Favorable Effects of Virgin Coconut Oil on Neuronal Damage and Mortality after a Stroke Incidence in the Stroke-Prone Spontaneously Hypertensive Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals
2.3. Treatment
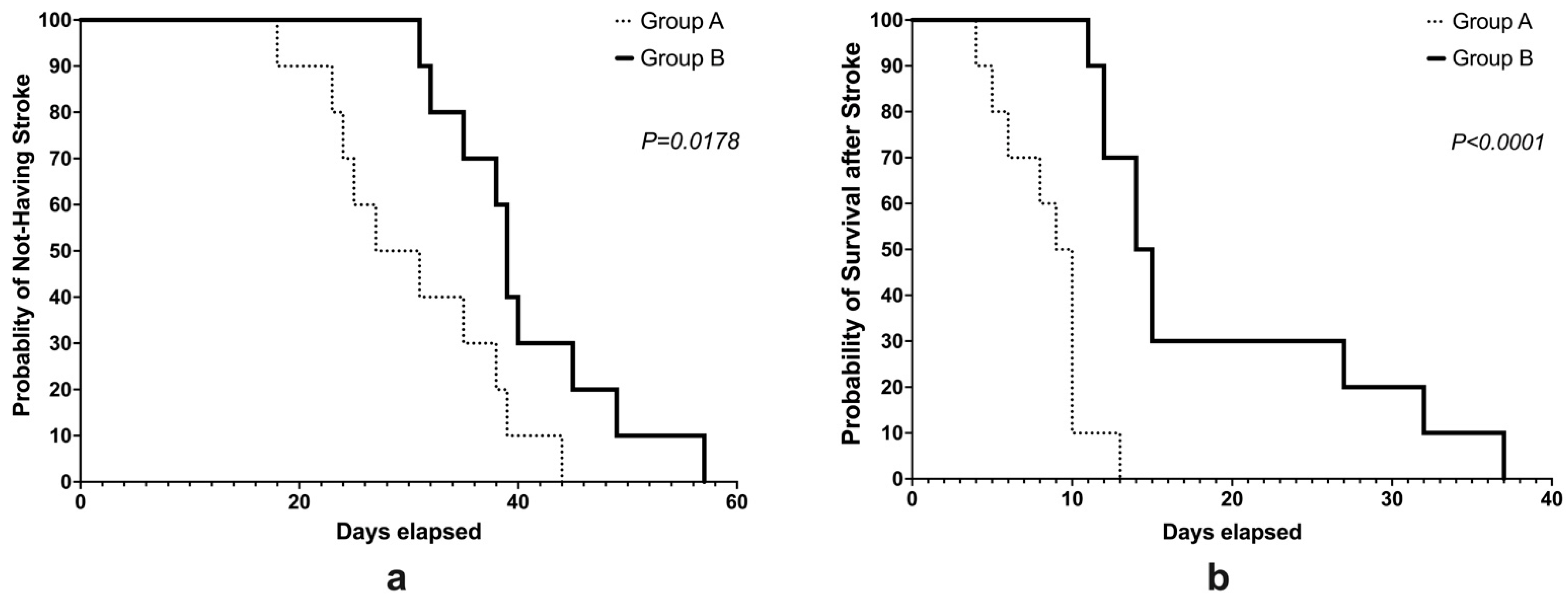
2.4. Incidence and Survival Rates
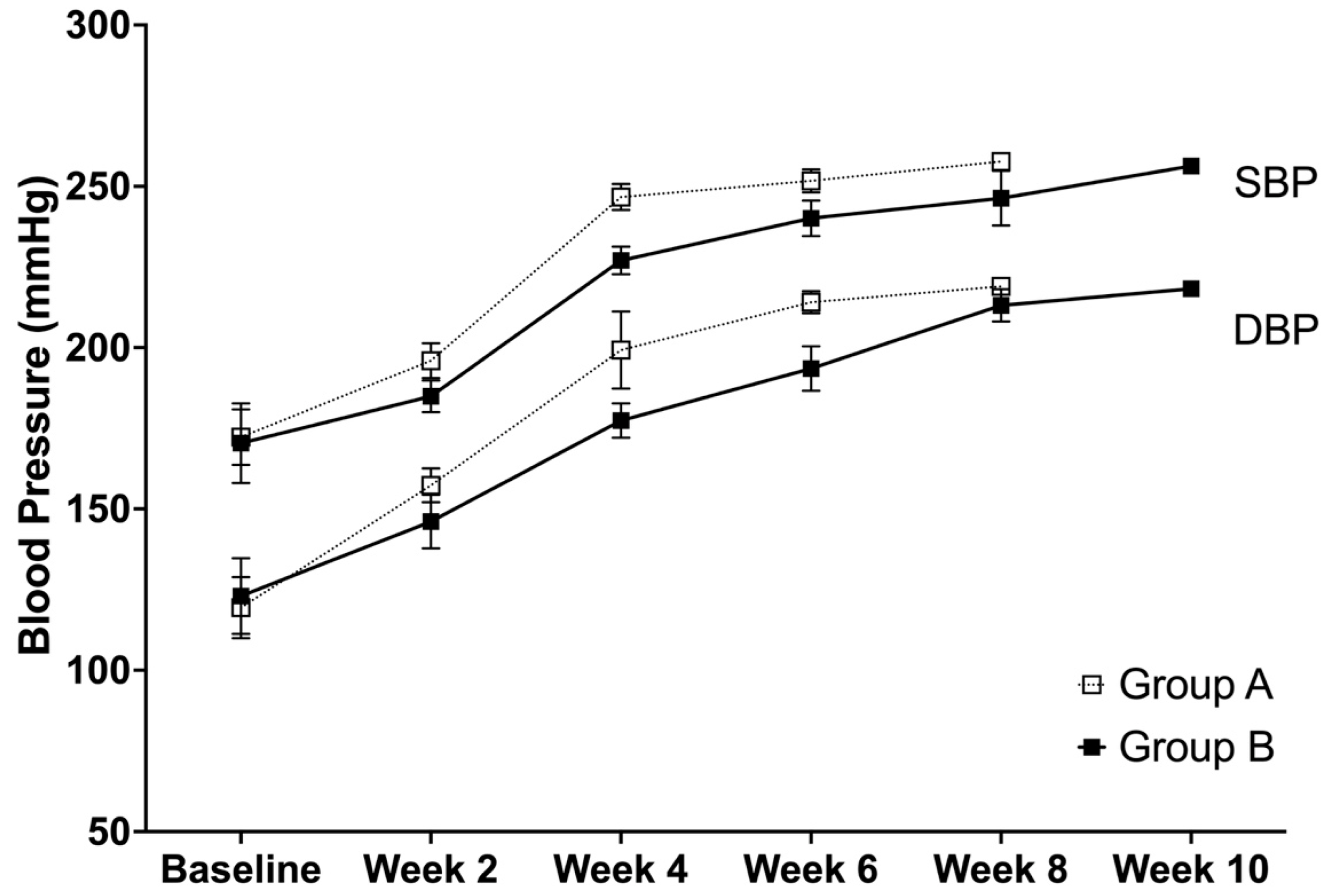
2.5. Systolic and Diastolic Blood Pressure and Heart Rate
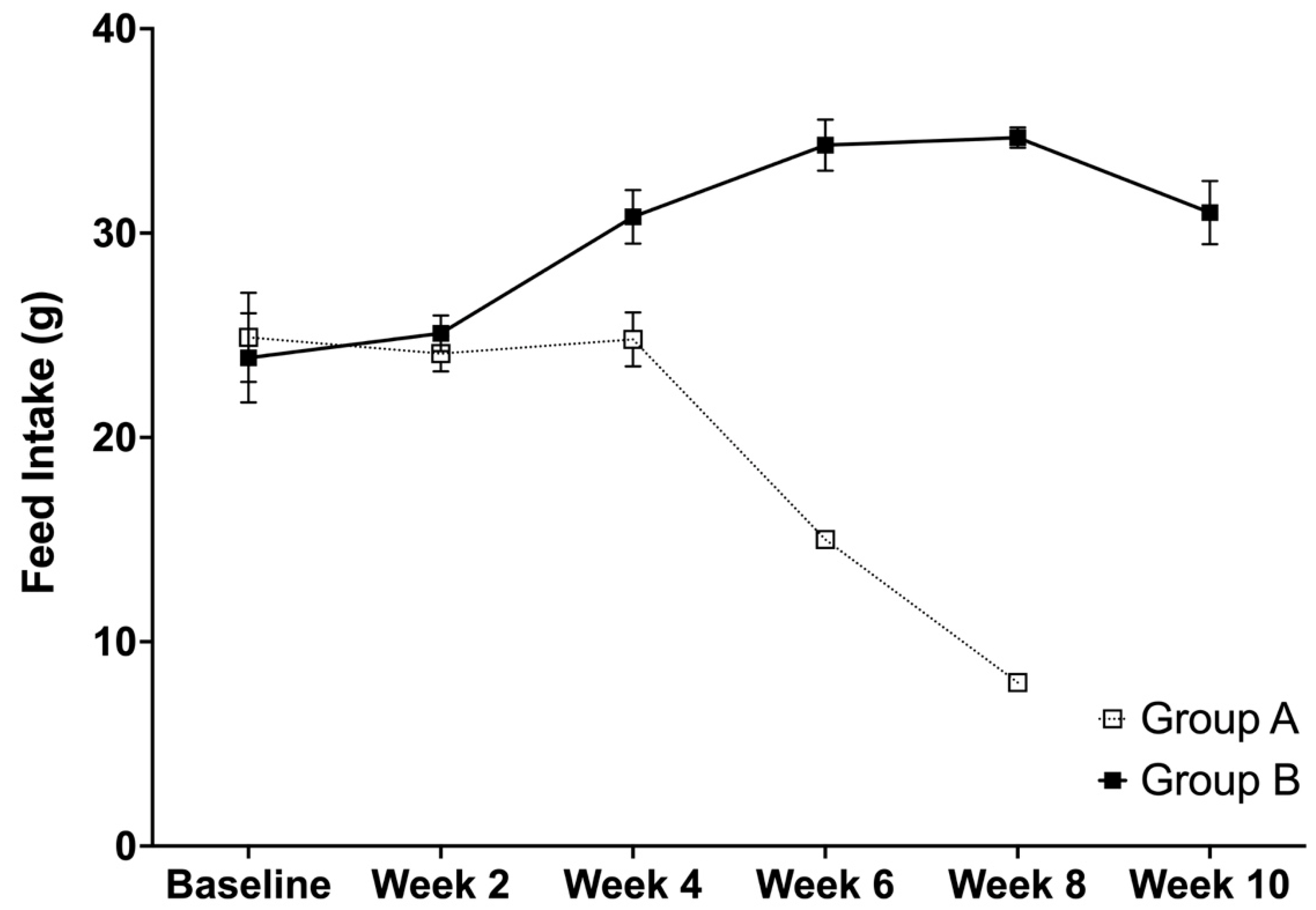
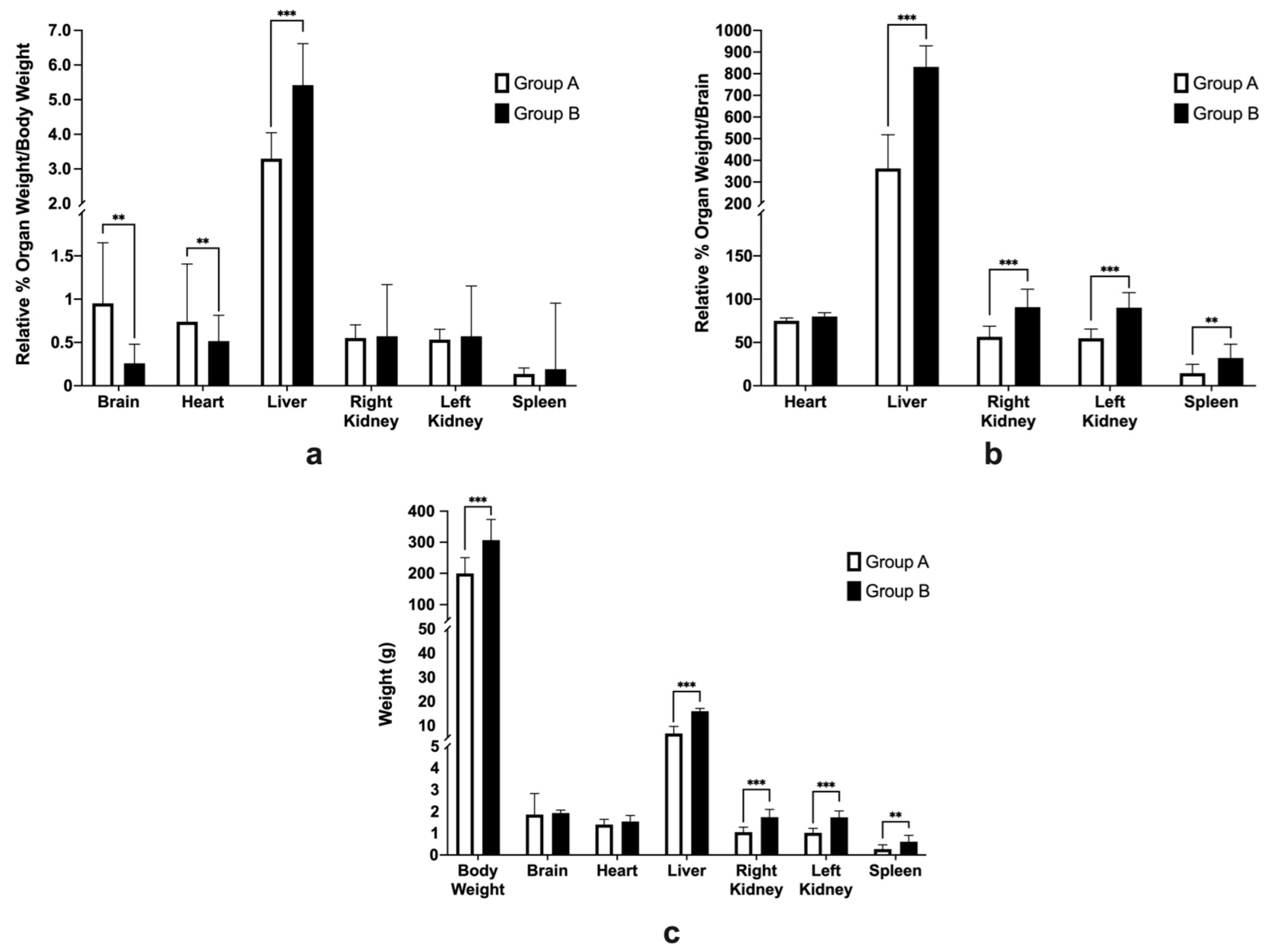
2.6. Average Food Intake, Body Weight, and Relative Organ Weights
2.7. Histopathological Analysis of Brain Tissues
2.8. Statistics
3. Results
3.1. Incidence and Survival Rates
3.2. Systolic and Diastolic Blood Pressure
3.3. Heart Rate
3.4. Average Feed Intake, Body Weight, and Relative Organ Weights
3.5. Histopathological Analysis of Brain Tissues
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Coupland, A.P.; Thapar, A.; Qureshi, M.I.; Jenkins, H.; Davies, A.H. The definition of stroke. J. R. Soc. Med. 2017, 110, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Truelsen, T.; Begg, S.; Mathers, C.D.; Satoh, T. Global Burden of Cardiovascular Disease in the Year 2000; 2000. Available online: http://www.who.int/healthinfo/statistics/bod_cerebrovasculardiseasestroke.pdf (accessed on 16 August 2022).
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics-2017 update: A report from the American heart association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global burden of stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.G.; Cadilhac, D.A.; Thayabaranathan, T.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Donnan, G.A. Global stroke statistics. Int. J. Stroke 2014, 9, 6–18. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics--2014 update: A report from the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar] [CrossRef]
- Hankey, G.J.; Sudlow, C.L.; Dunbabin, D.W. Thienopyridine derivatives (ticlopidine, clopidogrel) versus aspirin for preventing stroke and other serious vascular events in high vascular risk patients. Cochrane Database Syst. Rev. 2000, 2, 312–314. [Google Scholar]
- Aguilar, M.I.; Hart, R. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst. Rev. 2005, 3, Cd001927. [Google Scholar] [CrossRef]
- Chao, T.F.; Liu, C.J.; Liao, J.N.; Wang, K.L.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Chung, F.P.; et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation 2016, 133, 1540–1547. [Google Scholar] [CrossRef]
- Sandercock, P.; Gubitz, G.; Foley, P.; Counsell, C. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst. Rev. 2003, Cd000029. [Google Scholar] [CrossRef]
- Lansberg, M.G.; Schrooten, M.; Bluhmki, E.; Thijs, V.N.; Saver, J.L. Treatment time-specific number needed to treat estimates for tissue plasminogen activator therapy in acute stroke based on shifts over the entire range of the modified Rankin Scale. Stroke 2009, 40, 2079–2084. [Google Scholar] [CrossRef]
- Maas, M.B.; Safdieh, J.E. Ischemic stroke: Pathophysiology and principles of localization. Neurol. Board Rev. Man. 2009, 13, 1–16. [Google Scholar]
- Marina, A.M.; Che Man, Y.B.; Amin, I. Virgin coconut oil: Emerging functional food oil. Trends Food Sci. Technol. 2009, 20, 481–487. [Google Scholar] [CrossRef]
- Page, K.A.; Williamson, A.; Yu, N.; McNay, E.C.; Dzuira, J.; McCrimmon, R.J.; Sherwin, R.S. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes 2009, 58, 1237–1244. [Google Scholar] [CrossRef]
- Lim, S.; Chesser, A.S.; Grima, J.C.; Rappold, P.M.; Blum, D.; Przedborski, S.; Tieu, K. D-beta-hydroxybutyrate is protective in mouse models of Huntington’s disease. PLoS ONE 2011, 6, e24620. [Google Scholar] [CrossRef]
- Rahman, M.; Muhammad, S.; Khan, M.A.; Chen, H.; Ridder, D.A.; Muller-Fielitz, H.; Pokorna, B.; Vollbrandt, T.; Stolting, I.; Nadrowitz, R.; et al. The beta-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014, 5, 3944. [Google Scholar] [CrossRef]
- Maalouf, M.; Sullivan, P.G.; Davis, L.; Kim, D.Y.; Rho, J.M. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 2007, 145, 256–264. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Aja, P.M.; Maduagwuna, E.K.; Ekeleme-Egedigwe, C.A.; Ufebe, O.G.; Azubuike-Osu, S.O. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed. Pharmacother. 2017, 96, 905–911. [Google Scholar] [CrossRef]
- Keddy, P.G.; Dunlop, K.; Warford, J.; Samson, M.L.; Jones, Q.R.; Rupasinghe, H.P.; Robertson, G.S. Neuroprotective and anti-inflammatory effects of the flavonoid-enriched fraction AF4 in a mouse model of hypoxic-ischemic brain injury. PLoS ONE 2012, 7, e51324. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Somchit, M.N.; Mat Jais, A.M.; Teh, L.K.; Salleh, M.Z.; Long, K. In vivo antinociceptive and anti-inflammatory activities of dried and fermented processed virgin coconut oil. Med. Princ. Pract. 2011, 20, 231–236. [Google Scholar] [CrossRef]
- Intahphuak, S.; Khonsung, P.; Panthong, A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm. Biol. 2010, 48, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Shilling, M.; Matt, L.; Rubin, E.; Visitacion, M.P.; Haller, N.A.; Grey, S.F.; Woolverton, C.J. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J. Med. Food 2013, 16, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Handajani, N.S.; Dhamawan, R. Effect of VCO to leukocyte differential count, glucose levels and blood creatining of hyperglycemic and ovalbumin sensitized Mus musculus Balb/c. Nusuntara Biosci. 2009, 1, 1–8. [Google Scholar]
- Harini, M.; Astirin, O.P. Blood cholesterol levels of hypercholesterolemic rat (Rattus norvegicus) after VCO treatment. Nusant. Biosci. 2009, 1, 53–58. [Google Scholar] [CrossRef]
- Dayrit, C.S. Coconut oil: Atherogenic or not? Philipp. J. Cardiol. 2003, 31, 97–104. [Google Scholar]
- Zakaria, Z.A.; Rofiee, M.S.; Somchit, M.N.; Zuraini, A.; Sulaiman, M.R.; Teh, L.K.; Salleh, M.Z.; Long, K. Hepatoprotective activity of dried- and fermented-processed virgin coconut oil. Evid.-Based Complementary Altern. Med. 2011, 2011, 142739. [Google Scholar] [CrossRef]
- Otuechere, C.A.; Madarikan, G.; Simisola, T.; Bankole, O.; Osho, A. Virgin coconut oil protects against liver damage in albino rats challenged with the anti-folate combination, trimethoprim-sulfamethoxazole. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 249–253. [Google Scholar] [CrossRef]
- Babu, A.S.; Veluswamy, S.K.; Arena, R.; Guazzi, M.; Lavie, C.J. Virgin coconut oil and its potential cardioprotective effects. Postgrad. Med. 2014, 126, 76–83. [Google Scholar] [CrossRef]
- Gandotra, S.; Kour, J.; Van Der Waag, A. Efficacy of adjunctive extra virgin coconut oil use in moderate to severe alzheimer’s disease. Int. J. Sch. Cogn. Psychol. 2014, 1, 2. [Google Scholar]
- Dayrit, C.S. Coconut oil in health and disease: Its and monolaurins’s potential as cure for HIV/AIDS. In Proceedings of the 36th Cocotech Meeting, Chennai, India, 5–9 July 2000. [Google Scholar]
- Srivastava, Y.; Semwal, A.D.; Majumdar, A. Quantitative and qualitative analysis of bioactive components present in virgin coconut oil. Cogent Food Agric. 2016, 2, 1164929. [Google Scholar] [CrossRef]
- Bederson, J.B.; Pitts, L.H.; Tsuji, M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986, 17, 472–476. [Google Scholar] [CrossRef]
- Bailey, S.A.; Zidell, R.H.; Perry, R.W. Relationships between organ weight and body/brain weight in the rat: What is the best analytical endpoint? Toxicol. Pathol. 2004, 32, 448–466. [Google Scholar] [CrossRef]
- Ben Othman, M.; Han, J.; El Omri, A.; Ksouri, R.; Neffati, M.; Isoda, H. Antistress effects of the ethanolic extract from Cymbopogon schoenanthus growing wild in Tunisia. Evid.-Based Complementary Altern. Med. 2013, 2013, 737401. [Google Scholar] [CrossRef][Green Version]
- Narayanankutty, A.; Mukesh, R.K.; Ayoob, S.K.; Ramavarma, S.K.; Suseela, I.M.; Manalil, J.J.; Kuzhivelil, B.T.; Raghavamenon, A.C. Virgin coconut oil maintains redox status and improves glycemic conditions in high fructose fed rats. J. Food Sci. Technol. 2016, 53, 895–901. [Google Scholar] [CrossRef]
- Illam, S.P.; Narayanankutty, A.; Raghavamenon, A.C. Polyphenols of virgin coconut oil prevent pro-oxidant mediated cell death. Toxicol. Mech. Methods 2017, 27, 442–450. [Google Scholar] [CrossRef]
- White, A.R.; Collins, S.J.; Maher, F.; Jobling, M.F.; Stewart, L.R.; Thyer, J.M.; Beyreuther, K.; Masters, C.L.; Cappai, R. Prion protein-deficient neurons reveal lower glutathione reductase activity and increased susceptibility to hydrogen peroxide toxicity. Am. J. Pathol. 1999, 155, 1723–1730. [Google Scholar] [CrossRef]
- Chaiswing, L.; Oberley, T.D.; Zhong, W. Increasing discordant antioxidant protein levels and enzymatic activities contribute to increasing redox imbalance observed during human prostate cancer progression. Free. Radic. Biol. Med. 2014, 67, 342–352. [Google Scholar] [CrossRef][Green Version]
- Dickhout, J.G.; Lee, R.M. Blood pressure and heart rate development in young spontaneously hypertensive rats. Am. J. Physiol. 1998, 274, H794–H800. [Google Scholar] [CrossRef]
- Nurul-Iman, B.S.; Kamisah, Y.; Jaarin, K.; Qodriyah, H.M. Virgin coconut oil prevents blood pressure elevation and improves endothelial functions in rats fed with repeatedly heated palm oil. Evid. Based Complement Altern. Med. 2013, 2013, 629329. [Google Scholar] [CrossRef]
- Júnior, F.A.O.; Ruiz, C.R.; de Oliveira, Y.; Barros, M.A.V.; Silva, A.S.; Santos, M.S.B.; Martins, V.J.B.; Balarini, C.M.; Braga, V.A. Coconut Oil Supplementation Does Not Affect Blood Pressure Variability and Oxidative Stress: A Placebo-Controlled Clinical Study in Stage-1 Hypertensive Patients. Nutrients 2021, 13, 798. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Vitor, R.J.S., II. Neuroprotective Effects of Virgin Coconut Oil in a Sprague Dawley Rat Ischemic Stroke Model. Master’s Thesis, University of the Philippines Manila, Manila, Philippines, 2018. [Google Scholar]
- Yamagata, K.; Tagami, M. Prevention of ischemia-induced neuronal apoptosis by vitamin E in stroke-prone spontaneously hypertensive rats: Cellular mechanisms of antioxidants. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 399–402. [Google Scholar]
- Maalouf, M.; Rho, J.M.; Mattson, M.P. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Rev. 2009, 59, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Thaler, S.; Choragiewicz, T.J.; Rejdak, R.; Fiedorowicz, M.; Turski, W.A.; Tulidowicz-Bielak, M.; Zrenner, E.; Schuettauf, F.; Zarnowski, T. Neuroprotection by acetoacetate and β-hydroxybutyrate against NMDA-induced RGC damage in rat—possible involvement of kynurenic acid. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Mary, S.; Boder, P.; Rossitto, G.; Graham, L.; Scott, K.; Flynn, A.; Kipgen, D.; Graham, D.; Delles, C. Salt loading decreases urinary excretion and increases intracellular accumulation of uromodulin in stroke-prone spontaneously hypertensive rats. Clin. Sci. 2021, 135, 2749–2761. [Google Scholar] [CrossRef]
- Carnevale, D.; Lembo, G. Heart, Spleen, Brain. Circulation 2018, 138, 1917–1919. [Google Scholar] [CrossRef]
- McIntosh, T.K.; Saatman, K.E.; Raghupathi, R.; Graham, D.I.; Smith, D.H.; Lee, V.M.; Trojanowski, J.Q. The Dorothy Russell Memorial Lecture. The molecular and cellular sequelae of experimental traumatic brain injury: Pathogenetic mechanisms. Neuropathol. Appl. Neurobiol. 1998, 24, 251–267. [Google Scholar] [CrossRef]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef]
- Hua, F.; Ma, J.; Ha, T.; Xia, Y.; Kelley, J.; Williams, D.L.; Kao, R.L.; Browder, I.W.; Schweitzer, J.B.; Kalbfleisch, J.H.; et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J. Neuroimmunol. 2007, 190, 101–111. [Google Scholar] [CrossRef]
- Ning, R.; Chopp, M.; Zacharek, A.; Yan, T.; Zhang, C.; Roberts, C.; Lu, M.; Chen, J. Neamine induces neuroprotection after acute ischemic stroke in type one diabetic rats. Neuroscience 2014, 257, 76–85. [Google Scholar] [CrossRef]
- Broughton, B.R.S.; Reutens, D.C.; Sobey, C.G. Apoptotic mechanisms after cerebral ischemia. Stroke 2009, 40, e331–e339. [Google Scholar] [CrossRef]
- Diestro, J.D.B.; Omar, A.T., II; Climacosa, F.M.M.; Mondia, M.W.L.; Arbis, C.C.H.; Collantes, T.M.A.; Khu, K.J.O.; Roxas, A.A., Jr.; Estacio, M.A.C. Virgin coconut oil attenuates deficits in rats undergoing transient cerebral ischemia. Acta Med. Philipp. 2021, 55, 109–116. [Google Scholar] [CrossRef]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef]



| Score * | Description |
|---|---|
| 0 | No neurological deficit |
| 1 | Left forelimb flexion when suspended by the tail or failure to extend the right forepaw fully |
| 2 | Left shoulder adduction when suspended by the tail |
| 3 | Reduced resistance to lateral push toward the left side |
| 4 | Spontaneous movements in all directions with circling to the left exhibiting only if pulled by the tail |
| 5 | Circle or walk spontaneously only to the left |
| 6 | Walk only when stimulated |
| 7 | No response to stimulation |
| 8 | Stroke-related death |
| Group | Baseline | Week 2 | Week 4 | Week 6 | Week 8 | Week 10 |
|---|---|---|---|---|---|---|
| A | 10 | 10 | 10 | 4 | 1 | - |
| B | 10 | 10 | 10 | 10 | 9 | 4 |
| Total | 20 | 20 | 20 | 14 | 10 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitor, R.J.S., II; Tochinai, R.; Sekizawa, S.-I.; Kuwahara, M. Favorable Effects of Virgin Coconut Oil on Neuronal Damage and Mortality after a Stroke Incidence in the Stroke-Prone Spontaneously Hypertensive Rat. Life 2022, 12, 1857. https://doi.org/10.3390/life12111857
Vitor RJS II, Tochinai R, Sekizawa S-I, Kuwahara M. Favorable Effects of Virgin Coconut Oil on Neuronal Damage and Mortality after a Stroke Incidence in the Stroke-Prone Spontaneously Hypertensive Rat. Life. 2022; 12(11):1857. https://doi.org/10.3390/life12111857
Chicago/Turabian StyleVitor, Rodel Jonathan Santos, II, Ryota Tochinai, Shin-Ichi Sekizawa, and Masayoshi Kuwahara. 2022. "Favorable Effects of Virgin Coconut Oil on Neuronal Damage and Mortality after a Stroke Incidence in the Stroke-Prone Spontaneously Hypertensive Rat" Life 12, no. 11: 1857. https://doi.org/10.3390/life12111857
APA StyleVitor, R. J. S., II, Tochinai, R., Sekizawa, S.-I., & Kuwahara, M. (2022). Favorable Effects of Virgin Coconut Oil on Neuronal Damage and Mortality after a Stroke Incidence in the Stroke-Prone Spontaneously Hypertensive Rat. Life, 12(11), 1857. https://doi.org/10.3390/life12111857








