Fermented Soybean Meal (FSBM) in African Catfish (Clarias gariepinus) Diets: Effects on Growth Performance, Fish Gut Microbiota Analysis, Blood Haematology, and Liver Morphology
Abstract
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Ethic Approval
2.2. Experimental Fish Preparation
2.3. Preparation of Defatted SBM Protein Mixture
2.4. Fish Feed Preparation
2.5. Growth Performance of Experimental Fish
- Survival rate (%) = (Total number of survived fish/Total number of experimental fish at the beginning of the experiment) × 100%
- Weight gain (%) = (Final weight − initial weight) × 100%/initial weight
- Specific growth rate (%) = (log Final weight − log initial weight) × 100%/Experiment duration
- Hepatosomatic (%) = (Weight of liver/body weight) × 100%
- Visceral somatic (%) = (Visceral weight/body weight) × 100%
- Feed conversion rate (FCR) = Total feed consumption/Fish weight gain
2.6. Fish Gut Microbiology Analysis
2.7. Fish Blood Haematology and Biochemical Analysis
2.8. Histological Analysis
2.9. Next Generation Sequence (NGS) Metagenomics Data Analysis
2.9.1. Fish DNA Preparation
2.9.2. Library Data Preparation
2.9.3. DNA Sequencing Assay
2.9.4. Data DNA Sequencing Analysis
2.10. Statistical Analysis
3. Results
3.1. Fish Growth Performance
3.2. Fish Gut Microbiology Analysis
3.3. Blood Parameters Analysis
3.4. Blood Biochemical Parameters
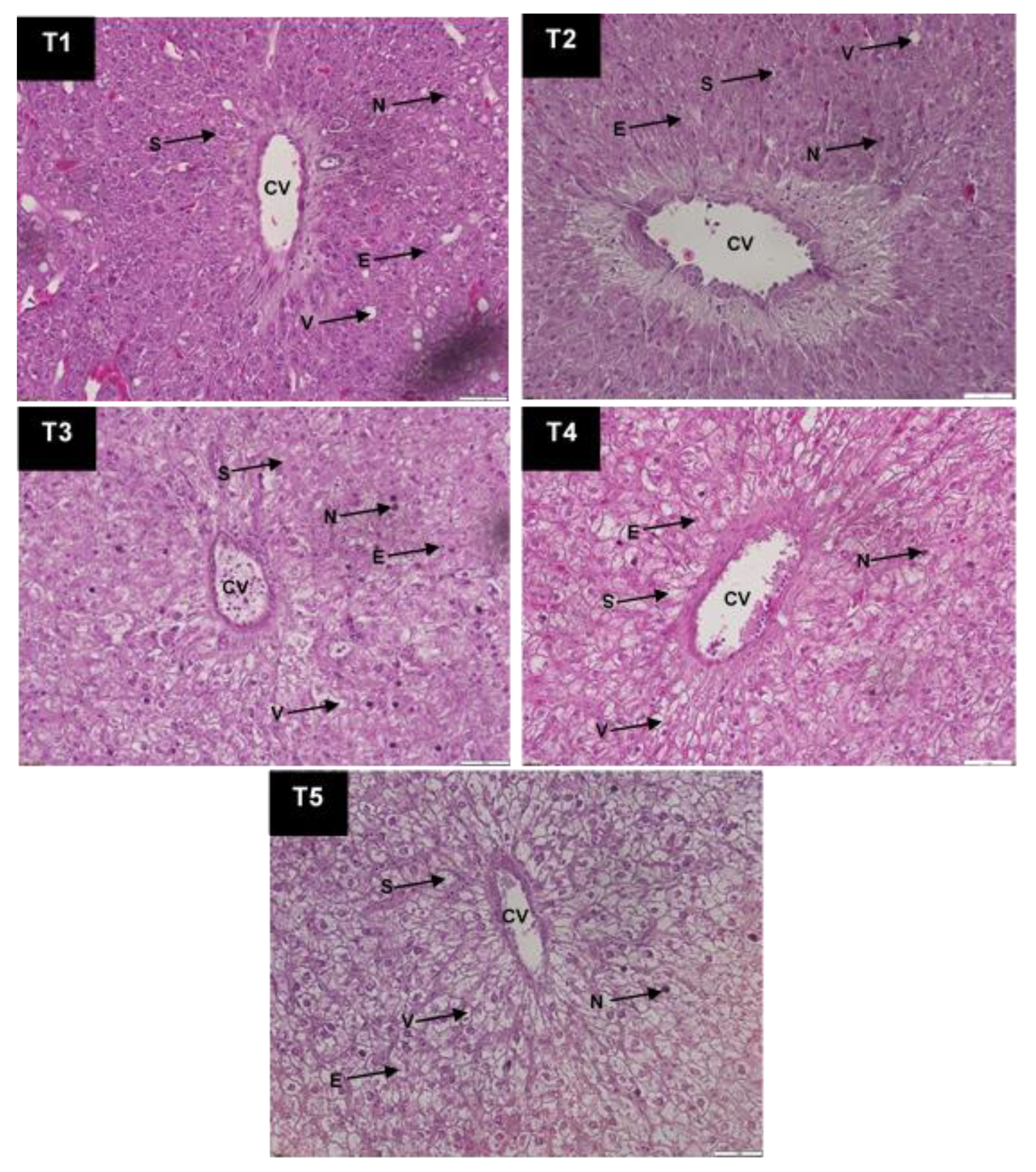
3.5. Experimental Fish Liver Histological Analysis
3.6. Experimental Fish Gut Microbiota Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Kari, Z.A.; Kabir, M.A.; Dawood, M.A.; Razab, M.K.A.A.; Ariff, N.S.N.A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Mat, K.; Ismail, T.A. Effect of fish meal substitution with fermented soy pulp on growth performance, digestive enzyme, amino acid profile, and immune-related gene expression of African catfish (Clarias gariepinus). Aquaculture 2022, 546, 737418. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquacult. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Dossou, S.; Dawood, M.A.O.; Zaineldin, A.I.; Abouelsaad, I.A.; Mzengereza, K.; Shadrack, R.S.; Zhang, Y.K.; El-Sharnouby, M.; Ahmed, H.A.; El Basuini, M.F. Dynamical Hybrid System for Optimizing and Controlling Efficacy of Plant-Based Protein in Aquafeeds. Complexity 2021, 2021, 9957723. [Google Scholar] [CrossRef]
- Amer, A.A.; El-Nabawy, E.M.; Gouda, A.H.; Dawood, M.A.O. The addition of insect meal from Spodoptera littoralis in the diets of Nile tilapia and its effect on growth rates, digestive enzyme activity and health status. Aquac. Res. 2021, 52, 5585–5594. [Google Scholar] [CrossRef]
- Elesho, F.E.; Krockel, S.; Ciavoni, E.; Sutter, D.A.H.; Verreth, J.A.J.; Schrama, J.W. Effect of feeding frequency on performance, nutrient digestibility, energy and nitrogen balances in juvenile African catfish (Clarias gariepinus) fed diets with two levels of crystalline methionine. Anim. Feed Sci. Tech. 2021, 281, 115098. [Google Scholar] [CrossRef]
- Kari, Z.A.; Goh, K.W.; Edinur, H.A.; Mat, K.; Khalid, H.-N.M.; Rusli, N.D.; Sukri, S.A.M.; Harun, H.C.; Wei, L.S.; Hanafiah, M.H.B.M.A. Palm date meal as a non-traditional ingredient for feeding aquatic animals: A review. Aquac. Rep. 2022, 25, 101233. [Google Scholar] [CrossRef]
- Kari, Z.A.; Wee, W.; Hamid, N.K.A.; Mat, K.; Rusli, N.D.; Khalid, H.N.M.; Sukri, S.A.M.; Harun, H.C.; Dawood, M.A.; Hakim, A.H. Recent Advances of Phytobiotic Utilization in Carp Farming: A Review. Aquac. Nutr. 2022, 2022, 7626675. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; El-Ashram, S.; Yilmaz, S.; Naiel, M.A.; Kari, Z.A.; Hamid, N.K.A.; Dawood, M.A.; Nowosad, J.; Kucharczyk, D. The effectiveness of Arthrospira platensis and microalgae in relieving stressful conditions affecting finfish and shellfish species: An overview. Aquac. Rep. 2022, 24, 101135. [Google Scholar] [CrossRef]
- Dawood, M.A.; Habotta, O.A.; Elsabagh, M.; Azra, M.N.; Van Doan, H.; Kari, Z.A.; Sewilam, H. Fruit processing by-products in the aquafeed industry: A feasible strategy for aquaculture sustainability. Rev. Aquac. 2022, 14, 1945–1965. [Google Scholar] [CrossRef]
- Azri, N.A.M.; Chun, L.K.; Hasan, H.A.; Jaya-Ram, A.; Kari, Z.A.; Hamid, N.K.A. The effects of partial replacement of fishmeal with hermetia meal on the growth and fatty acid profile of African catfish fry. Agric. Rep. 2022, 1, 17–27. [Google Scholar]
- Hazreen-Nita, M.K.; Kari, Z.A.; Mat, K.; Rusli, N.D.; Sukri, S.A.M.; Harun, H.C.; Lee, S.W.; Rahman, M.M.; Norazmi-Lokman, N.; Nur-Nazifah, M. Olive oil by-products in aquafeeds: Opportunities and challenges. Aquac. Rep. 2022, 22, 100998. [Google Scholar] [CrossRef]
- Maulu, S.; Langi, S.; Hasimuna, O.J.; Missinhoun, D.; Munganga, B.P.; Hampuwo, B.M.; Gabriel, N.N.; Elsabagh, M.; Van Doan, H.; Abdul Kari, Z.; et al. Recent advances in the utilization of insects as an ingredient in aquafeeds: A review. Anim. Nutr. 2022, 11, 334–349. [Google Scholar] [CrossRef]
- Rahman, M.M.; Nakagawa, T.; Bin Abdullah, R.; Embong, W.K.W.; Akashi, R. Feed intake and growth performance of goats supplemented with soy waste. Pesqui Agropecu Bras 2014, 49, 554–558. [Google Scholar] [CrossRef][Green Version]
- Azarm, H.M.; Lee, S.M. Effects of partial substitution of dietary fish meal by fermented soybean meal on growth performance, amino acid and biochemical parameters of juvenile black sea bream Acanthopagrus schlegeli. Aquac. Res. 2014, 45, 994–1003. [Google Scholar] [CrossRef]
- Elesho, F.E.; Krockel, S.; Sutter, D.A.H.; Nuraini, R.; Chen, I.J.; Verreth, J.A.J.; Schrama, J.W. Effect of feeding level on the digestibility of alternative protein-rich ingredients for African catfish (Clarias gariepinus). Aquaculture 2021, 544, 737108. [Google Scholar] [CrossRef]
- Zulhisyam, A.K.; Kabir, M.A.; Munir, M.B.; Wei, L.S. Using of fermented soy pulp as an edible coating material on fish feed pellet in African catfish (Clarias gariepinus) production. Aquac. Aquar. Conserv. Legis. 2020, 13, 296–308. [Google Scholar]
- Wu, P.; Golly, M.K.; Guo, Y.T.; Ma, H.L.; He, R.H.; Luo, X.; Luo, S.L.; Zhang, C.; Zhang, L.P.; Zhu, J.H. Effect of partial replacement of soybean meal with high-temperature fermented soybean meal in antibiotic-growth-promoter-free diets on growth performance, organ weights, serum indexes, intestinal flora and histomorphology of broiler chickens. Anim. Feed Sci. Tech. 2020, 269, 114616. [Google Scholar] [CrossRef]
- Manickavasagan, A.; Manickavasagan, A.; Lim, L.-T.; Ali, A. Plant Protein Foods, 1st ed.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.S.; Krogdahl, A.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Kari, Z.A.; Kabir, M.A.; Mat, K.; Rusli, N.D.; Razab, M.K.A.A.; Ariff, N.S.N.A.; Edinur, H.A.; Rahim, M.Z.A.; Pati, S.; Dawood, M.A. The possibility of replacing fish meal with fermented soy pulp on the growth performance, blood biochemistry, liver, and intestinal morphology of African catfish (Clarias gariepinus). Aquac. Rep. 2021, 21, 100815. [Google Scholar] [CrossRef]
- Habotta, O.A.; Dawood, M.A.; Kari, Z.A.; Tapingkae, W.; Van Doan, H. Antioxidative and immunostimulant potential of fruit derived biomolecules in aquaculture. Fish Shellfish. Immunol. 2022, 130, 317–322. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev. Aquacult. 2020, 12, 987–1002. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iwashita, Y.; Matsunari, H.; Sugita, T.; Furuita, H.; Akimoto, A.; Okamatsu, K.; Suzuki, N. Influence of fermentation conditions for soybean meal in a non-fish meal diet on the growth performance and physiological condition of rainbow trout Oncorhynchus mykiss. Aquaculture 2010, 309, 173–180. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, P.F.; Lin, S.M.; Tang, R.J.; Chen, Y.J.; Luo, L. Partial substitution of soybean meal with fermented soybean residue in diets for juvenile largemouth bass, Micropterus salmoides. Aquac. Nutr. 2018, 24, 1213–1222. [Google Scholar] [CrossRef]
- He, M.; Yu, Y.; Li, X.; Poolsawat, L.; Yang, P.; Bian, Y.; Guo, Z.; Leng, X. An evaluation of replacing fish meal with fermented soybean meal in the diets of largemouth bass (Micropterus salmoides): Growth, nutrition utilization and intestinal histology. Aquac. Res. 2020, 51, 4302–4314. [Google Scholar] [CrossRef]
- Shiu, Y.L.; Hsieh, S.L.; Guei, W.C.; Tsai, Y.T.; Chiu, C.H.; Liu, C.H. Using B acillus subtilis E20-fermented soybean meal as replacement for fish meal in the diet of orange-spotted grouper (E pinephelus coioides, H amilton). Aquac. Res. 2015, 46, 1403–1416. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; He, R.; Xu, W.; Mai, K.; He, G. Effects of soybean meal fermentation by Lactobacillus plantarum P8 on growth, immune responses, and intestinal morphology in juvenile turbot (Scophthalmus maximus L.). Aquaculture 2016, 464, 87–94. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.N.; Guan, D.; Wang, Y.P.; Zhang, Z.Y. Evaluation of Solid-State Fermentation by Ganoderma lucidum Using Soybean Curd Residue. Food Bioprocess Tech. 2013, 6, 1856–1867. [Google Scholar] [CrossRef]
- Archer, G.L. Staphylococcus aureus: A well-armed pathogen. Clin. Infect. Dis. 1998, 26, 1179–1181. [Google Scholar] [CrossRef]
- Heo, S.; Lee, J.H.; Jeong, D. Food-derived coagulase-negative Staphylococcus as starter cultures for fermented foods. Food Sci. Biotechnol. 2020, 29, 1023. [Google Scholar] [CrossRef]
- Guan, L.; Cho, K.H.; Lee, J.H. Analysis of the cultivable bacterial community in jeotgal, a Korean salted and fermented seafood, and identification of its dominant bacteria. Food Microbiol. 2011, 28, 101–113. [Google Scholar] [CrossRef]
- Leroy, F.; Verluyten, J.; De Vuyst, L. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 2006, 106, 270–285. [Google Scholar] [CrossRef]
- Jeong, D.W.; Lee, J.H. Complete Genome Sequence of Staphylococcus succinus 14BME20 Isolated from a Traditional Korean Fermented Soybean Food. Genome Announc. 2017, 5, e01731-16. [Google Scholar] [CrossRef]
- Jeong, D.W.; Han, S.; Lee, J.H. Safety and technological characterization of Staphylococcus equorum isolates from jeotgal, a Korean high-salt-fermented seafood, for starter development. Int. J. Food Microbiol. 2014, 188, 108–115. [Google Scholar] [CrossRef]
- Lee, S.W.; Farid, M.R.; Wendy, W.; Zulhisyam, A.K. Water hyacinth, Eichhornia crassipes (Mart.), leaf as an Alternative Protein Source for Siamese Gourami, Trichogaster pectoralis. Int. J. Aquat. Sci. 2016, 7, 58–62. [Google Scholar]
- Thomson, P.; García, P.; Miles, J.; Isla, D.; Yáñez, C.; Santibáñez, R.; Núñez, A.; Flores-Yáñez, C.; Del Río, C.; Cuadra, F. Isolation and Identification of Staphylococcus Species Obtained from Healthy Companion Animals and Humans. Vet. Sci. 2022, 9, 79. [Google Scholar] [CrossRef]
- Shen, G.; Zheng, L.; Li, S.; Wu, H.; Li, M.; Luo, Q.; Yu, G.; Chen, A.; Zhang, Z. The role of soy protein degradation caused by spoilage Bacillus amyloliquefaciens in texture deterioration of yuba, a soy product. LWT 2020, 123, 109108. [Google Scholar] [CrossRef]
- Thiex, N.J.; Manson, H.; Anderson, S.; Persson, J.A. Determination of crude protein in animal feed, forage, grain, and oilseeds by using block digestion with a copper catalyst and steam distillation into boric acid: Collaborative study. J. Aoac Int. 2002, 85, 309–317. [Google Scholar] [CrossRef]
- Kader, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Bulbul, M. Supplemental effects of some crude ingredients in improving nutritive values of low fishmeal diets for red sea bream, Pagrus major. Aquaculture 2010, 308, 136–144. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Fish; National Academies Press: Washington, DC, USA, 1993. [Google Scholar]
- Seong Wei, L.; Shareef, M.H.Z.; Aweng, E.R.; Wee, W. The effectiveness of developed depuration system in controling bacteria colonized in Asian clam, Corbicula fluminea tissue. Int. J. Aquat. Sci. 2021, 12, 9–13. [Google Scholar]
- He, Q.; Xiao, S.Q.; Zhang, C.L.; Zhang, Y.F.; Shi, H.R.; Zhang, H.F.; Lin, F.M.; Liu, X.C.; Yang, H.R.; Wang, Q.; et al. Modulation of the growth performance, biochemical parameters, and non-specific immune responses of the hybrid grouper (Epinephelus fuscoguttatus female xE. lanceolatus male) by two kinds of Chinese herb. Aquacult. Rep. 2021, 19, 100604. [Google Scholar]
- Lee, S.W.; Tey, H.C.; Wendy, W.; Wan Zahari, M. The effect of house cricket (Acheta domesticus) meal on growth performance of red hybrid tilapia (Oreochromis sp.). Int. J. Aquat. Sci. 2017, 8, 78–82. [Google Scholar]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A Simple Salting out Procedure for Extracting DNA from Human Nucleated Cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Inglis, P.W.; Pappas, M.D.R.; Resende, L.V.; Grattapaglia, D. Fast and inexpensive protocols for consistent extraction of high quality DNA and RNA from challenging plant and fungal samples for high-throughput SNP genotyping and sequencing applications. PLoS ONE 2018, 13, e0206085. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Garcia-Lopez, R.; Cornejo-Granados, F.; Lopez-Zavala, A.A.; Sanchez-Lopez, F.; Cota-Huizar, A.; Sotelo-Mundo, R.R.; Guerrero, A.; Mendoza-Vargas, A.; Gomez-Gil, B.; Ochoa-Leyva, A. Doing More with Less: A Comparison of 16S Hypervariable Regions in Search of Defining the Shrimp Microbiota. Microorganisms 2020, 8, 134. [Google Scholar] [CrossRef]
- Glenn, T.C.; Nilsen, R.A.; Kieran, T.J.; Sanders, J.G.; Bayona-Vásquez, N.J.; Finger, J.W.; Pierson, T.W.; Bentley, K.E.; Hoffberg, S.L.; Louha, S. Adapterama I: Universal stubs and primers for 384 unique dual-indexed or 147,456 combinatorially-indexed Illumina libraries (iTru & iNext). PeerJ 2019, 7, e7755. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Chaumeil, P.A.; Rinke, C.; Mussig, A.J.; Hugenholtz, P. A complete domain-to-species taxonomy for Bacteria and Archaea. Nat. Biotechnol. 2020, 38, 1079–1086. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using Microbiome Analyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat Protoc 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Friedman, J.; Alm, E.J. Inferring Correlation Networks from Genomic Survey Data. PloS Comput. Biol. 2012, 8, e1002687. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liang, G.; Yang, H.; Liu, Y.; Tian, L. Replacement of fish meal by fermented soybean meal and crystal amino acid in diets for hybrid tilapia. South China Fish. Sci. 2009, 5, 28–33. [Google Scholar]
- Uczay, J.; Battisti, E.K.; Lazzari, R.; Pessatti, M.L.; Schneider, T.L.S.; Hermes, L.B.; Peixoto, N.C.; Fabregat, T.E.H.P. Fish meal replaced by hydrolysed soybean meal in diets increases growth and improves the antioxidant defense system of silver catfish (Rhamdia quelen). Aquac. Res. 2019, 50, 1438–1447. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Khaoian, P.; Fukada, H.; Suzuki, N.; Masumoto, T. Feeding fermented soybean meal diet supplemented with taurine to yellowtail Seriola quinqueradiata affects growth performance and lipid digestion. Aquac. Res. 2015, 46, 1101–1110. [Google Scholar] [CrossRef]
- Zhou, F.; Song, W.; Shao, Q.; Peng, X.; Xiao, J.; Hua, Y.; Owari, B.N.; Zhang, T.; Ng, W.-K. Partial Replacement of Fish Meal by Fermented Soybean Meal in Diets for Black Sea Bream, Acanthopagrus schlegelii, Juveniles. J. World Aquac. Soc. 2011, 42, 184–197. [Google Scholar] [CrossRef]
- Cherdkeattipol, K.; Chuchird, N.; Chonudomkul, D.; Yongmanitchai, W.; Pichitkul, P. Effect of partial replacement of fish meal by Bacillus sp-fermented soybean meal on growth performance, immunity, hepatopancreas microbiota and disease resistance in pacific White Shrimp (Litopenaeus vannamei). J. Fish. Environ. 2021, 45, 32–42. [Google Scholar]
- Liu, X.; Ju, Y.; Huang, L.; Liu, M.; Bo, J.; Zhou, T.; Zhang, Y.; Liu, C.; Feng, M.; Zhang, S.; et al. Effects of a new fermented soya bean meal on growth performance, serum biochemistry profile, intestinal immune status and digestive enzyme activities in piglets. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1046–1059. [Google Scholar] [CrossRef]
- Novriadi, R. A Meta-analysis approach toward fish meal replacement with fermented soybean meal: Effects on fish growth performance and feed conversion ratio. Asian Fish Sci. 2017, 30, 227–244. [Google Scholar] [CrossRef]
- Poleksic, V.; Lenhardt, M.; Jaric, I.; Djordjevic, D.; Gacic, Z.; Cvijanovic, G.; Raskovic, B. Liver, gills, and skin histopathology and heavy metal content of the Danube sterlet (Acipenser ruthenus Linnaeus, 1758). Environ. Toxicol. Chem. Int. J. 2010, 29, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Rašković, B.; Stanković, M.; Marković, Z.; Poleksić, V. Histological methods in the assessment of different feed effects on liver and intestine of fish. J. Agric. Sci. 2011, 56, 87–100. [Google Scholar]
- Zhang, Y.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dossou, S.; Wang, W.; Zhang, X.; Shadrack, R.S.; Mzengereza, K.; Zhu, K. Optimization of soybean meal fermentation for aqua-feed with Bacillus subtilis natto using the response surface methodology. Fermentation 2021, 7, 306. [Google Scholar] [CrossRef]
- Trushenski, J.T.; Rombenso, A.N.; Page, M.; Jirsa, D.; Drawbridge, M. Traditional and fermented soybean meals as ingredients in feeds for white seabass and yellowtail jack. North Am. J. Aquac. 2014, 76, 312–322. [Google Scholar] [CrossRef]
- De Oliveira, N.S.; Ha, N.; Da Cunha, L.; Cipriani, L.A.; Neto, A.T.; Skoronski, E.; Gisbert, E.; Perez Fabregat, T.E.H. Fermentation of Soybean Meal with Lactobacillus acidophilus Allows Greater Inclusion of Vegetable Protein in the Diet and Can Reduce Vibrionacea in the Intestine of the South American Catfish (Rhamdia quelen). Animals 2022, 12, 690. [Google Scholar] [CrossRef]
- Ozgur, Y.N.; Tulay, D. Use of fermented soybean meal with whey as a protein source for feeding juvenile tilapia (Oreochromis niloticus). Isr. J. Aquac. Bamidgeh 2016, 68, 20787. [Google Scholar]
- Li, C.Q.; Zhang, B.L.; Liu, C.D.; Zhou, H.H.; Wang, X.; Mai, K.S.; He, G. Effects of dietary raw or Enterococcus faecium fermented soybean meal on growth, antioxidant status, intestinal microbiota, morphology, and inflammatory responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immun. 2020, 100, 261–271. [Google Scholar] [CrossRef]
- Silva-Carrillo, Y.; Hernandez, C.; Hardy, R.W.; Gonzalez-Rodriguez, B.; Castillo-Vargasmachuca, S. The effect of substituting fish meal with soybean meal on growth, feed efficiency, body composition and blood chemistry in juvenile spotted rose snapper Lutjanus guttatus (Steindachner, 1869). Aquaculture 2012, 364, 180–185. [Google Scholar] [CrossRef]
- Yang, H.; Bian, Y.H.; Huang, L.L.; Lan, Q.; Ma, L.Z.; Li, X.Q.; Leng, X.J. Effects of replacing fish meal with fermented soybean meal on the growth performance, intestinal microbiota, morphology and disease resistance of largemouth bass (Micropterus salmoides). Aquacult. Rep. 2022, 22, 100954. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, Y.T. Lactobacillus spp. fermented soybean meal partially substitution to fish meal enhances innate immune responses and nutrient digestibility of white shrimp (Litopenaeus vannamei) fed diet with low fish meal. Aquaculture 2022, 548, 737634. [Google Scholar] [CrossRef]
- Robaina, L.; Izquierdo, M.; Moyano, F.; Socorro, J.; Vergara, J.; Montero, D.; Fernandez-Palacios, H. Soybean and lupin seed meals as protein sources in diets for gilthead seabream (Sparus aurata): Nutritional and histological implications. Aquaculture 1995, 130, 219–233. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Hemre, G.I.; Mommsen, T. Carbohydrates in fish nutrition: Digestion and absorption in postlarval stages. Aquac. Nutr. 2005, 11, 103–122. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Davies, S.J.; Sweetman, J.; Divanach, P.; Chatzifotis, S. Dietary supplementation of mannan oligosaccharide on white sea bream (Diplodus sargus L.) larvae: Effects on development, gut morphology and salinity tolerance. Aquac. Res. 2010, 41, e245–e251. [Google Scholar] [CrossRef]
- Ighwela, K.A.; Ahmad, A.B.; Abol-Munafi, A. The selection of viscerosomatic and hepatosomatic indices for the measurement and analysis of Oreochromis niloticus condition fed with varying dietary maltose levels. Int. J. Fauna Biol. Stud. 2014, 1, 18–20. [Google Scholar]
- Ahmad, M.; Qureshi, T.; Singh, A. Effect of dietary protein, lipid and carbohydrate contents on the viscera composition and organ Indices of Cyprinus carpio communis fingerlings. Afr. J. Biotechnol. 2012, 11, 8361–8366. [Google Scholar]
- Kapka-Skrzypczak, L.; Niedźwiecka, J.; Wojtyła, A.; Kruszewski, M. Probiotics and prebiotics as a bioactive component of functional food. Pediatric Endocrinol. Diabetes Metab. 2012, 18, 79–83. [Google Scholar]
- Webster, C.D.; Thompson, K.R.; Morgan, A.M.; Grisby, E.J.; Dasgupta, S. Feeding frequency affects growth, not fillet composition, of juvenile sunshine bass Morone chrysops × M. saxatilis grown in cages. J. World Aquac. Soc. 2001, 32, 79–88. [Google Scholar] [CrossRef]
- Shiu, Y.L.; Wong, S.L.; Guei, W.C.; Shin, Y.C.; Liu, C.H. Increase in the plant protein ratio in the diet of white shrimp, Litopenaeus vannamei (Boone), using Bacillus subtilis E20-fermented soybean meal as a replacement. Aquac. Res. 2015, 46, 382–394. [Google Scholar] [CrossRef]
- Heikkinen, J.; Vielma, J.; Kemilainen, O.; Tiirola, M.; Eskelinen, P.; Kiuru, T.; Navia-Paldanius, D.; Von Wright, A. Effects of soybean meal based diet on growth performance, gut histopathology and intestinal microbiota of juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 2006, 261, 259–268. [Google Scholar] [CrossRef]
- Lilleeng, E.; Froystad, M.K.; Ostby, G.C.; Valen, E.C.; Krogdahl, A. Effects of diets containing soybean meal on trypsin mRNA expression and activity in Atlantic salmon (Salmo salar L). Comp. Biochem. Phys. A 2007, 147, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Nagel, F.; Von Danwitz, A.; Tusche, K.; Kroeckel, S.; Van Bussel, C.G.J.; Schlachter, M.; Adem, H.; Tressel, R.P.; Schulz, C. Nutritional evaluation of rapeseed protein isolate as fish meal substitute for juvenile turbot (Psetta maxima L.)—Impact on growth performance, body composition, nutrient digestibility and blood physiology. Aquaculture 2012, 356, 357–364. [Google Scholar] [CrossRef]
- Feng, J.; Liu, X.; Xu, Z.R.; Liu, Y.Y.; Lu, Y.P. Effects of Aspergillus oryzae 3.042 fermented soybean meal on growth performance and plasma biochemical parameters in broilers. Anim. Feed Sci. Tech. 2007, 134, 235–242. [Google Scholar] [CrossRef]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Tech. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Cheng, A.C.; Lin, H.L.; Shiu, Y.L.; Tyan, Y.C.; Liu, C.H. Isolation and characterization of antimicrobial peptides derived from Bacillus subtilis E20-fermented soybean meal and its use for preventing Vibrio infection in shrimp aquaculture. Fish Shellfish Immunol. 2017, 67, 270–279. [Google Scholar] [CrossRef]
- Faustino, M.; Durao, J.; Pereira, C.F.; Pintado, M.E.; Carvalho, A.P. Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae-A sustainable source of functional ingredients. Carbohyd. Polym. 2021, 272, 118467. [Google Scholar] [CrossRef]
- Pessione, E.; Mazzoli, R.; Giuffrida, M.G.; Lamberti, C.; Garcia-Moruno, E.; Barello, C.; Conti, A.; Giunta, C. A proteomic approach to studying biogenic amine producing lactic acid bacteria. Proteomics 2005, 5, 687–698. [Google Scholar] [CrossRef]
- Ismail, A.; Goncalves, B.L.; De Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.S.C.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A.F. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- Taufek, N.M.; Aspani, F.; Muin, H.; Raji, A.A.; Razak, S.A.; Alias, Z. The effect of dietary cricket meal (Gryllus bimaculatus) on growth performance, antioxidant enzyme activities, and haematological response of African catfish (Clarias gariepinus). Fish Physiol. Biochem. 2016, 42, 1143–1155. [Google Scholar] [CrossRef]
- Svobodová, Z.; Máchová, J.; Drastichová, J.; Groch, L.; Lusková, V.; Poleszczuk, G.; Velíšek, J.; Kroupová, H. Haematological and biochemical profiles of carp blood following nitrite exposure at different concentrations of chloride. Aquac. Res. 2005, 36, 1177–1184. [Google Scholar] [CrossRef]
- Ozovehe, B.N. Growth performance, haematological indices and some biochemical enzymes of juveniles Clarias gariepinus (Burchell 1822) fed varying levels of Moringa oleifera leaf meal diet. J. Aquac. Res. Dev. 2013, 4, 166. [Google Scholar] [CrossRef]
- Erhunmwunse, N.; Ainerua, M. Characterization of some blood parameters of African Catfish (Clarias gariepinus). Am. Eurasian J. Toxicol. Sci. 2013, 5, 72–76. [Google Scholar]
- Dienye, H.; Olumuji, O. Growth performance and haematological responses of African mud catfish Clarias gariepinus fed dietary levels of Moringa oleifera leaf meal. Net J. Agric. Sci. 2014, 2, 79–88. [Google Scholar]
- Nya, E.J.; Austin, B. Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2009, 32, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Chen, H.C. Serum metabolic enzyme activities and hepatocyte ultrastructure of common carp after gallium exposure. Zool. Stud. 2003, 42, 455–461. [Google Scholar]
- Siddik, M.A.B.; Foysal, M.J.; Fotedar, R.; Francis, D.S.; Gupta, S.K. Probiotic yeast Saccharomyces cerevisiae coupled with Lactobacillus casei modulates physiological performance and promotes gut microbiota in juvenile barramundi, Lates calcarifer. Aquaculture 2022, 546, 737346. [Google Scholar] [CrossRef]
- Ashrafian, F.; Keshavarz Azizi Raftar, S.; Shahryari, A.; Behrouzi, A.; Yaghoubfar, R.; Lari, A.; Moradi, H.R.; Khatami, S.; Omrani, M.D.; Vaziri, F.; et al. Comparative effects of alive and pasteurized Akkermansia muciniphila on normal diet-fed mice. Sci. Rep. 2021, 11, 17898. [Google Scholar] [CrossRef]
- Cani, P.D.; De Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; De Vos, W.M. Akkermansia muciniphila gen. nov. sp. nov. a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- Zhai, Q.X.; Feng, S.S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. 2019, 59, 3227–3236. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubfar, R.; Behrouzi, A.; Fateh, A.; Nojoumi, S.A.; Vaziri, F.; Khatami, S.; Siadat, S.D. Effects of Akkermansia muciniphila and Faecalibacterium prausnitzii on serotonin transporter expression in intestinal epithelial cells. J. Diabetes Metab. Disord. 2021, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dehghanbanadaki, H.; Aazami, H.; Keshavarz Azizi Raftar, S.; Ashrafian, F.; Ejtahed, H.S.; Hashemi, E.; Hoseini Tavassol, Z.; Ahmadi Badi, S.; Siadat, S.D. Global scientific output trend for Akkermansia muciniphila research: A bibliometric and scientometric analysis. BMC Med. Inf. Decis. Mak. 2020, 20, 291. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]

| Ingredients (g/kg) | Diets (%) | ||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | |
| Fish meal 1 | 40 | 30 | 20 | 10 | 0 |
| SBM | 30 | 0 | 0 | 0 | 0 |
| FSBM 2 | 0 | 40 | 50 | 60 | 70 |
| Wheat | 20 | 20 | 20 | 20 | 20 |
| Fish oil | 6 | 6 | 6 | 6 | 6 |
| CMC 3 | 2 | 2 | 2 | 2 | 2 |
| Vitamin premix 4 | 1 | 1 | 1 | 1 | 1 |
| Mineral premix 5 | 1 | 1 | 1 | 1 | 1 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Proximate analysis (%) | |||||
| Protein | 31.34 | 32.32 | 32.98 | 32.92 | 33.44 |
| Carbohydrate | 47.70 | 46.67 | 46.32 | 46.63 | 46.98 |
| Lipid | 5.50 | 5.21 | 5.01 | 4.78 | 4.63 |
| Fibre | 4.50 | 4.45 | 4.34 | 4.21 | 4.09 |
| Ash | 5.66 | 5.99 | 5.86 | 5.92 | 5.38 |
| Moisture | 5.30 | 5.36 | 5.49 | 5.54 | 5.48 |
| Amino Acid (%) | T1 | T2 | T3 | T4 | T5 | (%) |
|---|---|---|---|---|---|---|
| Lysine | 1.53 ± 0.04 | 1.51 ± 0.32 | 1.49 ± 0.04 | 1.46 ± 0.23 | 1.45 ± 0.28 | 1.43 * |
| Methionine | 0.98 ± 0.03 | 0.82 ± 0.03 | 0.76 ± 0.04 | 0.73 ± 0.06 | 0.68 ± 0.04 | 0.64 * |
| Arginine | 6.64 ± 0.32 | 6.31 ± 0.22 | 5.81 ± 0.54 | 5.51 ± 0.11 | 4.98 ± 0.32 | 1.20 * |
| Phenylalanine | 1.23 ± 0.21 | 2.44 ± 0.32 | 2.56 ± 0.12 | 2.78 ± 0.45 | 3.68 ± 0.32 | 1.40 * |
| Parameters | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| IW (g) | 10.3 ± 0.06 | 10.3 ± 0.10 | 10.2 ± 0.12 | 10.3 ± 0.06 | 10.2 ± 0.10 |
| FW (g) | 195.4 ± 14.47 d | 245.5 ± 8.35 a | 227.0 ± 4.80 b | 214.7 ± 3.88 b | 206.9 ± 5.03 c |
| WG (%) | 1791.1 ± 148.79 d | 2283.8 ± 104.21 a | 2118.4 ± 67.93 b | 1991.4 ± 49.50 c | 1928.3 ± 58.60 c |
| SGR (%) | 2.28 ± 0.061 d | 2.46 ± 0.034 a | 2.40 ± 0.024 b | 2.36 ± 0.018 c | 2.33 ± 0.022 c |
| VSI (%) | 3.59 ± 0.48 b | 2.85 ± 0.278 a | 3.53 ± 0.131 b | 3.69 ± 0.218 c | 4.13 ± 0.147 d |
| HIS (%) | 1.34 ± 0.183 b | 1.15 ± 0.047 a | 1.28 ± 0.071 c | 1.33 ± 0.118 b | 1.47 ± 0.084 d |
| FCR | 1.36 ± 0.106 d | 1.06 ± 0.038 a | 1.15 ± 0.026 b | 1.22 ± 0.023 c | 1.27 ± 0.033 c |
| Parameters | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| Total bacteria CFU/g intestine × 109 | 2.78 ± 0.454 a | 3.00 ± 0.498 b | 3.20 ± 0.225 b | 2.80 ± 0.427 a | 3.67 ± 0.110 c |
| Staphylococcus succinus CFU/g intestine × 106 | 1.58 ± 0.332 a | 4.03 ± 0.164 b | 2.98 ± 0.023 c | 1.70 ± 0.203 a | 2.77 ± 0.210 c |
| Blood Parameters | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| WBC (103/µL) | 121.2 ± 3.23 a | 132.9 ± 2.21 b | 121.3 ± 2.31 a | 116.7 ± 1.42 c | 113.9 ± 2.31 d |
| LYM (%) | 89.4 ± 4.51 a | 109.8 ± 3.53 b | 98.5 ± 2.67 c | 96.7 ± 3.41 c | 97.8 ± 5.67 c |
| MON (%) | 13.8 ± 3.21 | 12.42 ± 2.21 | 13.8 ± 1.81 | 16.9 ± 3.34 | 12.4 ± 2.87 |
| GRA (103/µL) | 3.98 ± 0.36 a | 2.34 ± 0.32 b | 3.32 ± 0.28 c | 3.46 ± 0.31 d | 3.56 ± 0.23 e |
| RBC (103/µL) | 2.23 ± 0.13 a | 2.78 ± 0.23 b | 2.56 ± 0.34 c | 2.21 ± 0.19 a | 1.89 ± 0.21 d |
| HGB (g/dL) | 6.28 ± 1.08 a | 9.53 ± 0.68 b | 9.83 ± 0.72 b | 8.54 ± 0.34 c | 8.34 ± 0.56 c |
| HCT (%) | 26.5 ± 1.21 | 27.8 ± 1.89 | 25.6 ± 2.21 | 26.2 ± 2.31 | 27.2 ± 1.16 |
| MCV (µm3) | 125.3 ± 2.29 | 120.3 ± 3.18 | 125.7 ± 2.31 | 121.4 ± 3.32 | 126.8 ± 4.24 |
| MCH (pg) | 38.3 ± 3.18 a | 44.5 ± 2.43b | 40.1 ± 2.56 c | 38.7 ± 2.45 a | 40.3 ± 3.78 c |
| MCHC (g/dL) | 28.7 ± 4.52 a | 36.4 ± 3.86 b | 35.2 ± 3.12 b | 34.3 ± 2.86 c | 33.2 ± 3.42 c |
| RDW (%) | 7.6 ± 0.45 a | 5.3 ± 0.34 b | 5.4 ± 0.28 b | 7.2 ± 1.08 a | 7.5 ± 0.86 a |
| PLT (103/µL) | 42.3 ± 2.42 a | 30.2 ± 3.46 b | 32.5 ± 2.86 b | 31.7 ± 3.48 b | 39.4 ± 2.68 a |
| MPV (µm3) | 7.12 ± 0.78 a | 6.32 ± 1.34 b | 6.48 ± 0.86 b | 5.54 ± 0.98 c | 5.48 ± 0.72 c |
| PCT (%) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 |
| PDW (%) | 7.68 ± 0.82 a | 9.32 ± 1.44 b | 7.42 ± 0.68 c | 9.54 ± 0.76 b | 9.42 ± 0.68 b |
| Blood Chemical Profiles | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| ALB (g/dL) | 0.74 ± 0.12 a | 1.12 ± 0.10 b | 1.14 ± 0.15 b | 1.13 ± 0.17 b | 1.21 ± 0.13 b |
| GLOB (g/dL) | 1.98 ± 0.14 a | 2.34 ± 0.10 b | 2.45 ± 0.08 b | 2.41 ± 0.16 b | 2.56 ± 0.06 b |
| TP (g/dL) | 2.98 ± 0.20 a | 3.34 ± 0.24 b | 3.45 ± 0.38 b | 3.41 ± 0.42 b | 3.56 ± 0.54 b |
| BUN/urea (mg/dL) | 3.46 ± 0.18 | 3.56 ± 0.12 | 3.64 ± 0.46 | 3.34 ± 0.34 | 3.68 ± 0.24 |
| Crea (mg/dL) | 0.14 ± 0.03 | 0.13 ± 0.05 | 0.12 ± 0.05 | 0.13 ± 0.01 | 0.12 ± 0.02 |
| ALKP (µ/L) | 11.24 ± 0.52 | 12.34 ± 0.51 | 11.18 ± 0.64 | 12.26 ± 0.74 | 12.17 ± 0.48 |
| ALT (µ/L) | 13.36 ± 1.48 a | 14.28 ± 2.32 a | 20.76 ± 4.68 b | 21.78 ± 3.78 b | 21.22 ± 5.64 b |
| AST (µ/L) | 68.18 ± 4.82 a | 69.46 ± 5.36 a | 84.62 ± 6.42 b | 88.72 ± 6.24 b | 86.24 ± 7.48 b |
| GGT (µ/L) | 0.98 ± 0.13 | 0.96 ± 0.23 | 0.95 ± 0.12 | 0.94 ± 0.14 | 0.97 ± 0.24 |
| GLU (mg/dL) | 56.32 ± 3.42 a | 70.42 ± 4.82 b | 71.68 ± 5.62 b | 72.14 ± 3.21 b | 81.38 ± 3.12 c |
| CHOL (g/dL) | 12.26 ± 0.14 a | 7.22 ± 0.32 b | 8.22 ± 0.32 c | 9.43 ± 0.42 d | 9.86 ± 0.23 d |
| TBIL (mg/dL) | 0.13 ± 0.02 | 0.13 ± 0.01 | 0.12 ± 0.02 | 0.12 ± 0.02 | 0.13 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, M.K.; Kari, Z.A.; Van Doan, H.; Kabir, M.A.; Che Harun, H.; Mohamad Sukri, S.A.; Goh, K.W.; Wee, W.; Khoo, M.I.; Wei, L.S. Fermented Soybean Meal (FSBM) in African Catfish (Clarias gariepinus) Diets: Effects on Growth Performance, Fish Gut Microbiota Analysis, Blood Haematology, and Liver Morphology. Life 2022, 12, 1851. https://doi.org/10.3390/life12111851
Zakaria MK, Kari ZA, Van Doan H, Kabir MA, Che Harun H, Mohamad Sukri SA, Goh KW, Wee W, Khoo MI, Wei LS. Fermented Soybean Meal (FSBM) in African Catfish (Clarias gariepinus) Diets: Effects on Growth Performance, Fish Gut Microbiota Analysis, Blood Haematology, and Liver Morphology. Life. 2022; 12(11):1851. https://doi.org/10.3390/life12111851
Chicago/Turabian StyleZakaria, Muhammad Khairulanam, Zulhisyam Abdul Kari, Hien Van Doan, Muhammad Anamul Kabir, Hasnita Che Harun, Suniza Anis Mohamad Sukri, Khang Wen Goh, Wendy Wee, Martina Irwan Khoo, and Lee Seong Wei. 2022. "Fermented Soybean Meal (FSBM) in African Catfish (Clarias gariepinus) Diets: Effects on Growth Performance, Fish Gut Microbiota Analysis, Blood Haematology, and Liver Morphology" Life 12, no. 11: 1851. https://doi.org/10.3390/life12111851
APA StyleZakaria, M. K., Kari, Z. A., Van Doan, H., Kabir, M. A., Che Harun, H., Mohamad Sukri, S. A., Goh, K. W., Wee, W., Khoo, M. I., & Wei, L. S. (2022). Fermented Soybean Meal (FSBM) in African Catfish (Clarias gariepinus) Diets: Effects on Growth Performance, Fish Gut Microbiota Analysis, Blood Haematology, and Liver Morphology. Life, 12(11), 1851. https://doi.org/10.3390/life12111851









