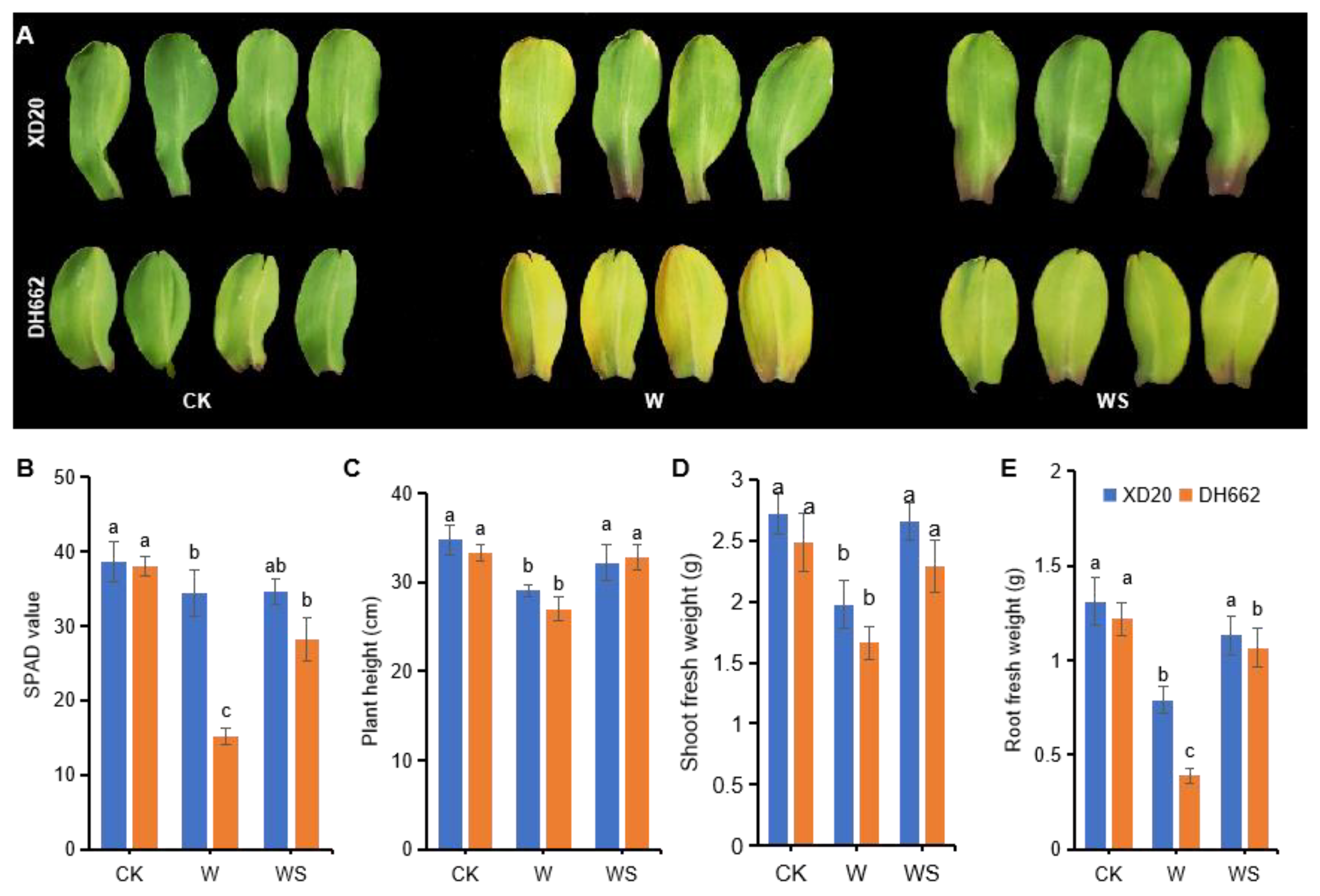
3.1. Variations in Chlorophyll Content and Growth Attributes
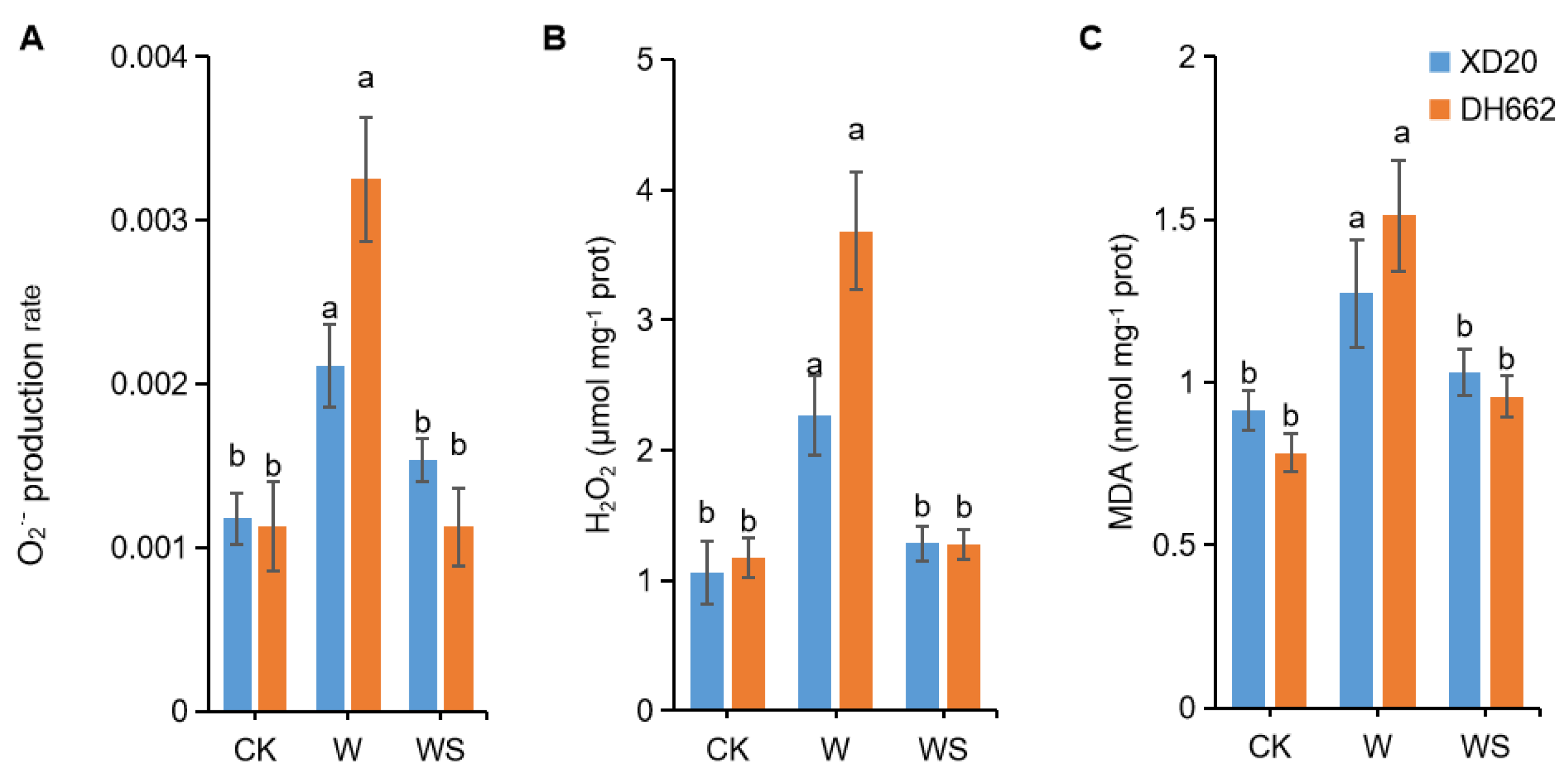
3.2. Impacts of Exogenous Spd on O2− Production, H2O2, and MDA Content
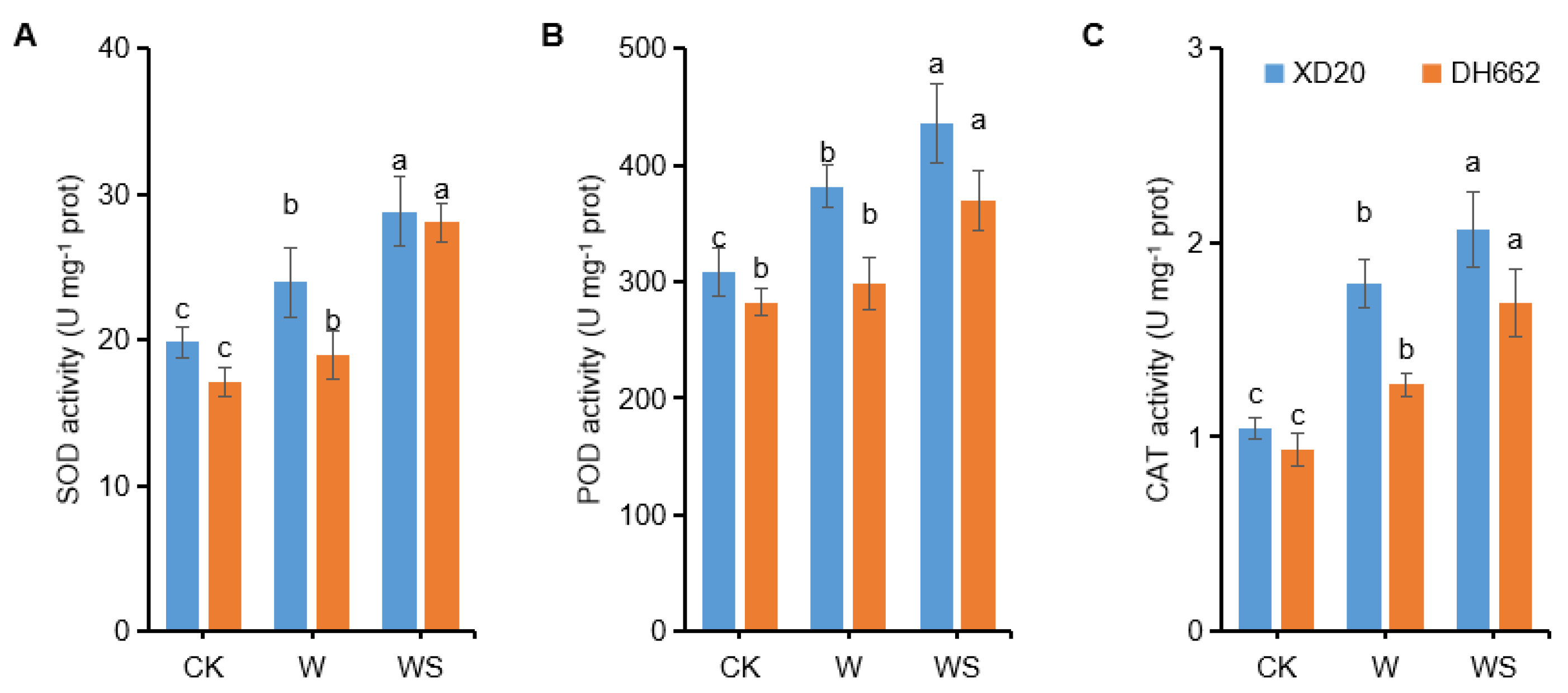
3.3. Variations in the Activities of SOD, POD, and CAT
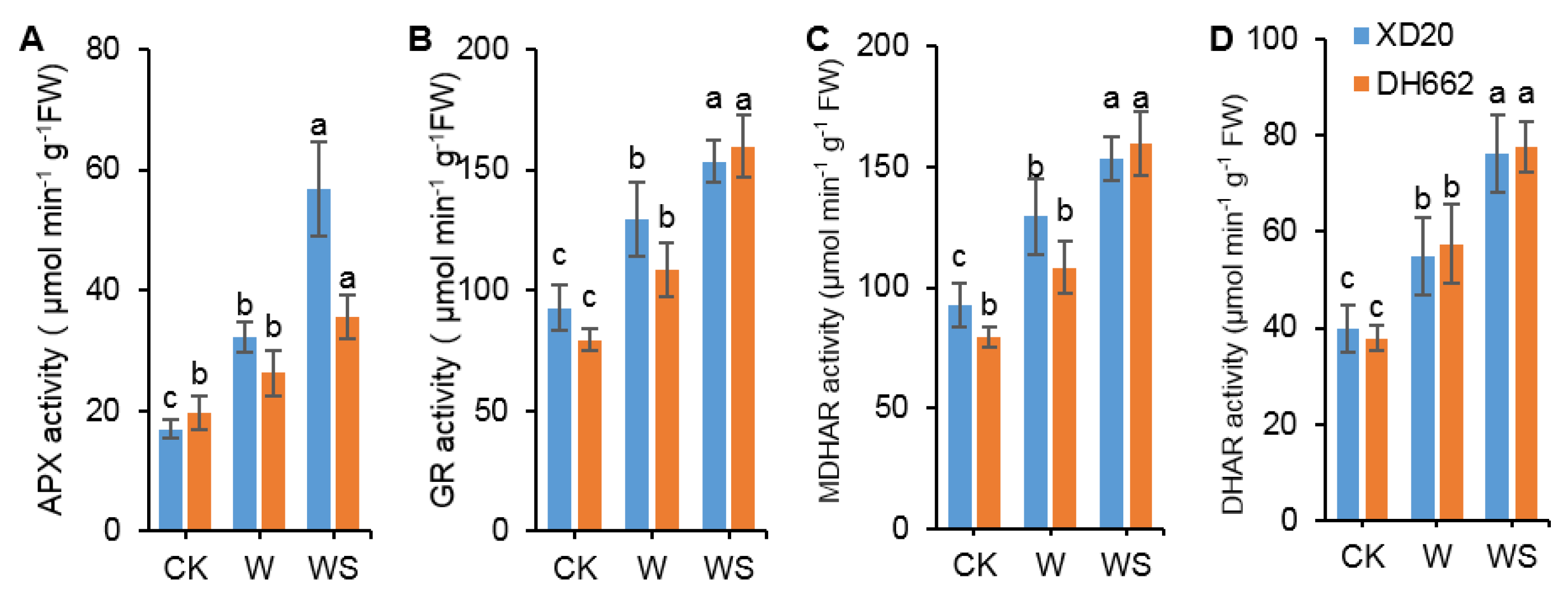
3.4. Variations in the Activities of APX, GR, MDHAR, and DHAR
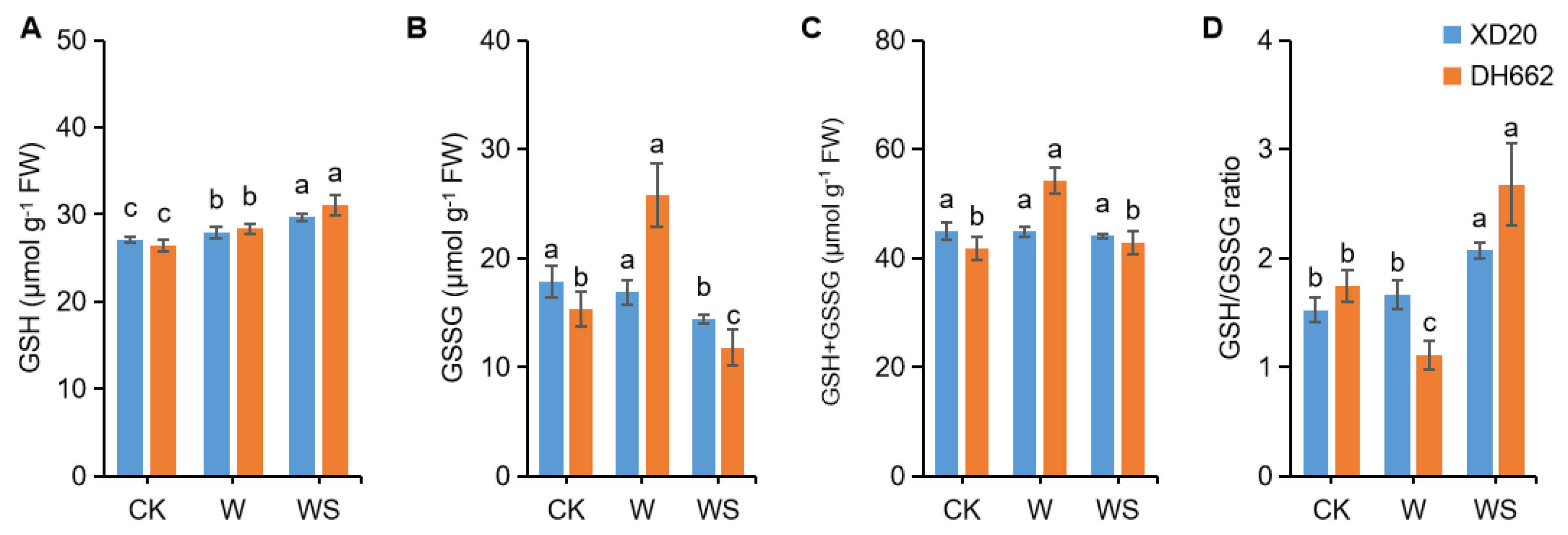
3.5. Effects of Exogenous Spd on the Non-Enzymatic Antioxidants
3.6. Effects of Exogenous Spd on Endogenous Put, Spd, and Spm
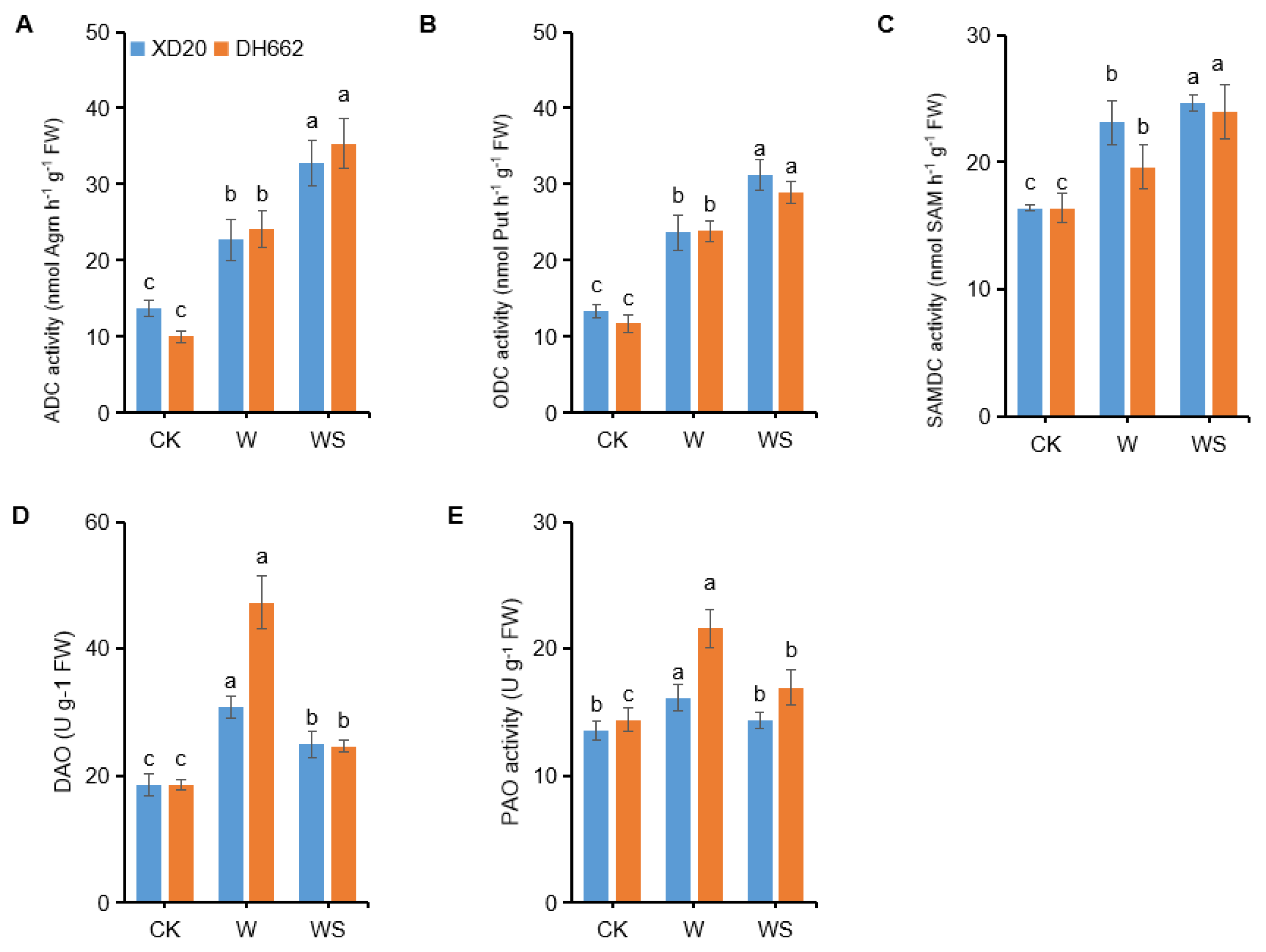
3.7. Effects of Exogenous Spd on PAs Metabolic Enzyme Activities
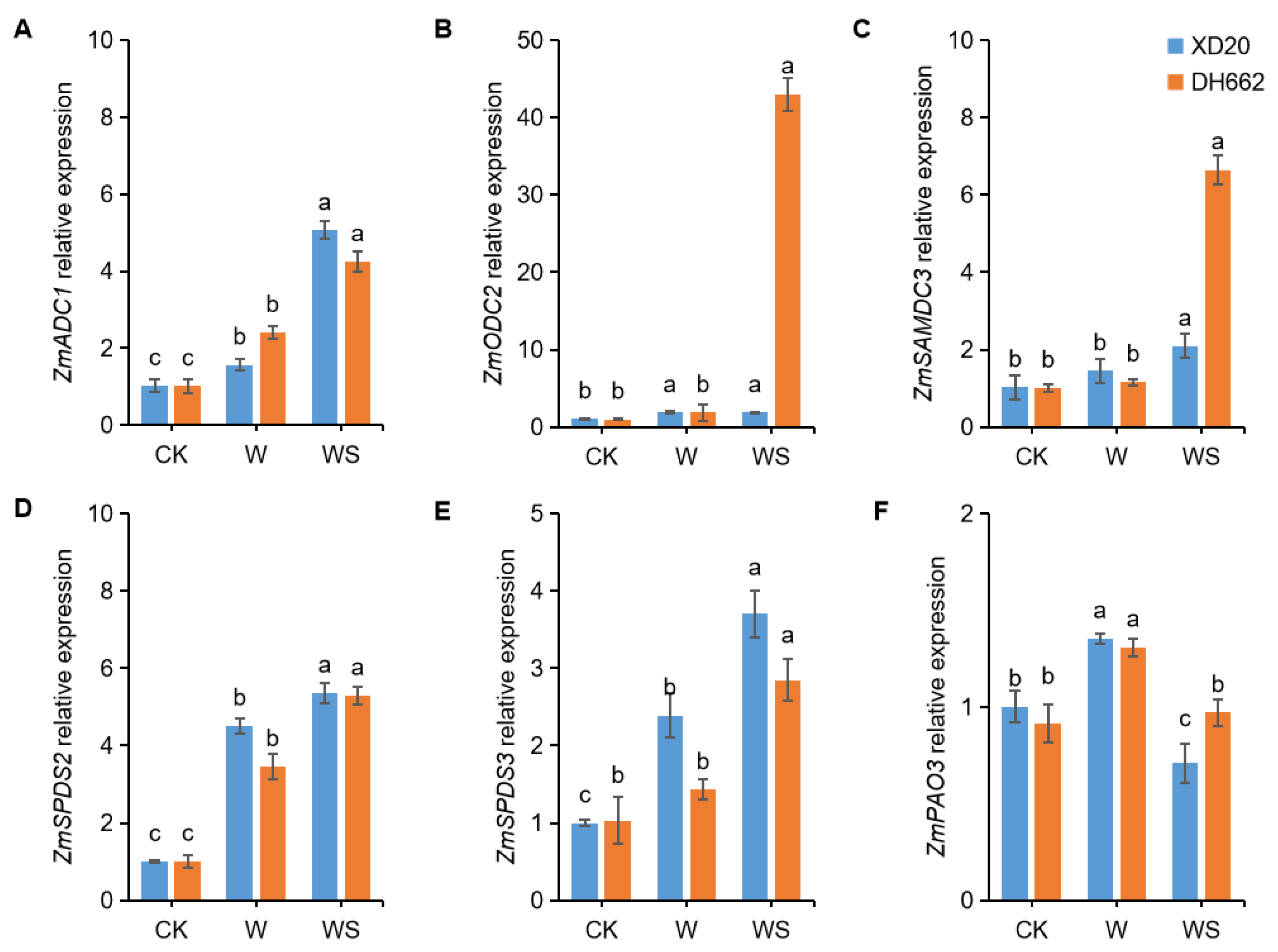
3.8. Effects of Exogenous Spd on the Expression of PA-Related Genes
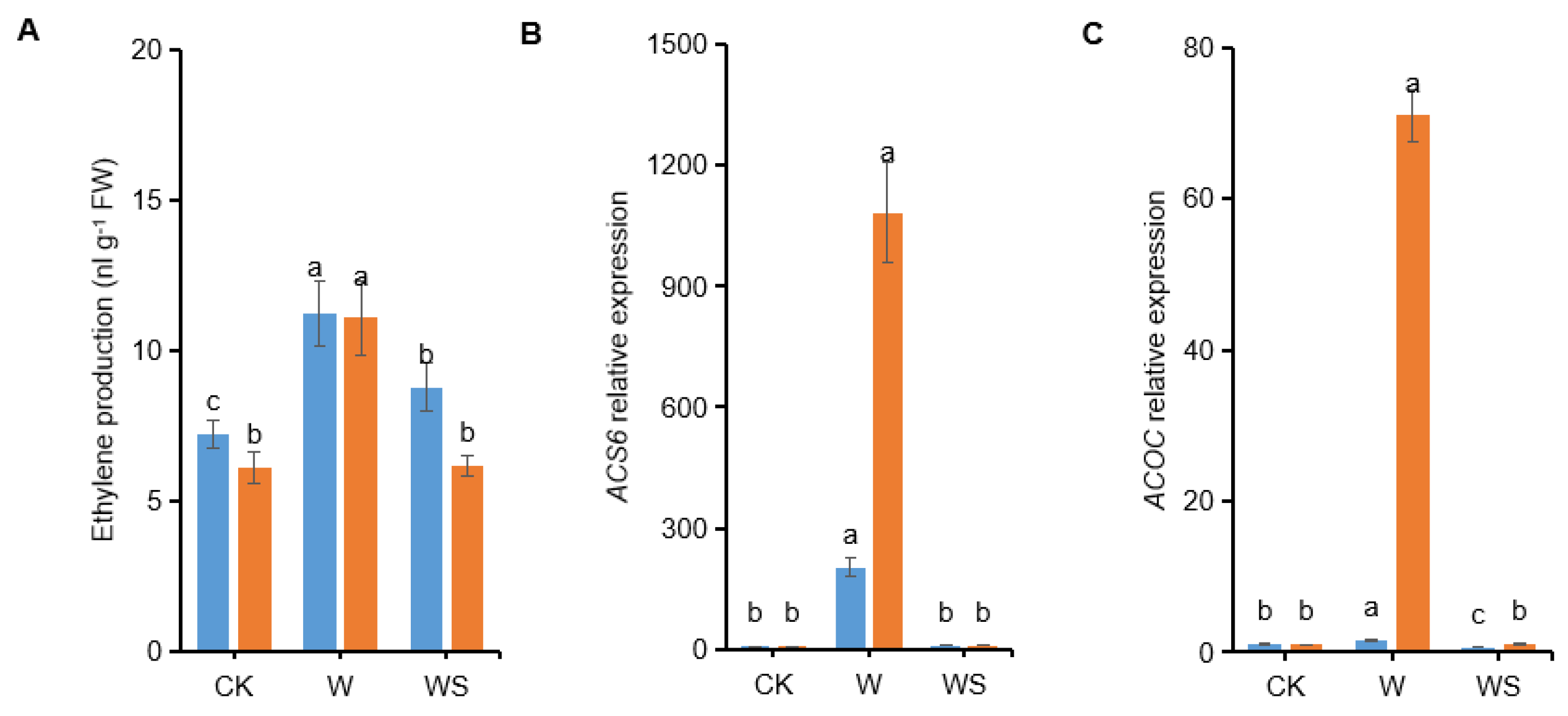
3.9. Exogenous Spd Application Down-Regulates Ethylene Biosynthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, N.; Laxman, R.; Shivashankara, K. Physiological and morphological responses of horticultural crops to abiotic stresses. In Abiotic Stress Physiology of Horticultural Crops; Springer: Berlin/Heidelberg, Germany, 2016; pp. 3–17. [Google Scholar]
- Colmer, T.D.; Flowers, T.J. Flooding tolerance in halophytes. New Phytol. 2008, 179, 964–974. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, Y.; Shabala, S.; Koutoulis, A.; Shabala, L.; Johnson, P.; Hu, H.; Zhou, M. A new major-effect QTL for waterlogging tolerance in wild barley (H. spontaneum). Theor. Appl. Genet. 2017, 130, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.; Van, V.H.; Sasidharan, R. Learning from nature: The use of non-model species to identify novel acclimations to flooding stress. AoB Plants 2014, 6, 490–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, G.; Shabala, S.; Koutoulis, A.; Shabala, L.; Johnson, P.; Li, C.; Zhou, M. Identification of aerenchyma formation-related QTL in barley that can be effective in breeding for waterlogging tolerance. Theor. Appl. Genet. 2016, 129, 1167–1177. [Google Scholar] [CrossRef]
- Boru, G.; Vantoai, T.; Alves, J.; Hua, D.; Knee, M. Responses of soybean to oxygen deficiency and elevated root-zone carbon dioxide concentration. Ann. Bot. 2003, 91, 447–453. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Pan, X.; Najeeb, U.; Tan, D.K.Y.; Fahad, S.; Zahoor, R.; Luo, H. Coping with drought: Stress and adaptive mechanisms, and management through cultural and molecular alternatives in cotton as vital constituents for plant stress resilience and fitness. Biol. Res. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Peltonen-Sainio, P.; Rajala, A. Chlormequat chloride and ethephon affect growth and yield formation of conventional, naked and dwarf oat. Agric. Food Sci. Finl. 2001, 10, 165–174. [Google Scholar] [CrossRef]
- Gommers, C.M.; Visser, E.J.; St Onge, K.R.; Voesenek, L.A.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhang, Z.; Liu, N.; Li, D.; Hu, L. Melatonin improved waterlogging tolerance in alfalfa (Medicago sativa) by reprogramming polyamine and ethylene metabolism. Front. Plant Sci. 2019, 10, 44. [Google Scholar] [CrossRef]
- Shi, H.; Chan, Z. Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J. Integr. Plant Biol. 2014, 56, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ariyaratne, M. A New Perspective on Polyamine Biosynthesis and Transport in Arabidopsis thaliana; Bowling Green State University: Bowling Green, OH, USA, 2019. [Google Scholar]
- Bouchereau, A.; Aziz, A.; Larher, F.; Martin-Tanguy, J. Polyamines and environmental challenges: Recent development. Plant Sci. 1999, 140, 103–125. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Varshney, P.; Yusuf, M.; Ahmad, A. Polyamines: Potent modulators of plant responses to stress. J. Plant Interact. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Fatima, T.; Handa, A.K.; Mattoo, A.K. Polyamines and their biosynthesis/catabolism genes are differentially modulated in response to heat versus cold stress in tomato leaves (Solanum lycopersicum L.). Cells 2020, 9, 1749. [Google Scholar] [CrossRef] [PubMed]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and abiotic stresses in plants. Abiotic Biot. Stress Plants 2019, 1–19. [Google Scholar]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Liu, B.; Peng, X.; Han, L.; Hou, L.; Li, B. Effects of exogenous spermidine on root metabolism of cucumber seedlings under salt stress by GC-MS. Agronomy 2020, 10, 459. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Li, W.; Lu, J. Effects of ammonium on the antioxidative response in Hydrilla verticillata (Lf) Royle plants. Ecotoxicol. Environ. Saf. 2010, 73, 189–195. [Google Scholar] [CrossRef]
- Zor, T.; Selinger, Z. Linearization of the Bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef]
- Charles, B.; Irwin, F. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar]
- Kochba, J.; Lavee, S.; Spiegel-Roy, P. Differences in peroxidase activity and isoenzymes in embryogenic ane non-embryogenic ‘Shamouti’orange ovular callus lines. Plant Cell Physiol. 1977, 18, 463–467. [Google Scholar] [CrossRef]
- Shang, D.; Yu, Q.; Liu, W.; Zhang, S.; Li, Y.; Chen, J.; Zhang, Z.; Lu, X. Enhanced porphyrin-based fluorescence imaging-guided photodynamic/photothermal synergistic cancer therapy by mitochondrial targeting. Sci. China Mater. 2022, 65, 527–535. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zhang, R.; Huang, R. The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012, 71, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.; Fujita, M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol. Plant. 2015, 59, 745–756. [Google Scholar] [CrossRef]
- Kotzabasis, K.; Christakishampsas, M.D.; Roubelakisangelakis, K.A. A narrow-bore HPLC method for the identification and quantitation of free, conjugated, and bound polyamines. Anal. Biochem. 1993, 214, 484–489. [Google Scholar] [CrossRef]
- Zhao, F.-G.; Zhang, G.-Z.; Zhang, Z.-F. Changes of free polyamines levels and activities of some enzymes during senescence stage of peanut leaves. Plant Physiol. Commun. 1996, 351–353. [Google Scholar]
- Hu, X.; Zhang, Y.; Shi, Y.; Zhang, Z.; Zou, Z.; Zhang, H.; Zhao, J. Effect of exogenous spermidine on polyamine content and metabolism in tomato exposed to salinity–alkalinity mixed stress. Plant Physiol. Biochem. 2012, 57, 200–209. [Google Scholar] [CrossRef]
- Yamauchi, T.; Shiono, K.; Nagano, M.; Fukazawa, A.; Ando, M.; Takamure, I.; Mori, H.; Nishizawa, N.K.; Kawai-Yamada, M.; Tsutsumi, N. Ethylene biosynthesis is promoted by very-long-chain fatty acids during lysigenous aerenchyma formation in rice roots. Plant Physiol. 2015, 169, 180–193. [Google Scholar] [CrossRef]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and Waterlogging Stress in Plants: A Review Highlighting Research Opportunities and Understudied Aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef]
- Jackson, M.; Colmer, T. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Striker, G.G.; Colmer, T.D. Flooding tolerance of forage legumes. J. Exp. Bot. 2017, 68, 1851–1872. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Hossain, Z.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant. 2008, 132, 452–466. [Google Scholar] [CrossRef]
- Hai, X.; Mi, J.; Zhao, B.; Zhang, B.; Zhao, Z.; Liu, J. Foliar Application of Spermidine Reduced the Negative Effects of Salt Stress on Oat Seedlings. Front. Plant Sci. 2022, 13, 846280. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Exogenous spermidine alleviates low temperature injury in mung bean (Vigna radiata L.) seedlings by modulating ascorbate-glutathione and glyoxalase pathway. Int. J. Mol. Sci. 2015, 16, 30117–30132. [Google Scholar] [CrossRef]
- Yetisir, H.; Caliskan, M.E.; Soylu, S.; Sakar, M. Some physiological and growth responses of watermelon [Citrullus lanatus (Thunb.) Matsum. and Nakai] grafted onto Lagenaria siceraria to flooding. Environ. Exp. Bot. 2006, 58, 1–8. [Google Scholar] [CrossRef]
- He, N.; Umer, M.J.; Yuan, P.; Wang, W.; Zhu, H.; Zhao, S.; Lu, X.; Xing, Y.; Gong, C.; Liu, W. Expression dynamics of metabolites in diploid and triploid watermelon in response to flooding. PeerJ 2022, 10, e13814. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Ines, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Latef, A.A.A.; Hasanuzzaman, M.; Tahjib-Ul-Arif, M. Mitigation of salinity stress by exogenous application of cytokinin in faba bean (Vicia faba L.). Not. Bot. Horti Agrobot. Cluj Napoca 2021, 49, 12192. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, B.; Peng, Y.; Zhang, Y. Adaptability to abiotic stress regulated by γ-aminobutyric acid in relation to alterations of endogenous polyamines and organic metabolites in creeping bentgrass. Plant Physiol. Biochem. 2020, 157, 185–194. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 2012, 149, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, S.S.; Gill, R.; Hasanuzzaman, M.; Duarte, A.C.; Pereira, E.; Ahmad, I.; Tuteja, R.; Tuteja, N. Metal/metalloid stress tolerance in plants: Role of ascorbate, its redox couple, and associated enzymes. Protoplasma 2014, 251, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Beckett, R.P.; Wornik, S.; Zorn, M.; Pfeifhofer, H.W. Revival of a resurrection plant correlates with its antioxidant status. Plant J. 2002, 31, 13–24. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Szalai, G.; Kellős, T.; Galiba, G.; Kocsy, G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Sang, Q.; Shu, S.; Shan, X.; Guo, S.; Sun, J. Effects of exogenous spermidine on antioxidant system of tomato seedlings exposed to high temperature stress. Russ. J. Plant Physiol. 2016, 63, 645–655. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Galston, A.W.; Kaur-Sawhney, R. Polyamines as endogenous growth regulators. In Plant Hormones; Springer: Berlin/Heidelberg, Germany, 1995; pp. 158–178. [Google Scholar]
- Groppa, M.; Benavides, M. Polyamines and abiotic stress: Recent advances. Amino Acids 2008, 34, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Dey, A.; Gupta, B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 2013, 35, 2015–2036. [Google Scholar] [CrossRef]
- Shao, G.; Lan, J.; Yu, S.; Liu, N.; Guo, R.; She, D. Photosynthesis and growth of winter wheat in response to waterlogging at different growth stages. Photosynthetica 2013, 51, 429–437. [Google Scholar] [CrossRef]
- Casierra-Posada, F.; Cutler, J. Photosystem II fluorescence and growth in cabbage plants (Brassica oleracea var. capitata) grown under waterlogging stress. Rev. UDCA Actual. Divulg. Científica 2017, 20, 321–328. [Google Scholar] [CrossRef]
- Chen, H. Ecophysiology of Lepidium latifolium, an Invasive Exotic, in Response to Root Oxygen Stress; University of Nevada: Reno, NV, USA, 2002. [Google Scholar]
- Najeeb, U.; Bange, M.P.; Tan, D.K.; Atwell, B.J. Consequences of waterlogging in cotton and opportunities for mitigation of yield losses. AoB Plants 2015, 7, plv080. [Google Scholar] [CrossRef]
- Sasidharan, R.; Voesenek, L.A. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef]


 ” points to inhibition and “
” points to inhibition and “ ” points to activation.
” points to activation.
 ” points to inhibition and “
” points to inhibition and “ ” points to activation.
” points to activation.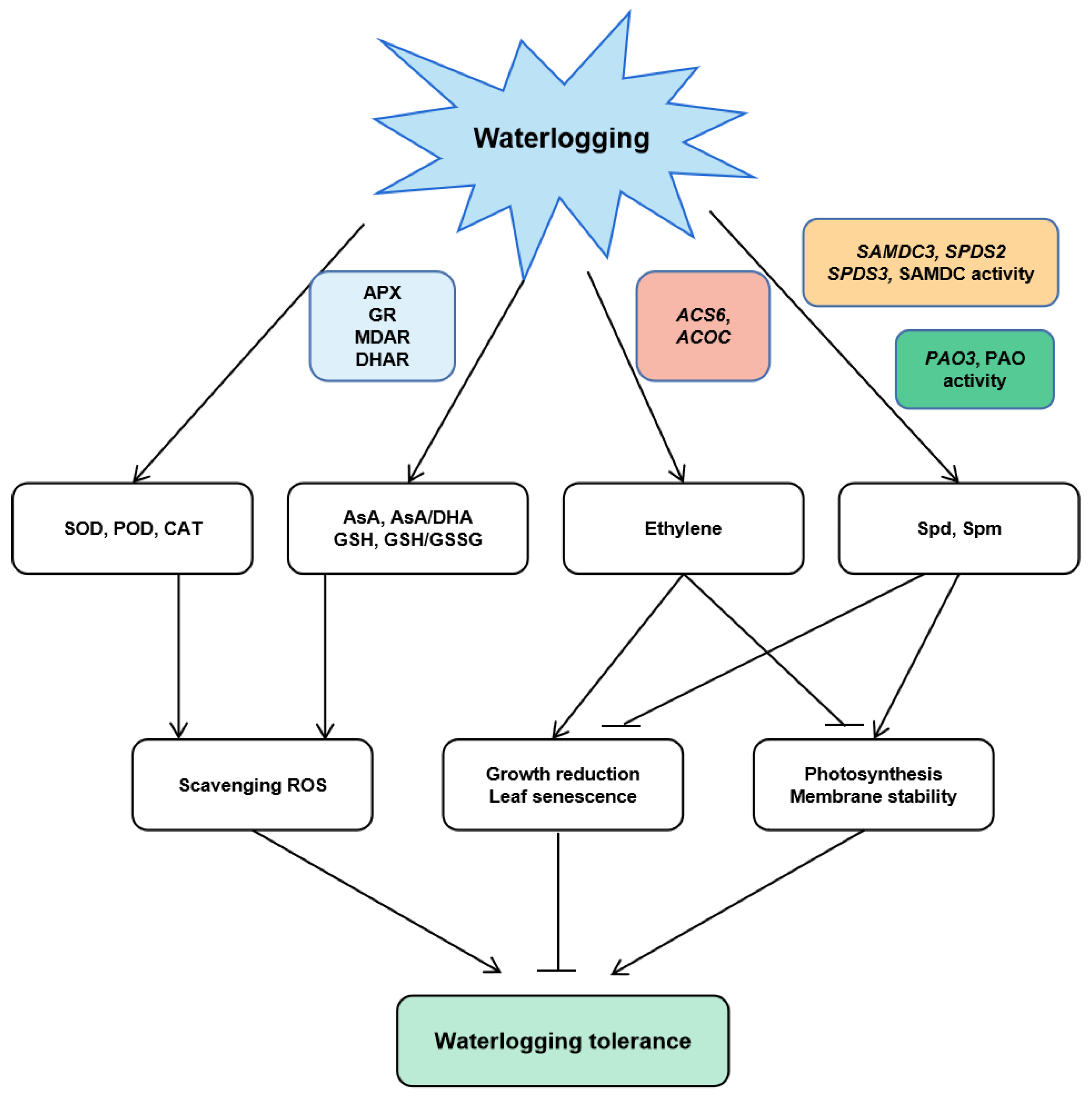
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, Q.; Zhang, M.; Zhao, Y.; Dong, P.; Zhao, Y.; Li, H.; Jia, X.; An, P.; Tang, Y.; et al. Foliar Application of Spermidine Alleviates Waterlogging-Induced Damages to Maize Seedlings by Enhancing Antioxidative Capacity, Modulating Polyamines and Ethylene Biosynthesis. Life 2022, 12, 1921. https://doi.org/10.3390/life12111921
Wang X, Wang Q, Zhang M, Zhao Y, Dong P, Zhao Y, Li H, Jia X, An P, Tang Y, et al. Foliar Application of Spermidine Alleviates Waterlogging-Induced Damages to Maize Seedlings by Enhancing Antioxidative Capacity, Modulating Polyamines and Ethylene Biosynthesis. Life. 2022; 12(11):1921. https://doi.org/10.3390/life12111921
Chicago/Turabian StyleWang, Xiuling, Qun Wang, Moubiao Zhang, Yulong Zhao, Pengfei Dong, Yali Zhao, Hongping Li, Xucun Jia, Panpan An, Yulou Tang, and et al. 2022. "Foliar Application of Spermidine Alleviates Waterlogging-Induced Damages to Maize Seedlings by Enhancing Antioxidative Capacity, Modulating Polyamines and Ethylene Biosynthesis" Life 12, no. 11: 1921. https://doi.org/10.3390/life12111921
APA StyleWang, X., Wang, Q., Zhang, M., Zhao, Y., Dong, P., Zhao, Y., Li, H., Jia, X., An, P., Tang, Y., & Li, C. (2022). Foliar Application of Spermidine Alleviates Waterlogging-Induced Damages to Maize Seedlings by Enhancing Antioxidative Capacity, Modulating Polyamines and Ethylene Biosynthesis. Life, 12(11), 1921. https://doi.org/10.3390/life12111921



