Blood Vessels as a Key Mediator for Ethanol Toxicity: Implication for Neuronal Damage
Abstract
1. Introduction: Ethanol Effect on the Nervous System
2. Effect on Ethanol on Blood Vessel Endothelial Cells
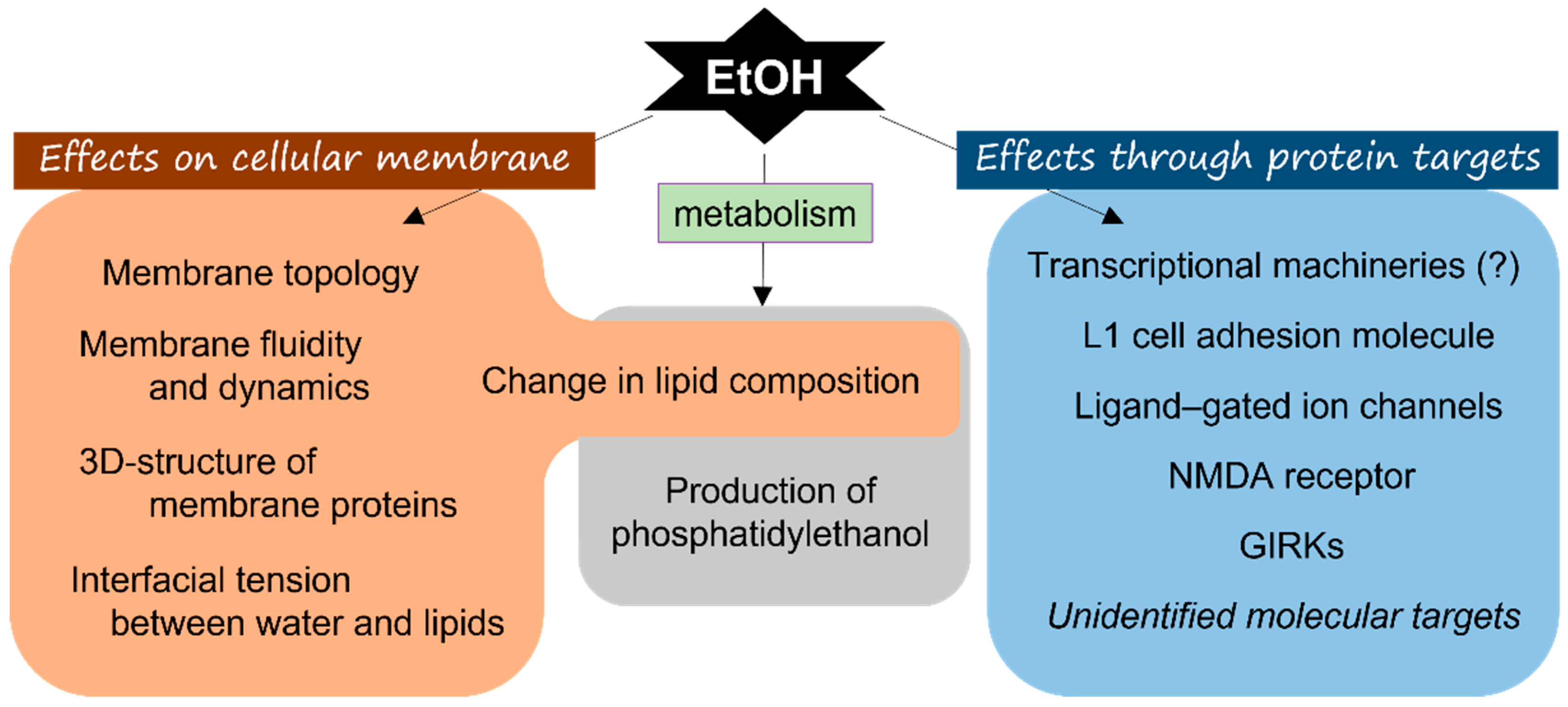
2.1. Molecular Target of Ethanol
2.1.1. Direct Action of Ethanol on Membrane Lipids
2.1.2. “Receptor” Proteins of Ethanol
2.2. Effect of Ethanol on Endothelial Functions
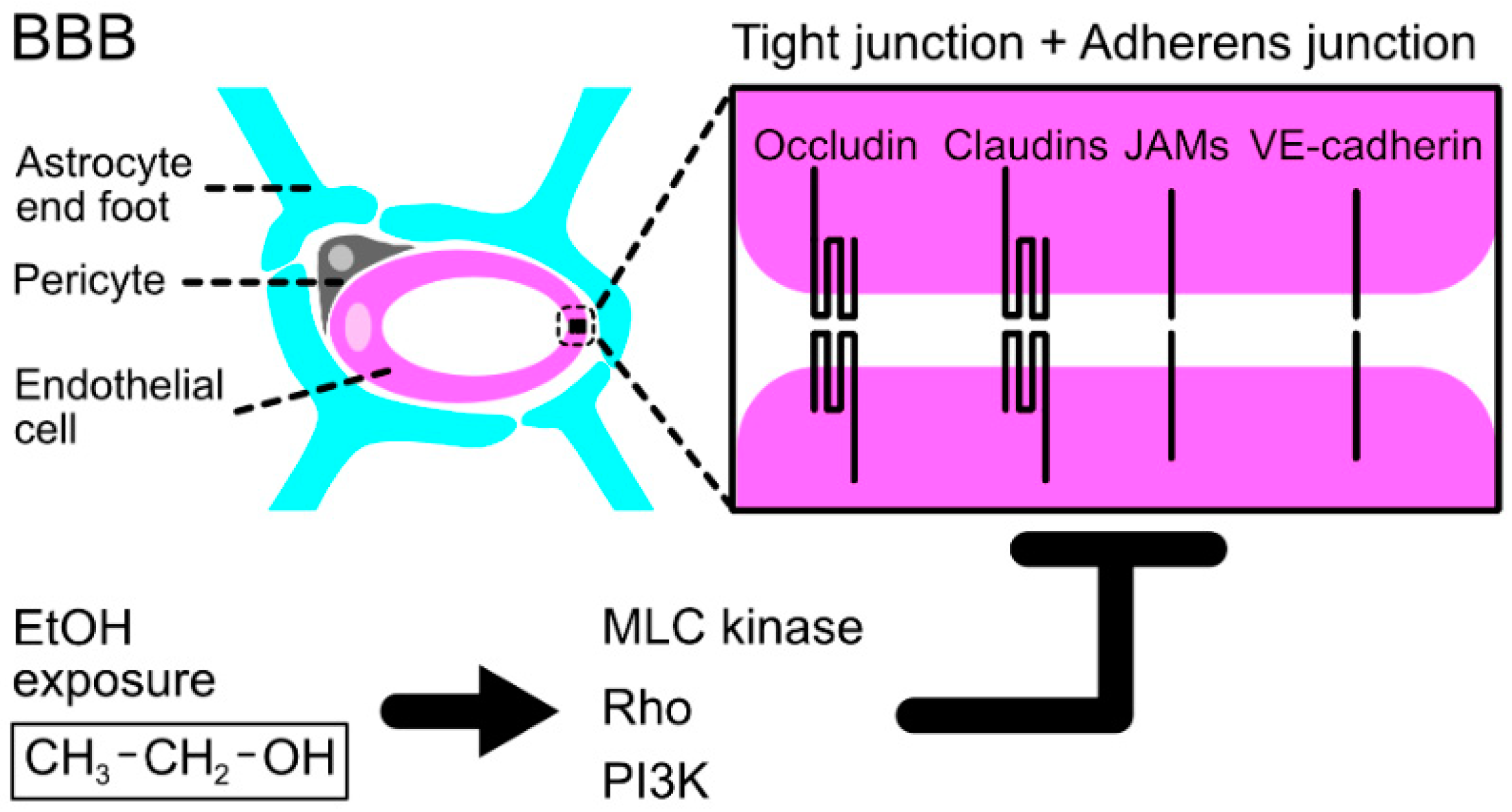
2.3. Ethanol Toxicity through the Impairment of the Blood–Brain Barrier
2.4. Ethanol Toxicity through Nitric Oxide Production
2.4.1. Roles of NO in Ethanol Toxicity in the Nervous System
2.4.2. Contribution of eNOS to Action of Ethanol on Endothelial Cells
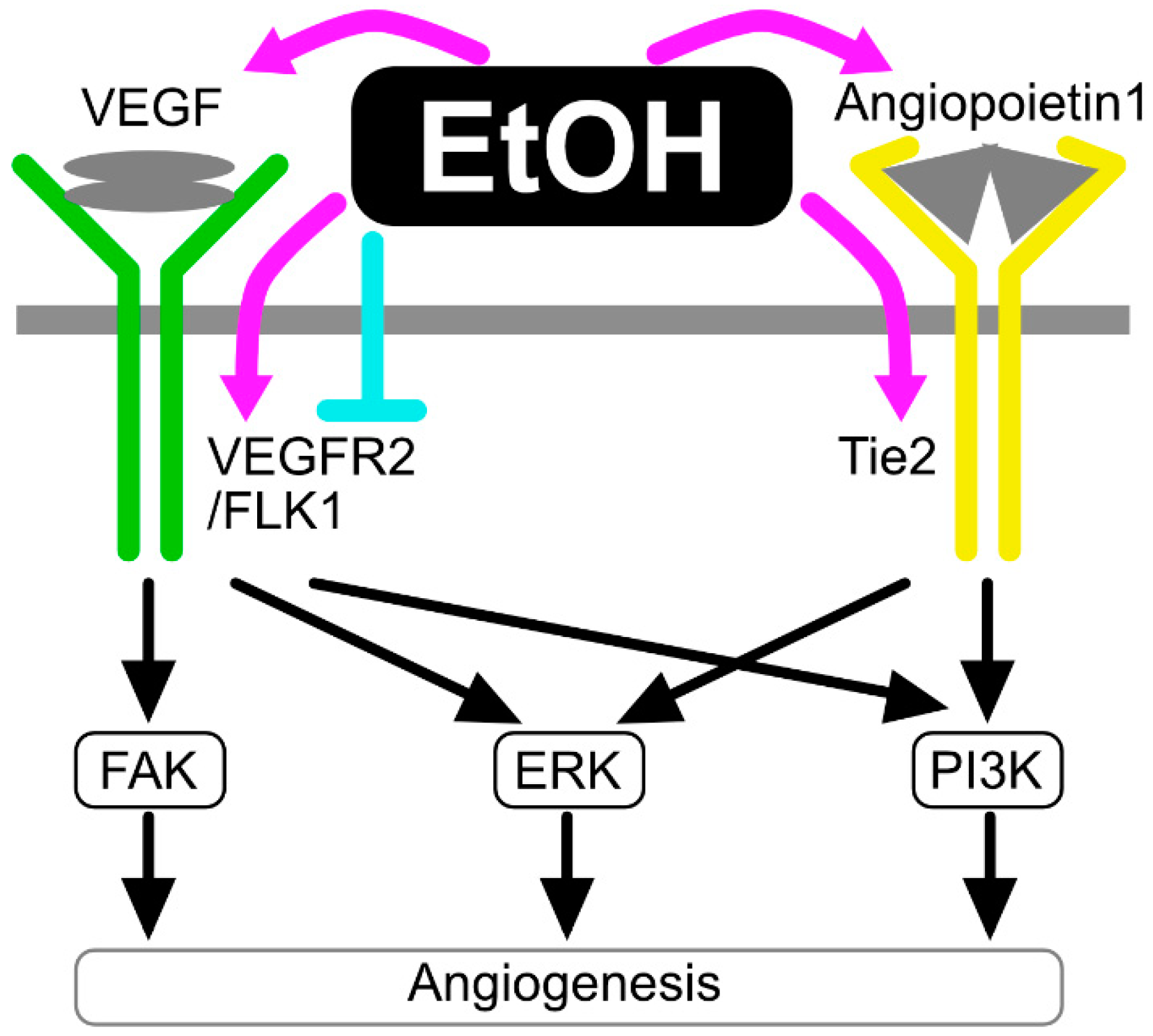
2.5. The Effects of Ethanol on Angiogenesis/Blood Vessel Remodeling
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruno, A. Cerebrovascular complications of alcohol and sympathomimetic drug abuse. Curr. Neurol. Neurosci. Rep. 2003, 3, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Witte, K.; Schrödl, W.; Schütt, C. Chronic alcoholism causes deleterious conditioning of innate immunity. Alcohol Alcohol. 2004, 39, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Jiang, Y. Differential activation of NF kappa B/RelA-p50 and NF kappa B/p50-p50 in control and alcohol-drinking rats subjected to carrageenin-induced pleurisy. Mediat. Inflamm. 2004, 13, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.W.; Drews-Botsch, C.; Falek, A.; Coles, C.; Brown, L.A.S. Maternal alcohol abuse and neonatal infection. Alcohol. Clin. Exp. Res. 2005, 29, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Gullo, L.; Migliori, M.; Brunetti, M.A.; Manca, M. Alcoholic pancreatitis: New insights into an old disease. Curr. Gastroenterol. Rep. 2005, 7, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, A.C.; Zhao, S.; Young, C.L.; Lam, L.; Jones, S.H.; Edwards, D.R.V.; Hartmann, K.E. Alcohol use in pregnancy and miscarriage: A systematic review and meta-analysis. Alcohol. Clin. Exp. Res. 2019, 43, 1606–1616. [Google Scholar] [CrossRef]
- Sulik, K.K.; Johnston, M.C.; Webb, M.A. Fetal alcohol syndrome: Embryogenesis in a mouse model. Science 1981, 214, 936–938. [Google Scholar] [CrossRef]
- Miller, M.W. Effect of early exposure to ethanol on the protein and DNA contents of specific brain regions in the rat. Brain Res. 1996, 734, 286–294. [Google Scholar] [CrossRef]
- Lipinski, R.J.; Hammond, P.; O’Leary-Moore, S.K.; Ament, J.J.; Pecevich, S.J.; Jiang, Y.; Budin, F.; Parnell, S.E.; Suttie, M.; Godin, E.A.; et al. Ethanol-induced face-brain dysmorphology patterns are correlative and exposure-stage dependent. PLoS ONE 2012, 7, e43067. [Google Scholar] [CrossRef]
- Moulder, K.L.; Fu, T.; Melbostad, H.; Cormier, R.J.; Isenberg, K.E.; Zorumski, C.F.; Mennerick, S. Ethanol-induced death of postnatal hippocampal neurons. Neurobiol. Dis. 2002, 10, 396–409. [Google Scholar] [CrossRef]
- Isayama, R.N.; Leite, P.E.; Lima, J.P.; Uziel, D.; Yamasaki, E.N. Impact of ethanol on the developing GABAergic system. Anat. Rec. 2009, 292, 1922–1939. [Google Scholar] [CrossRef] [PubMed]
- de Wit, H.; Crean, J.; Richards, J.B. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav. Neurosci. 2000, 114, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Boix, J.; Felipo, V.; Guerri, C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J. Neurochem. 2009, 108, 920–931. [Google Scholar] [CrossRef]
- Ehlers, C.L.; Criado, J.R.; Wills, D.N.; Liu, W.; Crews, F.T. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: Correlation with behavioral pathology. Neuroscience 2011, 199, 333–345. [Google Scholar] [CrossRef]
- Crews, F.T.; Vetreno, R.P.; Broadwater, M.A.; Robinson, D.L. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacol. Rev. 2016, 68, 1074–1109. [Google Scholar] [CrossRef] [PubMed]
- Spear, L.P. Effects of adolescent alcohol consumption on the brain and behaviour. Nat. Rev. Neurosci. 2018, 19, 197–214. [Google Scholar] [CrossRef]
- Kahkonen, S.; Wilenius, J.; Nikulin, V.V.; Ollikainen, M.; Ilmoniemi, R.J. Alcohol reduces prefrontal cortical excitability in humans: A combined TMS and EEG study. Neuropsychopharmacology 2003, 28, 747–754. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Jacobus, J.; Tapert, S.F. The effect of alcohol use on human adolescent brain structures and systems. Handb. Clin. Neurol. 2014, 125, 501–510. [Google Scholar] [CrossRef]
- Müller-Oehring, E.M.; Kwon, D.; Nagel, B.J.; Sullivan, E.V.; Chu, W.; Rohlfing, T.; Prouty, D.; Nichols, B.N.; Poline, J.B.; Tapert, S.F.; et al. Influences of age, sex, and moderate alcohol drinking on the intrinsic functional architecture of adolescent brains. Cereb. Cortex 2018, 28, 1049–1063. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Kwon, D.; Brumback, T.; Thompson, W.K.; Cummins, K.; Tapert, S.F.; Brown, S.A.; Colrain, I.M.; Baker, F.C.; Prouty, D.; et al. Altered brain developmental trajectories in adolescents after initiating drinking. Am. J. Psychiatry 2018, 175, 370–380. [Google Scholar] [CrossRef]
- Salling, M.C.; Skelly, M.J.; Avegno, E.; Regan, S.; Zeric, T.; Nichols, E.; Harrison, N.L. Alcohol consumption during adolescence in a mouse model of binge drinking alters the intrinsic excitability and function of the prefrontal cortex through a reduction in the hyperpolarization-activated cation current. J. Neurosci. 2018, 38, 6207–6222. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine: Wireless Information System for Emergency Use. Available online: https://webwiser.nlm.nih.gov/substance?substanceId=18&catId=86 (accessed on 4 September 2022).
- Peana, A.T.; Sánchez-Catalán, M.J.; Hipólito, L.; Rosas, M.; Porru, S.; Bennardini, F.; Romualdi, P.; Caputi, F.F.; Candeletti, S.; Polache, A.; et al. Mystic Acetaldehyde: The Never-Ending Story on Alcoholism. Front. Behav. Neurosci. 2017, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Foddai, M.; Dosia, G.; Spiga, S.; Diana, M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology 2004, 29, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Enrico, P.; Peana, A.T.; Diana, M. Acetaldehyde mediates alcohol activation of the mesolimbic dopamine system. Eur. J. Neurosci. 2007, 26, 2824–2833. [Google Scholar] [CrossRef]
- Enrico, P.; Sirca, D.; Mereu, M.; Peana, A.T.; Lintas, A.; Golosio, A.; Diana, M. Acetaldehyde sequestering prevents ethanol-induced stimulation of mesolimbic dopamine transmission. Drug Alcohol Depend. 2009, 100, 265–271. [Google Scholar] [CrossRef]
- Dellarco, V.L. A mutagenicity assessment of acetaldehyde. Mutat. Res./Rev. Genet. Toxicol. 1988, 195, 1–20. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Acetaldehyde as an underestimated risk factor for cancer development: Role of genetics in ethanol metabolism. Genes Nutr. 2010, 5, 121–128. [Google Scholar] [CrossRef]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef]
- Closon, C.; Didone, V.; Tirelli, E.; Quertemont, E. Acetaldehyde and the hypothermic effects of ethanol in mice. Alcohol. Clin. Exp. Res. 2009, 33, 2005–2014. [Google Scholar] [CrossRef]
- Chapp, A.D.; Behnke, J.E.; Driscoll, K.M.; Fan, Y.; Hoban, E.; Shan, Z.; Zhang, L.; Chen, Q.-H. Acetate Mediates Alcohol Excitotoxicity in Dopaminergic-like PC12 Cells. ACS Chem. Neurosci. 2019, 10, 235–245. [Google Scholar] [CrossRef]
- Sun, G.Y.; Sun, A.Y. Ethanol and membrane lipids. Alcohol. Clin. Exp. Res. 1985, 9, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.; Rottenberg, H. Ethanol-induced injury and adaptation in biological membranes. Fed. Proc. 1982, 41, 2465–2471. [Google Scholar] [PubMed]
- Barry, J.A.; Gawrisch, K. Effects of ethanol on lipid bilayers containing cholesterol, gangliosides, and sphingomyelin. Biochemistry 1995, 34, 8852–8860. [Google Scholar] [CrossRef] [PubMed]
- Chanda, J.; Bandyopadhyay, S. Distribution of ethanol in a model membrane: A computer simulation study. Chem. Phys. Lett. 2004, 392, 249–254. [Google Scholar] [CrossRef]
- Terama, E.; Ollila, O.H.S.; Salonen, E.; Rowat, A.C.; Trandum, C.; Westh, P.; Patra, M.; Karttunen, M.; Vattulainen, I. Influence of ethanol on lipid membranes: From lateral pressure profiles to dynamics and partitioning. J. Phys. Chem. B 2008, 112, 4131–4139. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Anwar, J. Interaction of ethanol with biological membranes: The formation of non-bilayer structures within the membrane interior and their significance. J. Phys. Chem. B 2009, 113, 1983–1992. [Google Scholar] [CrossRef]
- Cantor, R.S. The lateral pressure profile in membranes: A physical mechanism of general anesthesia. Biochemistry 1997, 36, 2339–2344. [Google Scholar] [CrossRef]
- Kondela, T.; Gallová, J.; Hauß, T.; Barnoud, J.; Marrink, S.-J.; Kučerka, N. Alcohol interactions with lipid bilayers. Molecules 2017, 22, 2078. [Google Scholar] [CrossRef]
- Hoek, J.B.; Rubin, E. Alcohol and membrane-associated signal transduction. Alcohol Alcohol. 1990, 25, 143–156. [Google Scholar] [CrossRef]
- Magai, R.M.; Shukla, S.D. Metabolic fate of [14C]-ethanol into endothelial cell phospholipids including platelet-activating factor, sphingomyelin and phosphatidylethanol. J. Biomed. Sci. 2001, 8, 143–150. [Google Scholar] [CrossRef]
- Torres, M.; Rosselló, C.A.; Fernández-García, P.; Lladó, V.; Kakhlon, O.; Escribá, P.V. The implications for cells of the lipid switches driven by protein-membrane interactions and the development of membrane lipid therapy. Int. J. Mol. Sci. 2020, 21, 2322. [Google Scholar] [CrossRef] [PubMed]
- Alling, C.; Gustavsson, L.; Månsson, J.E.; Benthin, G.; Änggård, E. Phosphatidylethanol formation in rat organs after ethanol treatment. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1984, 793, 119–122. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kanfer, J.N. Phosphatidylethanol formation via transphosphatidylation by rat brain synaptosomal phospholipase D. J. Neurochem. 1987, 48, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Liisanantti, M.K.; Savolainen, M.J. Phosphatidylethanol mediates its effects on the vascular endothelial growth factor via HDL receptor in endothelial cells. Alcohol. Clin. Exp. Res. 2009, 33, 283–288. [Google Scholar] [CrossRef]
- Catalá, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef]
- Catalá, A. Lipid peroxidation modifies the picture of membranes from the “Fluid Mosaic Model” to the “Lipid Whisker Model”. Biochimie 2012, 94, 101–109. [Google Scholar] [CrossRef]
- Cordeiro, R.M. Reactive oxygen species at phospholipid bilayers: Distribution, mobility and permeation. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 438–444. [Google Scholar] [CrossRef]
- Sergent, O.; Morel, I.; Chevanne, M.; Cillard, P.; Cillard, J. Oxidative stress induced by ethanol in rat hepatocyte cultures. Biochem. Mol. Biol. Int. 1995, 35, 575–583. [Google Scholar]
- Bailey, S.M.; Cunningham, C.C. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology 1998, 28, 1318–1326. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.-L.; Yu, K.-K.; Xu, M.; Xu, Y.-Z.; Chen, L.; Lu, Y.-M.; Fang, H.-S.; Wang, X.-Y.; Hu, Z.-Q.; et al. Activation of the NF-κB pathway as a mechanism of alcohol enhanced progression and metastasis of human hepatocellular carcinoma. Mol. Cancer 2015, 14, 10. [Google Scholar] [CrossRef]
- Haft, R.J.F.; Keating, D.H.; Schwaegler, T.; Schwalbach, M.S.; Vinokur, J.; Tremaine, M.; Peters, J.M.; Kotlajich, M.V.; Pohlmann, E.L.; Ong, I.M.; et al. Correcting direct effects of ethanol on translation and transcription machinery confers ethanol tolerance in bacteria. Proc. Natl. Acad. Sci. USA 2014, 111, E2576–E2585. [Google Scholar] [CrossRef] [PubMed]
- Cahill, A.; Sykora, P. Alcoholic liver disease and the mitochondrial ribosome: Methods of analysis. In Alcohol. Methods in Molecular Biology; Nagy, L.E., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 381–394. [Google Scholar] [CrossRef]
- Ramanathan, R.; Wilkemeyer, M.F.; Mittal, B.; Perides, G.; Charness, M.E. Alcohol inhibits cell-cell adhesion mediated by human L1. J. Cell Biol. 1996, 133, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, E.; Shanmugasundararaj, S.; Wilkemeyer, M.F.; Dou, X.; Chen, S.; Charness, M.E.; Miller, K.W. An alcohol binding site on the neural cell adhesion molecule L1. Proc. Natl. Acad. Sci. USA 2008, 105, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Lovinger, D.M.; Roberto, M. Synaptic effects induced by alcohol. Curr. Top. Behav. Neurosci. 2010, 13, 31–86. [Google Scholar] [CrossRef]
- Söderpalm, B.; Lidö, H.H.; Ericson, M. The glycine receptor-A functionally important primary brain target of ethanol. Alcohol. Clin. Exp. Res. 2017, 41, 1816–1830. [Google Scholar] [CrossRef]
- Howard, R.J.; Murail, S.; Ondricek, K.E.; Corringer, P.-J.; Lindahl, E.; Trudell, J.R.; Harris, R.A. Structural basis for alcohol modulation of a pentameric ligand-gated ion channel. Proc. Natl. Acad. Sci. USA 2011, 108, 12149–12154. [Google Scholar] [CrossRef]
- Sauguet, L.; Howard, R.J.; Malherbe, L.; Lee, U.S.; Corringer, P.-J.; Harris, R.A.; Delarue, M. Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat. Commun. 2013, 4, 1697. [Google Scholar] [CrossRef]
- Förstera, B.; Castro, P.A.; Moraga-Cid, G.; Aguayo, L.G. Potentiation of Gamma Aminobutyric Acid Receptors (GABAAR) by Ethanol: How Are Inhibitory Receptors Affected? Front. Cell. Neurosci. 2016, 10, 114. [Google Scholar] [CrossRef]
- Smothers, C.T.; Jin, C.; Woodward, J.J. Deletion of the N-terminal domain alters the ethanol inhibition of N-methyl-D-aspartate receptors in a subunit-dependent manner. Alcohol. Clin. Exp. Res. 2013, 37, 1882–1890. [Google Scholar] [CrossRef][Green Version]
- Smothers, C.T.; Woodward, J.J. Differential effects of TM4 tryptophan mutations on inhibition of N-methyl-d-aspartate receptors by ethanol and toluene. Alcohol 2016, 56, 15–19. [Google Scholar] [CrossRef]
- Bukiya, A.N.; Kuntamallappanavar, G.; Edwards, J.; Singh, A.K.; Shivakumar, B.; Dopico, A.M. An alcohol-sensing site in the calcium- and voltage-gated, large conductance potassium (BK) channel. Proc. Natl. Acad. Sci. USA 2014, 111, 9313–9318. [Google Scholar] [CrossRef] [PubMed]
- Toyama, Y.; Kano, H.; Mase, Y.; Yokogawa, M.; Osawa, M.; Shimada, I. Structural basis for the ethanol action on G-protein-activated inwardly rectifying potassium channel 1 revealed by NMR spectroscopy. Proc. Natl. Acad. Sci. USA 2018, 115, 3858–3863. [Google Scholar] [CrossRef] [PubMed]
- Aryal, P.; Dvir, H.; Choe, S.; Slesinger, P.A. A discrete alcohol pocket involved in GIRK channel activation. Nat. Neurosci. 2009, 12, 988–995. [Google Scholar] [CrossRef]
- Dwyer, D.; Bradley, R. Chemical properties of alcohols and their protein binding sites. Cell. Mol. Life Sci. 2000, 57, 265–275. [Google Scholar] [CrossRef]
- Olsen, R.W.; Li, G.-D.; Wallner, M.; Trudell, J.R.; Bertaccini, E.J.; Lindahl, E.; Miller, K.W.; Alkana, R.L.; Davies, D.L. Structural Models of Ligand-Gated Ion Channels: Sites of Action for Anesthetics and Ethanol. Alcohol. Clin. Exp. Res. 2014, 38, 595–603. [Google Scholar] [CrossRef]
- Tas, P.W.L.; Stöel, C.; Roewer, N. The volatile anesthetic isoflurane inhibits the histamine-induced Ca2+ influx in primary human endothelial cells. Anesth. Analg. 2003, 97, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ham, A.; Kim, K.Y.; Brown, K.M.; Lee, H.T. The volatile anesthetic isoflurane increases endothelial adenosine generation via microparticle ecto-5′-nucleotidase (CD73) release. PLoS ONE 2014, 9, e99950. [Google Scholar] [CrossRef]
- Shi, L.; Rodríguez-Contreras, A. The general anesthetic isoflurane inhibits calcium activity in cerebrovascular endothelial cells and disrupts vascular tone. bioRxiv 2022. [Google Scholar] [CrossRef]
- Levitt, M.D.; Li, R.; DeMaster, E.G.; Elson, M.; Furne, J.; Levitt, D.G. Use of measurements of ethanol absorption from stomach and intestine to assess human ethanol metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 273, G951–G957. [Google Scholar] [CrossRef]
- Tanaka, A.; Cui, R.; Kitamura, A.; Liu, K.; Imano, H.; Yamagishi, K.; Kiyama, M.; Okada, T.; Iso, H.; CIRCS Investigators. Heavy alcohol consumption is associated with impaired endothelial function. J. Atheroscler. Thromb. 2016, 23, 1047–1054. [Google Scholar] [CrossRef]
- Xu, M.; Chen, G.; Fu, W.; Liao, M.; Frank, J.A.; Bower, K.A.; Fang, S.; Zhang, Z.; Shi, X.; Luo, J. Ethanol disrupts vascular endothelial barrier: Implication in cancer metastasis. Toxicol. Sci. 2012, 127, 42–53. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 729–756. [Google Scholar] [CrossRef] [PubMed]
- Simet, S.M.; Wyatt, T.A.; DeVasure, J.; Yanov, D.; Allen-Gipson, D.; Sisson, J.H. Alcohol increases the permeability of airway epithelial tight junctions in Beas-2B and NHBE cells. Alcohol. Clin. Exp. Res. 2012, 36, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Doggett, T.M.; Breslin, J.W. Acute alcohol intoxication-induced micro-vascular leakage. Alcohol. Clin. Exp. Res. 2014, 38, 2414–2426. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, J.; Chang, B.; Wang, B.; Zhang, D.; Wang, B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol. Med. Rep. 2014, 9, 2352–2356. [Google Scholar] [CrossRef]
- Reed, M.J.; Damodarasamy, M.; Banks, W.A. The extracellular matrix of the blood-brain barrier: Structural and functional roles in health, aging, and Alzheimer’s disease. Tissue Barriers 2019, 7, 1651157. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Yu, H.; Wang, C.; Wang, X.; Wang, H.; Zhang, C.; You, J.; Wang, P.; Feng, C.; Xu, G.; Zhao, R.; et al. Long-term exposure to ethanol downregulates tight junction proteins through the protein kinase Cα signaling pathway in human cerebral microvascular endothelial cells. Exp. Ther. Med. 2017, 14, 4789–4796. [Google Scholar] [CrossRef]
- Haorah, J.; Knipe, B.; Leibhart, J.; Ghorpade, A.; Persidsky, Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J. Leukoc. Biol. 2005, 78, 1223–1232. [Google Scholar] [CrossRef]
- Schreibelt, G.; Kooij, G.; Reijerkerk, A.; van Doorn, R.; Gringhuis, S.I.; van der Pol, S.; Weksler, B.B.; Romero, I.A.; Couraud, P.-O.; Piontek, J.; et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007, 21, 3666–3676. [Google Scholar] [CrossRef]
- Minagar, A.; Alexander, J.S. Blood-brain barrier disruption in multiple sclerosis. Mult. Scler. J. 2003, 9, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Macías-Islas, M.Á.; Flores-Alvarado, L.J.; Mireles-Ramírez, M.A.; González-Renovato, E.D.; Hernández-Navarro, V.E.; Sánchez-López, A.L.; Alatorre-Jiménez, M.A. Role of the blood-brain barrier in multiple sclerosis. Arch. Med. Res. 2014, 45, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Guttmann, C.R.; Rousset, M.; Roch, J.A.; Hannoun, S.; Durand-Dubief, F.; Belaroussi, B.; Cavallari, M.; Rabilloud, M.; Sappey-Marinier, D.; Vukusic, S.; et al. Multiple sclerosis lesion formation and early evolution revisited: A weekly high-resolution magnetic resonance imaging study. Mult. Scler. J. 2016, 22, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, T.; Shibutani, M.; Okeda, R. Blood-brain barrier disruption and exacerbation of ischemic brain edema after restoration of blood flow in experimental focal cerebral ischemia. Acta Neuropathol. 1988, 76, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.G.; Xue, D.; Karbalai, H.; Buchan, A.M.; Preston, E. Biphasic opening of the blood-brain barrier following transient focal ischemia: Effects of hypothermia. Can. J. Neurol. Sci. 1999, 26, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Cornford, E.M.; Oldendorf, W.H. Epilepsy and the blood-brain barrier. Adv. Neurol. 1986, 44, 787–812. [Google Scholar]
- Horowitz, S.W.; Merchut, M.; Fine, M.; Azar-Kia, B. Complex partial seizure-induced transient MR enhancement. J. Comput. Assist. Tomogr. 1992, 16, 814–816. [Google Scholar] [CrossRef]
- Marchi, N.; Angelov, L.; Masaryk, T.; Fazio, V.; Granata, T.; Hernandez, N.; Hallene, K.; Diglaw, T.; Franic, L.; Najm, I.; et al. Seizure-promoting effect of blood–brain barrier disruption. Epilepsia 2007, 48, 732–742. [Google Scholar] [CrossRef]
- van de Haar, H.J.; Burgmans, S.; Jansen, J.F.; van Osch, M.J.; van Buchem, M.A.; Muller, M.; Hofman, P.A.; Verhey, F.R.; Backes, W.H. Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology 2016, 281, 527–535. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Uprety, A.; Kang, Y.; Kim, S.Y. Blood-brain barrier dysfunction as a potential therapeutic target for neurodegenerative disorders. Arch. Pharm. Res. 2021, 44, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Molina, P.; Souza-Smith, F.M. Ethanol-induced lymphatic endothelial cell permeability via MAP-kinase regulation. Am. J. Physiol. Cell Physiol. 2021, 321, C104–C116. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.L.; et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018, 21, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.; Rayasam, A.; Kijak, J.A.; Choi, Y.H.; Harding, J.S.; Marcus, S.A.; Karpus, W.J.; Sandor, M.; Fabry, Z. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 2019, 10, 229. [Google Scholar] [CrossRef]
- Kwon, S.; Moreno-Gonzalez, I.; Taylor-Presse, K.; Edwards, G., III; Gamez, N.; Calderon, O.; Zhu, B.; Velasquez, F.C.; Soto, C.; Sevick-Muraca, E.M. Impaired peripheral lymphatic function and cerebrospinal fluid outflow in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2019, 69, 585–593. [Google Scholar] [CrossRef]
- Zou, W.; Pu, T.; Feng, W.; Lu, M.; Zheng, Y.; Du, R.; Xiao, M.; Hu, G. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Transl. Neurodegener. 2019, 8, 7. [Google Scholar] [CrossRef]
- Ding, X.B.; Wang, X.X.; Xia, D.H.; Liu, H.; Tian, H.Y.; Fu, Y.; Chen, Y.K.; Qin, C.; Wang, J.Q.; Xiang, Z.; et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat. Med. 2021, 27, 411–418. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- Duncan, A.J.; Heales, S.J. Nitric oxide and neurological disorders. Mol. Asp. Med. 2005, 26, 67–96. [Google Scholar] [CrossRef]
- Yassin, L.; Radtke-Schuller, S.; Asraf, H.; Grothe, B.; Hershfinkel, M.; Forsythe, I.D.; Kopp-Scheinpflug, C. Nitric oxide signaling modulates synaptic inhibition in the superior paraolivary nucleus (SPN) via cGMP-dependent suppression of KCC2. Front. Neural Circuits 2014, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Takei, H.; Koyanagi, Y.; Koshikawa, N.; Kobayashi, M. Presynaptic cell type-dependent regulation of GABAergic synaptic transmission by nitric oxide in rat insular cortex. Neuroscience 2015, 284, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Steinert, J.R.; Chernova, T.; Forsythe, I.D. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist 2010, 16, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Levecque, C.; Elbaz, A.; Clavel, J.; Richard, F.; Vidal, J.-S.; Amouyel, P.; Tzourio, C.; Alpérovitch, A.; Chartier-Harlin, M.-C. Association between Parkinson’s disease and polymorphisms in the nNOS and iNOS genes in a community-based case–control study. Hum. Mol. Genet. 2003, 12, 79–86. [Google Scholar] [CrossRef]
- O’Gallagher, K.; Puledda, F.; O’Daly, O.; Ryan, M.; Dancy, L.; Chowienczyk, P.J.; Zelaya, F.; Goadsby, P.J.; Shah, A.M. Neuronal nitric oxide synthase regulates regional brain perfusion in healthy humans. Cardiovasc. Res. 2022, 118, 1321–1329. [Google Scholar] [CrossRef]
- Yokochi, A.; Nara, K.; Iwai, S.; Oguchi, K. Nitric oxide is increased in the rat hypothalamus by repetitive ethanol administration. Showa Univ. J. Med. Sci. 2005, 17, 129–136. [Google Scholar] [CrossRef]
- Finnerty, N.; O’Riordan, S.L.; Klamer, D.; Lowry, J.; Pålsson, E. Increased brain nitric oxide levels following ethanol administration. Nitric Oxide 2015, 47, 52–57. [Google Scholar] [CrossRef]
- Itzhak, Y.; Roger-Sánchez, C.; Anderson, K.L. Role of the nNOS gene in ethanol-induced conditioned place preference in mice. Alcohol 2009, 43, 285–291. [Google Scholar] [CrossRef]
- Blanco, A.M.; Pascual, M.; Valles, S.L.; Guerri, C. Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-κB. Neuroreport 2004, 15, 681–685. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Qin, J.; Lv, Y.; Ma, X.; Liu, C. Ethanol upregulates iNOS expression in colon through activation of nuclear factor-kappa B in rats. Alcohol. Clin. Exp. Res. 2010, 34, 57–63. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.B.P.; Ceron, C.S.; Mendes, A.S.; de Martinis, B.S.; Castro, M.M.; Tirapelli, C.R. Inducible nitric oxide synthase (iNOS) mediates ethanol-induced redox imbalance and upregulation of inflammatory cytokines in the kidney. Can. J. Physiol. Pharmacol. 2021, 99, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Syapin, P.J.; Militante, J.D.; Garrett, D.K.; Ren, L. Cytokine-induced iNOS expression in C6 glial cells: Transcriptional inhibition by ethanol. J. Pharmacol. Exp. Ther. 2001, 298, 744–752. [Google Scholar] [PubMed]
- Davis, R.L.; Syapin, P.J. Acute ethanol exposure modulates expression of inducible nitric-oxide synthase in human astroglia: Evidence for a transcriptional mechanism. Alcohol 2004, 32, 195–202. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, X.; Belfield, K.D.; Haorah, J. Biphasic effects of ethanol exposure on waste metabolites clearance in the CNS. Mol. Neurobiol. 2021, 58, 3953–3967. [Google Scholar] [CrossRef]
- Venkov, C.D.; Myers, P.R.; Tanner, M.A.; Su, M.; Vaughan, D.E. Ethanol increases endothelial nitric oxide production through modulation of nitric oxide synthase expression. Thromb. Haemost. 1999, 81, 638–642. [Google Scholar] [CrossRef]
- Polikandriotis, J.A.; Rupnow, H.L.; Hart, C.M. Chronic ethanol exposure stimulates endothelial cell nitric oxide production through PI-3 kinase-and hsp90-dependent mechanisms. Alcohol. Clin. Exp. Res. 2005, 29, 1932–1938. [Google Scholar] [CrossRef]
- Kleinhenz, D.J.; Sutliff, R.L.; Polikandriotis, J.A.; Walp, E.R.; Dikalov, S.I.; Guidot, D.M.; Hart, C.M. Chronic ethanol ingestion increases aortic endothelial nitric oxide synthase expression and nitric oxide production in the rat. Alcohol. Clin. Exp. Res. 2008, 32, 148–154. [Google Scholar] [CrossRef]
- Tirapelli, C.R.; Leone, A.F.C.; Yogi, A.; Tostes, R.C.; Lanchote, V.L.; Uyemura, S.A.; Resstel, L.B.M.; Corrêa, F.M.A.; Padovan, C.M.; de Oliveira, A.M.; et al. Ethanol consumption increases blood pressure and alters the responsiveness of the mesenteric vasculature in rats. J. Pharm. Pharmacol. 2008, 60, 331–341. [Google Scholar] [CrossRef]
- Fish, J.E.; Matouk, C.C.; Rachlis, A.; Lin, S.; Tai, S.C.; D’Abreo, C.; Marsden, P.A. The expression of endothelial nitric-oxide synthase is controlled by a cell-specific histone code. J. Biol. Chem. 2005, 280, 24824–24838. [Google Scholar] [CrossRef]
- Chandrasekar, R. Alcohol and NMDA receptor: Current research and future direction. Front. Mol. Neurosci. 2013, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, Z.; Gao, B.; Kunos, G. Dose-dependent activation of antiapoptotic and proapoptotic pathways by ethanol treatment in human vascular endothelial cells: Differential involvement of adenosine. J. Biol. Chem. 2002, 277, 20927–20933. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Abdel-Rahman, A.A. Effect of chronic ethanol administration on hepatic eNOS activity and its association with caveolin-1 and calmodulin in female rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G579–G585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hendrickson, R.J.; Cahill, P.A.; Sitzmann, J.V.; Redmond, E.M. Ethanol enhances basal and flow-stimulated nitric oxide synthase activity in vitro by activating an inhibitory guanine nucleotide binding protein. J. Pharmacol. Exp. Ther. 1999, 289, 1293–1300. [Google Scholar] [PubMed]
- Bedini, G.; Blecharz, K.G.; Nava, S.; Vajkoczy, P.; Alessandri, G.; Ranieri, M.; Acerbi, F.; Ferroli, P.; Riva, D.; Esposito, S.; et al. Vasculogenic and angiogenic pathways in Moyamoya disease. Curr. Med. Chem. 2016, 23, 315–345. [Google Scholar] [CrossRef] [PubMed]
- Korn, C.; Augustin, H.G. Mechanisms of vessel pruning and regression. Dev. Cell 2015, 34, 5–17. [Google Scholar] [CrossRef]
- Tregub, P.P.; Averchuk, A.S.; Baranich, T.I.; Ryazanova, M.V.; Salmina, A.B. Physiological and pathological remodeling of cerebral microvessels. Int. J. Mol. Sci. 2022, 23, 12683. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Ninomiya, I.; Kanazawa, M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen. Res. 2020, 15, 16–19. [Google Scholar] [CrossRef]
- Yamada, M.K. Angiogenesis in refractory depression: A possible phenotypic target to avoid the blood brain barrier. Drug Discov. Ther. 2016, 10, 74–78. [Google Scholar] [CrossRef]
- Morrow, D.; Cullen, J.P.; Cahill, P.A.; Redmond, E.M. Ethanol stimulates endothelial cell angiogenic activity via a Notch- and angiopoietin-1-dependent pathway. Cardiovasc. Res. 2008, 79, 313–321. [Google Scholar] [CrossRef]
- Maniyar, R.; Chakraborty, S.; Suriano, R. Ethanol Enhances Estrogen Mediated Angiogenesis in Breast Cancer. J. Cancer 2018, 9, 3874–3885. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ni, F.; Xu, M.; Yang, J.; Chen, J.; Chen, Z.; Wang, X.; Luo, J.; Wang, S. Alcohol promotes mammary tumor growth through activation of VEGF-dependent tumor angiogenesis. Oncol. Lett. 2014, 8, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Bailey, A.P.; Shparago, M.; Busby, B.; Covington, J.; Johnson, J.W.; Young, E.; Gu, J.W. Chronic alcohol consumption stimulates VEGF expression, tumor angiogenesis and progression of melanoma in mice. Cancer Biol. Ther. 2007, 6, 1222–1228. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Schwarz, J.M.; Wright, C.L.; Dean, S.L. Mechanisms mediating oestradiol modulation of the developing brain. J. Neuroendocrinol. 2008, 20, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M. Estradiol: A protective factor in the adult brain. J. Pediatr. Endocrinol. Metab. 2000, 13, 1425–1429. [Google Scholar] [CrossRef]
- Radek, K.A.; Matthies, A.M.; Burns, A.L.; Heinrich, S.A.; Kovacs, E.J.; Dipietro, L.A. Acute ethanol exposure impairs angiogenesis and the proliferative phase of wound healing. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1084–H1090. [Google Scholar] [CrossRef] [PubMed]
- Radek, K.A.; Kovacs, E.J.; Gallo, R.L.; DiPietro, L.A. Acute ethanol exposure disrupts VEGF receptor cell signaling in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H174–H184. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, H.; Sun, Y.; Wang, Y.; Wang, A. The effects of ethanol on angiogenesis after myocardial infarction, and preservation of angiogenesis with rosuvastatin after heavy drinking. Alcohol 2016, 54, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.; Hatch, E.; Hamm, K.; Cahill, P.A.; Redmond, E.M. Flk-1/KDR mediates ethanol-stimulated endothelial cell Notch signaling and angiogenic activity. J. Vasc. Res. 2014, 51, 315–324. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Liu, W.; Chu, C.C.; Cahill, P.A.; Redmond, E.M. Moderate dose alcohol protects against serum amyloid protein A1-induced endothelial dysfunction via both notch-dependent and notch-independent pathways. Alcohol. Clin. Exp. Res. 2021, 45, 2217–2230. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, C.-L.; Song, F.-Y.; Zhao, X.-L.; Yu, L.-H.; Zhu, Z.-P.; Xie, K.-Q. PI3K/Akt pathway activation was involved in acute ethanol-induced fatty liver in mice. Toxicology 2012, 296, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Leung, C.A.; Douti, L.Y.; Jay, S.M. Ethanol induces enhanced vascularization bioactivity of endothelial cell-derived extracellular vesicles via regulation of microRNAs and long non-coding RNAs. Sci. Rep. 2017, 7, 13794. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, K.; Hasegawa, H. Blood Vessels as a Key Mediator for Ethanol Toxicity: Implication for Neuronal Damage. Life 2022, 12, 1882. https://doi.org/10.3390/life12111882
Nakayama K, Hasegawa H. Blood Vessels as a Key Mediator for Ethanol Toxicity: Implication for Neuronal Damage. Life. 2022; 12(11):1882. https://doi.org/10.3390/life12111882
Chicago/Turabian StyleNakayama, Kei, and Hiroshi Hasegawa. 2022. "Blood Vessels as a Key Mediator for Ethanol Toxicity: Implication for Neuronal Damage" Life 12, no. 11: 1882. https://doi.org/10.3390/life12111882
APA StyleNakayama, K., & Hasegawa, H. (2022). Blood Vessels as a Key Mediator for Ethanol Toxicity: Implication for Neuronal Damage. Life, 12(11), 1882. https://doi.org/10.3390/life12111882






