Gravi-Sensitivity of Mosses and Their Gravity-Dependent Ontogenetic Adaptations
Abstract
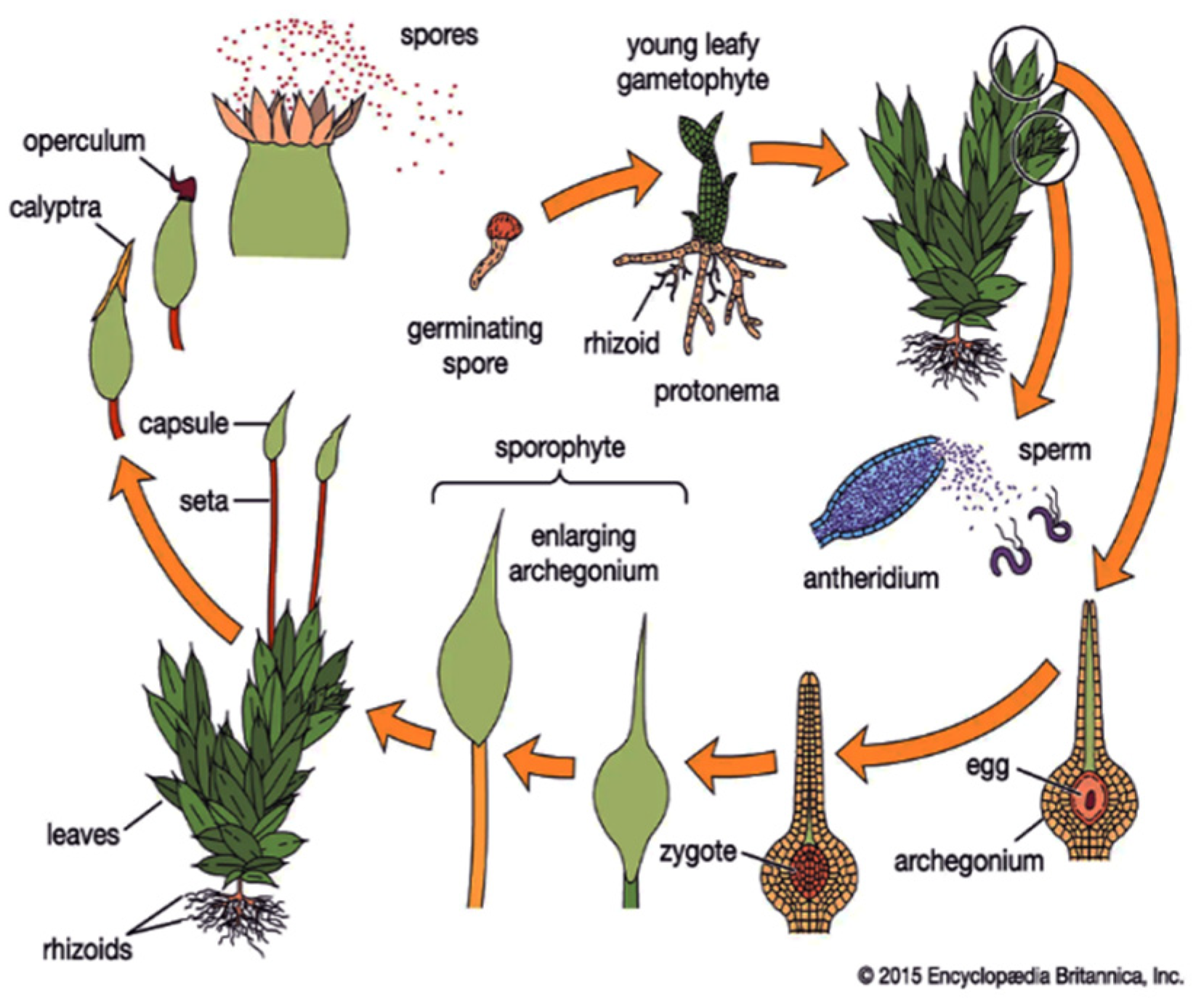
1. Introduction
2. Gravi-Sensitivity of Bryophytes Ontogenesis
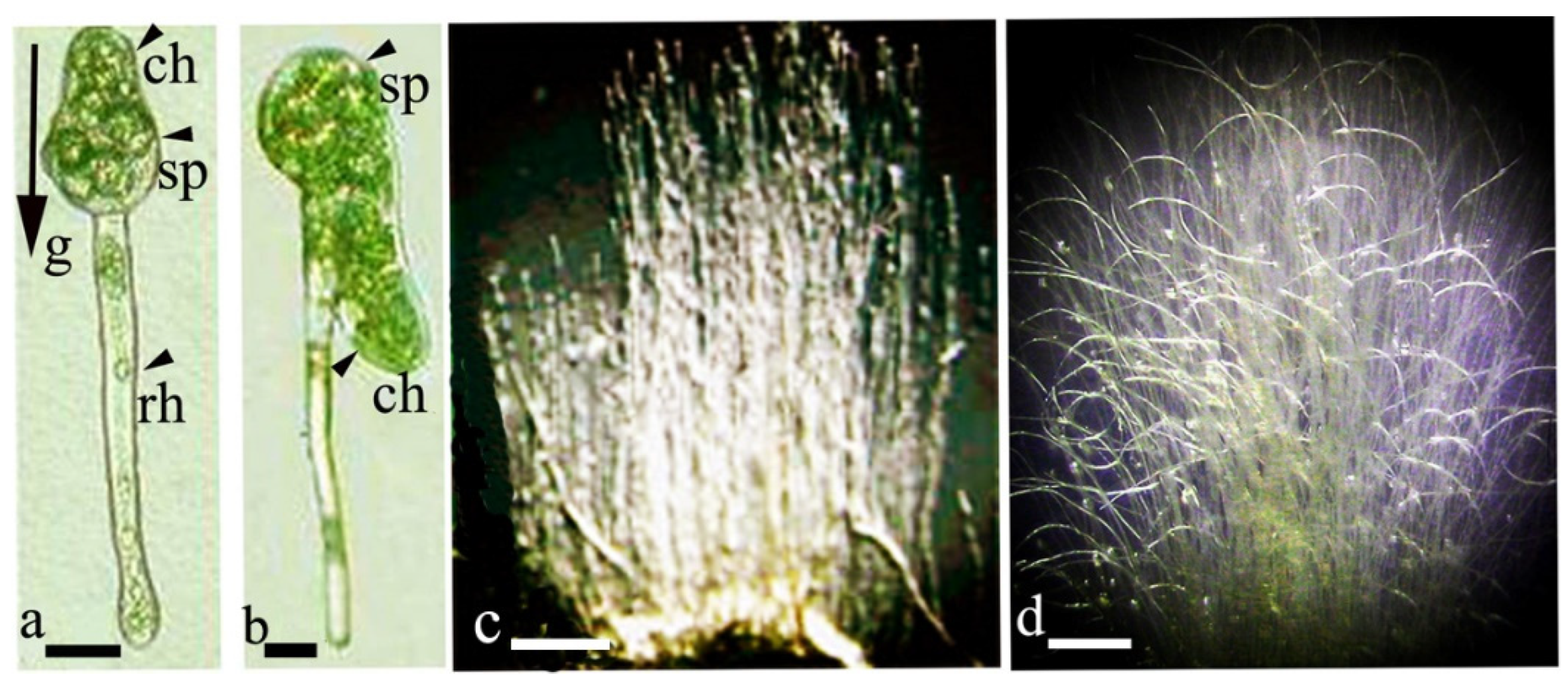
2.1. Gravi-Sensitivity of Sporelings
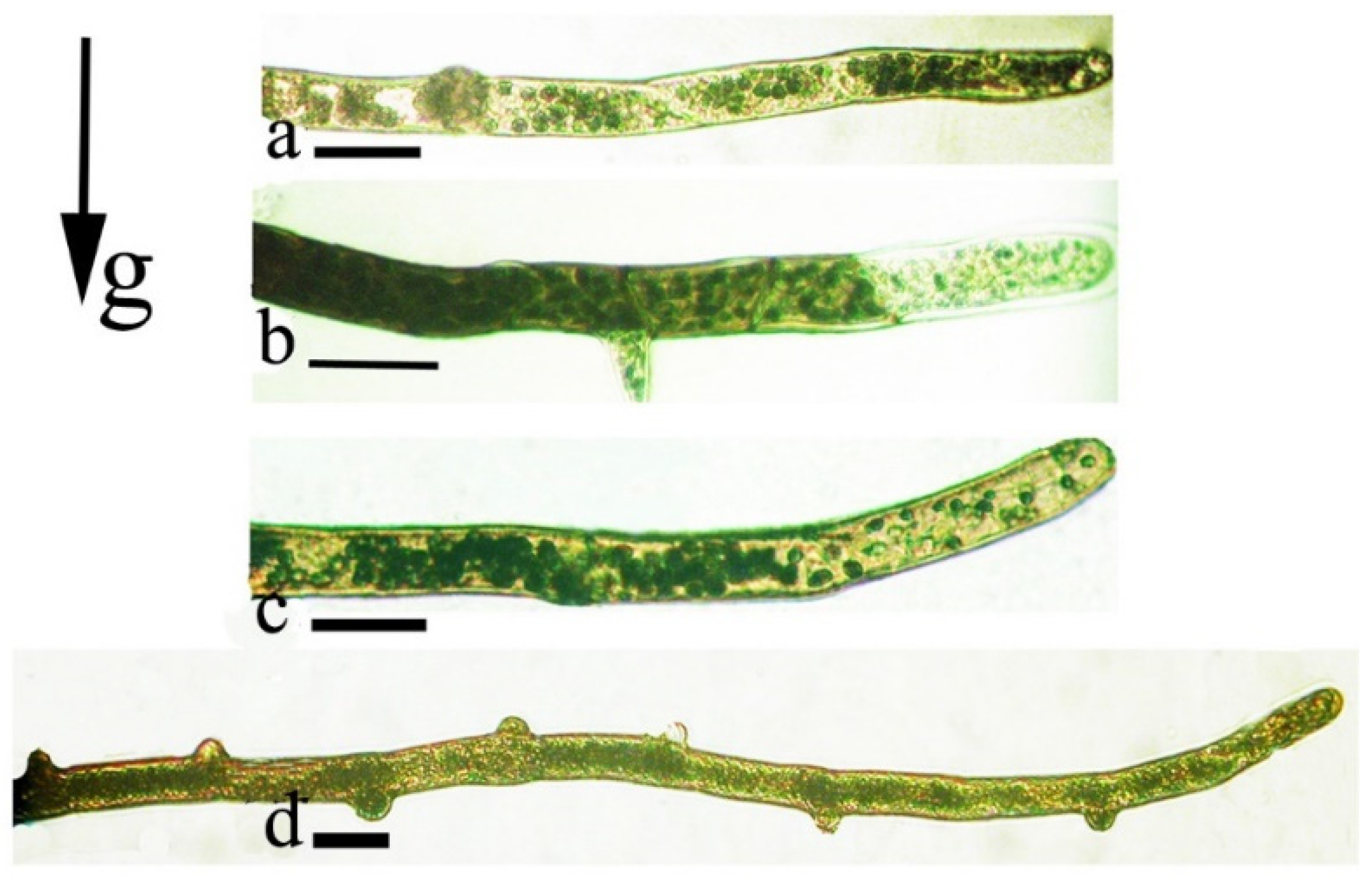
2.2. Gravi-Sensitivity of Protonemata—The Juvenile Stage of Moss Development
2.3. Gravity-Dependent Development of Bud Formation
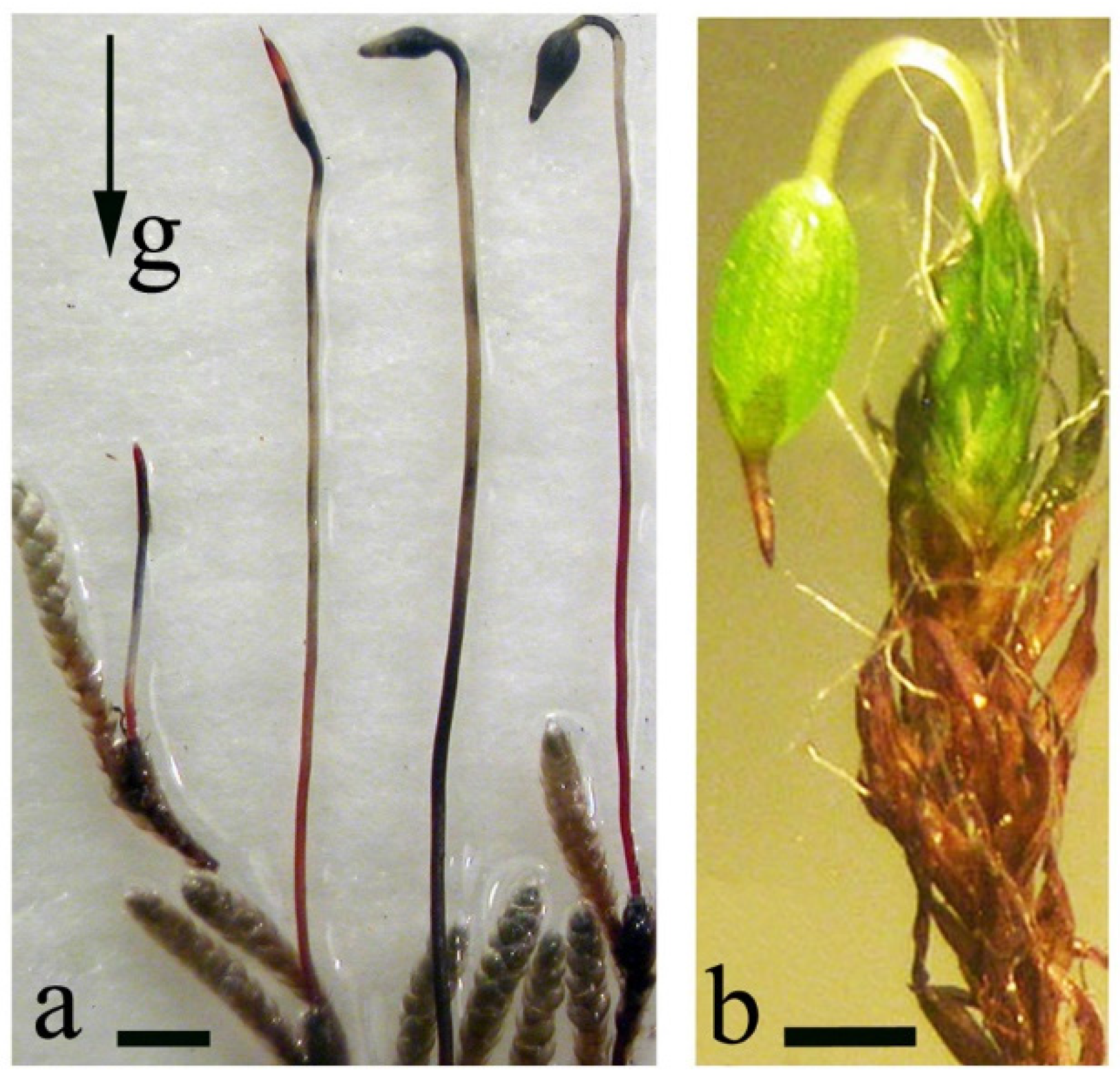
2.4. Gravi-Sensitivity of Mosses at the Stage of Leafy Shoots and Sporogonia Formation
3. Molecular Mechanisms of Gravi-Morphoses in Bryophytes
3.1. The Role of Auxins in Gravitropic Response Protonemata
3.2. Ca2+ Regulates in Gravitropism
3.3. The Role of Cytoskeleton in Gravitropic Growth
4. A Dominant Role of Moss Gravimorphoses in Adaptation to Extreme Conditions
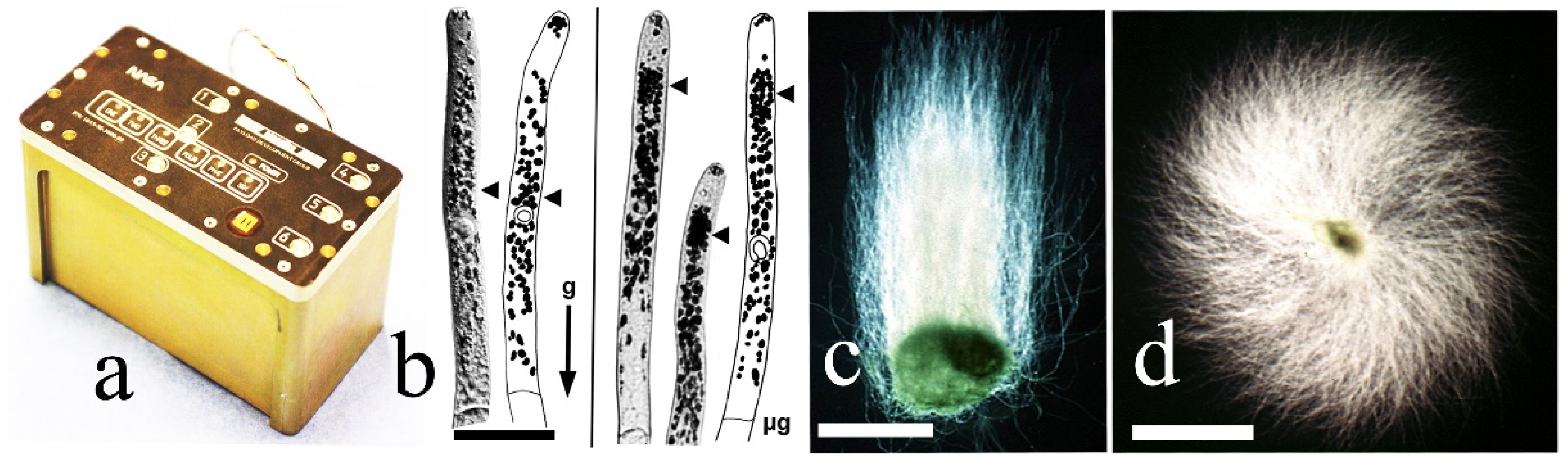
4.1. Effects of Microgravity on Apical Cell Morphology
4.2. Spiral Growth in 1 g, Microgravity during Spaceflight, and Clinorotation
4.3. Gravireaction of Mosses Are Influenced by Environmental Conditions
4.3.1. Adaptation of Weissia tortilis to Arid Conditions
4.3.2. Gravireaction of Moss Species from Antarctica
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demkiv, O.T.; Khorkavtsiv, Y.D.; Kardash, A.R.; Chaban, C.I. Vzaymodeistvye sveta y hravytatsyy v rostovukh dvyzhenyiakh protonemu mkhov (The interaction of light and gravity in growth movements of protonemata mosses). Fyzyologia Rastenyi 1997, 44, 205–211. [Google Scholar]
- Chebli, Y.; Geitmann, A. Gravity research on plants: Use of single-cell experimental models. Front. Plant Sci. 2011, 2, 56. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Bohmer, M.; Häder, D.-P.; Hemmersbach, R.; Palme, K. Gravitational Biology 1. Gravity sensing and graviorientation in microorganism and plants. In Springer Brief in Space Life Sciences; Ryuters, G., Braun, M., Eds.; Springer: Cham, Switzerland; German Aerospace Centre: Bonn, Germany, 2018. [Google Scholar]
- Barlow, P.W. Gravity perception in plants: A multiplicity. Pant Cell Environ. 1995, 18, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Hasenstein, K.H. Plant responses to gravity–insights and extrapolations from ground studies. Gravit. Space Res. 2009, 22, 21–33. Available online: https://link.gale.com/apps/doc/A348311756/AONE?u=googlescholar&sid=googleScholar&xid=916e9f0c (accessed on 11 September 2022).
- Pouliquen, O.; Forterre, Y.; Bérut, A.; Chauvet, H.; Bizet, F.; Legué, V.; Moulia, B. A new scenario for gravity detection in plants: The position sensor hypothesis. Phys. Biol. 2017, 14, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Girloy, S. Plant tropism. Curr. Biol. 2008, 18, 275–277. [Google Scholar] [CrossRef]
- Kordyum, E.L. Plant cell gravisensitivity and adaptation to microgravity. Plant Biol. 2014, 16, 79–90. [Google Scholar] [CrossRef]
- Hoson, T. Plant growth and morphogenesis under different gravity conditions: Relevance to plant life in space. Life 2014, 4, 205–216. [Google Scholar] [CrossRef]
- Chaban, C.I.; Kern, V.D.; Ripetskyj, R.T.; Demkiv, O.T.; Sack, F.D. Gravitropism in caulonemata of the moss Pottia intermedia. J. Bryol. 1998, 20, 287–299. [Google Scholar] [CrossRef]
- Demkiv, O.T.; Kordyum, E.L.; Khorkavtsiv, Y.D.; Kardash, O.R.; Chaban, C.I. Gravi-and photostimuli in moss protonema growth movements. Adv. Space Res. 1998, 21, 1191–1195. [Google Scholar] [CrossRef]
- Demkiv, O.T.; Kordyum, E.L.; Khorkavtsiv, Y.D.; Tairbekov, M.H. Umovy hravitatsii—Eksperymentalna baza dlia piznannia zakonomirnostei morfohenezu roslyn v hravitatsiinomu poli (Microgravity is the experimental basis for understanding of the peculiarities of plant morphogenesis in the gravitational field). Space Sci. Technol. 2006, 12, 30–35. [Google Scholar]
- Lobachevska, O.V.; Kyyak, N.Y.; Kordyum, E.L.; Khorkavtsiv, Y.D. Role of gravimorphoses in moss adaptation to extreme environment. Ukr. Bot. J. 2021, 78, 47–57. [Google Scholar] [CrossRef]
- Schofield, W.B. Bryophyte. Encyclopedia Britannica. 7 September 2022. Available online: https://www.britannica.com/plant/bryophyte (accessed on 20 October 2022).
- Lobachevska, O.V.; Khorkavtsiv, Y.D. Hravichutlyvist v ontohenezi mokhiv (Gravisensitivity in the moss ontogenesis). Space Sci. Technol. 2014, 20, 55–60. [Google Scholar] [CrossRef]
- Sabovljević, M.; Vujičić, M.; Sabovljević, A. Plant growth regulators in bryophytes. Bot. Serbica 2014, 38, 99–107. [Google Scholar]
- Lobachevska, O.V.; Demkiv, O.T.; Ripetskyj, R.T. Influence of gravity on spatial orientation and morphogenesis of moss sporophytes. Adv. Space Res. 1998, 21, 1141–1144. [Google Scholar] [CrossRef]
- Cove, D.; Bezanilla, M.; Harries, P.; Quatrano, R. Mosses as model systems for the study of metabolism and development. Annu. Rev. Plant Biol. 2006, 57, 497–520. [Google Scholar] [CrossRef][Green Version]
- Khorkavtsiv, Y.D.; Kordyum, E.L.; Lobachevska, O.V.; Kyyak, N.Y.; Kit, N.A. Haluzhennia protonemy Ceratodon purpureus v umovakh zminenoi syly tiazhinnia. (Branching of Ceratodon purpureus (Brid.) Hedw. protonemata effected under altered gravity conditions). Ukr. Bot. J. 2015, 72, 588–595. [Google Scholar] [CrossRef][Green Version]
- Lobachevska, O.V.; Kyyak, N.Y.; Khorkavtsiv, Y.D.; Kit, N.A. Hravizalezhna modyfikatsiia reproduktyvnoho rozvytku mokhiv (Gravity-dependent modification of reproductive development of mosses). Ukr. Bot. J. 2017, 74, 488–496. [Google Scholar] [CrossRef]
- Kyyak, N.Y.; Khorkavtsiv, Y.D. Otsinka okysniuvalnoho stresu mokhu Pohlia nutans (Hedw.) Lindb. zalezhno vid vplyvu hravitatsii (Estimation of the oxidative stress in moss Pohlia nutans (Hedw.) Lindb. depending on the influence of gravity). Space Sci. Technol. 2016, 22, 58–66. [Google Scholar]
- Lobachevska, O.V.; Kyyak, N.Y.; Khorkavtsiv, Y.D. Morphofunktsionalni ocoblyvosti klimyn protonemy Weissia tortulis Spreng. z riznoiu chutlyvistiu do hravitatsii (Morpho-functional peculiarities of the moss Weissia tortulis Spreng. protonemata cells with different gravisensitivity). Space Sci. Technol. 2019, 25, 60–70. [Google Scholar] [CrossRef]
- Wareing, P.F.; Phillips, I.D.J. Growth & Differentiation in Plants; Wareing, P., Ed.; Pergamon: Oxford, UK, 1981; p. 343. [Google Scholar]
- Moore, D.; Gange, A.C.; Gange, E.G.; Boddy, L. Fruit bodies: Their production and development in relation to environment. In Ecology of Saprotrophic Basidiomycetes; Elsevier Ltd.: Amsterdam, The Netherlands, 2008; Volume 28, pp. 79–103. [Google Scholar] [CrossRef]
- Wojtaszek, P. (Ed.) Mechanical Integration of Plant Cells and Plants; Springer: Berlin/Heidelberg, Germany, 2011; p. 345. [Google Scholar]
- Cove, D.J.; Quatrano, R.S. Agravitropic mutants of the moss Ceratodon purpureus do not complement mutants having a reversed gravitropic response. Plant Cell Environ. 2006, 29, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Schwuchow, J.M.; Kern, V.D.; White, N.J.; Sack, F.D. Conservation of the plastid sedimentation zone in all moss genera with known gravitropic protonemata. J. Plant Growth Regul. 2002, 21, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Haberlandt, G. Uber die Perzeption des geotropischen Reizes. Ber. Dtsch. Bot. Ges. 1900, 18, 261–272. [Google Scholar]
- Nèmec, B. Uber die Art der Wahrnehmung des Schwerkraftreizes bei den Pflanzen. Ber. Dtsch. Bot. Ges. 1900, XVIII, 241–245. [Google Scholar]
- Kern, V.D.; Smith, J.D.; Schwuchow, J.M.; Sack, F.D. Amyloplasts that sediment in protonemata of the moss Ceratodon purpureus are nonrandomly distributed in microgravity. Plant Physiol. 2001, 125, 2085–2094. [Google Scholar] [CrossRef][Green Version]
- Bramley-Alves, J.D.; King, D.; Miller, R.E. Dominating the Antarctic environment: Bryophytes in a time of change. Environ. Sci. 2014, 10, 5–17. [Google Scholar] [CrossRef]
- Kume, A.; Kamachi, H.; Onoda, Y.; Hanba, Y.T.; Hiwatashi, Y.; Karahara, I.; Fujita, T. How plants grow under gravity conditions besides 1 g: Perspectives from hypergravity and space experiments that employ bryophytes as a model organism. Plant Mol. Biol. 2021, 107, 279–291. [Google Scholar] [CrossRef]
- Gessel, N.; Lang, D.; Reski, R. Genetics and genomics of Physcomitrella patens. In Plant Cell Biology. The Plant Sciences; Assmann, S., Liu, B., Eds.; Springer: New York, NY, USA, 2017; pp. 1–32. [Google Scholar] [CrossRef]
- Ripetskyj, R.T.; Khorkavtsiv, Y.D. Epihenetychna adaptatsiia mokhiv i fenomen klitynnoi pamiati (Epigenetic adaptation in mosses and the phenomenon of cell memory). Ukr. Bot. J. 2012, 69, 302–314. [Google Scholar]
- Pundyak, O.I.; Demkiv, O.T.; Khorkavtsiv, Y.D.; Bagrii, B.B. Poliarnist prorostannia spor mokhu Funaria hygrometrica Hedw. (Polarity of spore germination in Funaria hygrometrica Hedw). Space Sci. Technol. 2002, 8, 96–100. [Google Scholar]
- Demkiv, O.T.; Khorkavtsiv, Y.D.; Pundyak, O.I. Hravitatsiia yak formotvorchyi faktor rozvytku mokhiv (Gravity as a formative factor in the moss development). In Physiology of Plants: Problems and Prospects of Development; Morgyn, V.V., Ed.; Logos: Kyiv, Ukraine, 2009; Volume 2, pp. 403–410. [Google Scholar]
- During, H.J. Life strategies of Bryophytes: A preliminary review. Lindbergia 1979, 5, 2–18. [Google Scholar]
- Glime, J.M. Physiological Ecology. In Bryophyte Ecology; Ebook Sponsored by Michigan Technological University and the International Association of Bryologists; Glime, J.M., Ed.; Michigan Technological University: Houghton, MI, USA, 2017; Volume 1, Available online: http://www.bryoecol.mtu.edu/ (accessed on 23 August 2022).
- Sack, F.D. Plant gravity sensing. Int. Rev. Cytol. 1991, 127, 193–252. [Google Scholar] [CrossRef]
- Kordyum, E.L.; Guikema, J.A. An active role of the amyloplasts and nuclei of root statocytes in graviperception. Adv. Space Res. 2001, 27, 951–956. [Google Scholar] [CrossRef]
- Ripetskyj, R.T.; Kit, N.A.; Chaban, C.I. Influence of gravity on the photomorphism of secondary moss protonemata. Adv. Space Res. 1999, 23, 2005–2010. [Google Scholar] [CrossRef]
- Bopp, M. Hormones of the moss protonemata. In Bryophyte Development: Physiology and Biochemistry; Chopra, R.N., Bhatla, S.C., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 55–77. [Google Scholar]
- Demkiv, O.T.; Khorkavtsiv, Y.D.; Kyyak, N.Y.; Kit, N.A. Vplyv hravitatsii na fotomorfohenez protonemy Pottia intermedia (Turn.) Furnr., Pottiales (Effect of gravity on photomorphogenesis of Pottia intermedia (Turn.) Fürn., Pottiales (Fleisch.) protonemata). Ukr. Bot. J. 2005, 62, 329–336. [Google Scholar]
- Roychoudhry, S.; Bianco, M.D.; Kieffer, M.; Kepinski, S. Auxin control gravitropic setpoint angle in higher plant lateral branches. Curr. Biol. 2013, 9, 1497–1504. [Google Scholar] [CrossRef]
- Demkiv, O.T.; Khorkavtsiv, Y.D. Vplyv inihibitoriv auksynovoho transportu na hravitropizm protonemy Pohlia nutans Hedw.) (The effects of auxin transport inhibitors on gravitropism in protonemata of the moss Pohlia nutans Hedw.). Ukr. Bot. J. 2003, 72, 77–82. [Google Scholar]
- Monshausen, G.B.; Miller, N.D.; Murphy, A.S.; Gilroy, S. Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J. 2011, 65, 309–318. [Google Scholar] [CrossRef]
- Baldwin, K.; Strohm, A.K.; Masson, P.H. Gravity sensing and signal transduction in vascular plant primary roots. Am. J. Bot. 2013, 100, 126–142. [Google Scholar] [CrossRef]
- Toyota, M.; Ikeda, N.; Sawai-Toyota, S.; Kato, T.; Gilroy, S.; Tasaka, M.; Morita, M.T. Amyloplast displacement is necessary for gravisensing in Arabidopsis shoots as revealed by a centrifuge microscope. Plant J. 2013, 76, 648–660. [Google Scholar] [CrossRef]
- Chatterjee, A.; Porterfield, D.M.; Smith, P.S.; Roux, S.J. Gravity-directed calcium current in germinating spores of Ceratopteris richardii. Planta 2000, 210, 607–610. [Google Scholar] [CrossRef]
- Bushart, T.J.; Cannon, A.E.; ul Haque, A.; Miguel, P.S.; Gregory, K.M.; Clark, B.; Porterfield, D.M.; Roux, S.J. RNA-seq analysis identifies potential modulators of gravity response in spores of Ceratopteris (Parkeriaceae): Evidence for modulation by calcium pumps and apyrase activity. Am. J. Bot. Adv. Plant Trop. 2013, 100, 161–174. [Google Scholar] [CrossRef]
- Salmi, M.L.; ul Haque, A.; Bushart, T.J.; Stout, S.C.; Roux, S.J.; Porterfield, D.M. Changes in gravity rapidly alter the magnitude and direction of a cellular calcium current. Planta 2011, 233, 911–920. [Google Scholar] [CrossRef]
- Demkiv, O.T.; Khorkavtsiv, Y.D.; Pundiak, O.I. Changes of protonemal cell growth related to cytoskeleton organization. Cell Biol. Int. 2003, 27, 187–189. [Google Scholar] [CrossRef]
- Demkiv, O.T.; Khorkavtsiv, Y.D.; Kordyum, E.L. Cytoskeleton organization in moss protonemal cells. In Proceedings of the Congress of Ukraine Societies Cell Biology, Lviv, Ukraine, 25–28 April 2004; p. 197. [Google Scholar]
- Kolesnikov, Y.S.; Kretynin, S.V.; Volotovsky, I.D.; Kordyum, E.L.; Ruelland, E.; Kravets, V.S. Molecular mechanisms of gravity perception and signal transduction in plants. Protoplasma 2016, 253, 987–1004. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kumasaki, S.; Soga, K.; Wakabayashi, K.; Hashimoto, T.; Hoson, T. Gravity-Induced Modifications to Development in Hypocotyls of Arabidopsis Tubulin Mutants. Plant Physiol. 2010, 152, 918. [Google Scholar] [CrossRef]
- Hoson, T.; Wakabayashi, K. Role of the plant cell wall in gravity resistance. Phytochemistry 2015, 112, 84–90. [Google Scholar] [CrossRef]
- Blancaflor, E.B. Regulation of plant gravity sensing and signaling by the actin cytoskeleton. Am. J. Bot. 2013, 100, 143–152. [Google Scholar] [CrossRef]
- He, F.; Chen, H.; Han, R. The Plant Cytoskeleton and Crosslinking Factors. Cell Biol. 2020, 9, 85–99. [Google Scholar] [CrossRef]
- Kraft, T.F.; van Loon, J.J.; Kiss, J.Z. Plastid position in Arabidopsis columella cells is similar in microgravity and on a random-positioning machine. Planta 2000, 211, 415–422. [Google Scholar] [CrossRef]
- Kordyum, E.L.; Nedukha, E.M.; Sytnik, K.M. Optical and electronmicroscopic studies of the Funaria hygrometrica protonema after cultivation for 96 days in Space. Adv. Space Res. 1981, 1, 159–162. [Google Scholar] [CrossRef]
- Hangarter, R.P. Gravity, light and plant form. Plant Cell Environ. 1997, 20, 796–800. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Kiss, J.Z.; Herranz, R.; Medina, F.J. Light and gravity signals synergize in modulating plant development. Front. Plant Sci. 2014, 5, 563. [Google Scholar] [CrossRef]
- Valbuena, M.A.; Manzano, A.; Vandenbrink, J.P.; Pereda-Loth, V.; Carnero-Diaz, E.; Edelmann, R.E.; Kiss, J.Z.; Herranz, R.; Medina, F.J. The combined effects of real or simulated microgravity and red-light photoactivation on plant root meristematic cells. Planta 2018, 248, 691–704. [Google Scholar] [CrossRef]
- Kern, D.V.; Sack, F.D. Irradiance-dependent regulation of gravitropism by red light in protonemata of the moss Ceratodon purpureus. Planta 1999, 209, 299–307. [Google Scholar] [CrossRef]
- Kofler, L. Sur quelques cas de spiralisation chez les végétaux. Bull. Société Bot. Fr. 1967, 114, 209–216. [Google Scholar] [CrossRef][Green Version]
- Bopp, M. Development Physiology of Bryophytes. In New Manual; Schuster, R.V., Ed.; The Hattori Botanical Laboratory: Nichinan, Japan, 1983; pp. 276–324. [Google Scholar]
- Lazarenko, A.S. Struktura Vydu i Mekhanizmy Vydoutvorennia Mokhiv (Species Structure and Mechanisms of Species Formation of Mosses); Holubets, M.A., Danulkiv, I.C., Eds.; Liha-Press: Lviv, Ukraine, 2001; p. 231. [Google Scholar]
- Kern, V.D.; Schwuchow, J.M.; Reed, D.W.; Nadeau, J.A.; Lucas, J.; Skripnikov, A.; Sack, F.D. Gravitropic moss cells default to spiral growth on the clinostat and in microgravity during spaceflight. Planta 2005, 221, 149–157. [Google Scholar] [CrossRef]
- Bopp, M. Entwicklungsphysiologie der Moose. In Encyclopedia of Plant Physiology; Ruhland, W., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1965; pp. 808–843. [Google Scholar]
- Sinnott, E. Plant Morphogenesis; Andesite Press: London, UK, 2015; p. 564. [Google Scholar]
- Tuba, Z. Bryophyte Physiological Processes in a Changing Climate: An Overview. In Bryophyte Ecology and Climate Change; Tuba, Z., Clack, N.G., Stark, L.R., Eds.; Cambridge University Press: New York, NY, USA, 2011; pp. 13–35. [Google Scholar]
- Müller, S.J.; Gütle, D.D.; Jacquot, J.-P.; Reski, R. Can mosses serve as model organisms for forest research? Ann. For. Sci. 2016, 73, 135–146. [Google Scholar] [CrossRef]
- Kyyak, N.Y.; Lobachevska, O.V.; Khorkavtsiv, Y.D. Morfofiziolohichni reaktsii hravichutlyvosti ta adaptatsii do UF-oprominennia mokhu Bryum caespiticium Hedw. z Antarktyky (Morpho-physiological reactions of gravisensitivity and adaptation to UV irradiance of the moss Bryum caespiticium Hedw. from Antarctica). Space Sci. Technol. 2021, 27, 47–49. [Google Scholar] [CrossRef]
- Lobachevska, O.; Kyyak, N.; Khorkavtsiv, Y.; Kordyum, E. Biological characteristics gravimorphoses that assist of moss species tolerance in Antarctica conditions. In Proceedings of the X International Antarctic Conference Dedicated to the 25th Anniversary of Raising of the National Flag of Ukraine at the Ukrainian Antarctic Akademik Vernadsky Station, Kyiv, Ukraine, 11–13 May 2021; pp. 108–109. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobachevska, O.V.; Kyyak, N.Y.; Kordyum, E.L.; Khorkavtsiv, Y.D.; Kern, V.D. Gravi-Sensitivity of Mosses and Their Gravity-Dependent Ontogenetic Adaptations. Life 2022, 12, 1782. https://doi.org/10.3390/life12111782
Lobachevska OV, Kyyak NY, Kordyum EL, Khorkavtsiv YD, Kern VD. Gravi-Sensitivity of Mosses and Their Gravity-Dependent Ontogenetic Adaptations. Life. 2022; 12(11):1782. https://doi.org/10.3390/life12111782
Chicago/Turabian StyleLobachevska, Oksana V., Natalia Y. Kyyak, Elizabeth L. Kordyum, Yaroslava D. Khorkavtsiv, and Volker D. Kern. 2022. "Gravi-Sensitivity of Mosses and Their Gravity-Dependent Ontogenetic Adaptations" Life 12, no. 11: 1782. https://doi.org/10.3390/life12111782
APA StyleLobachevska, O. V., Kyyak, N. Y., Kordyum, E. L., Khorkavtsiv, Y. D., & Kern, V. D. (2022). Gravi-Sensitivity of Mosses and Their Gravity-Dependent Ontogenetic Adaptations. Life, 12(11), 1782. https://doi.org/10.3390/life12111782



