Effects of the Probiotic Enterococcus faecium on Muscle Characteristics of Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Biochemical Analyses
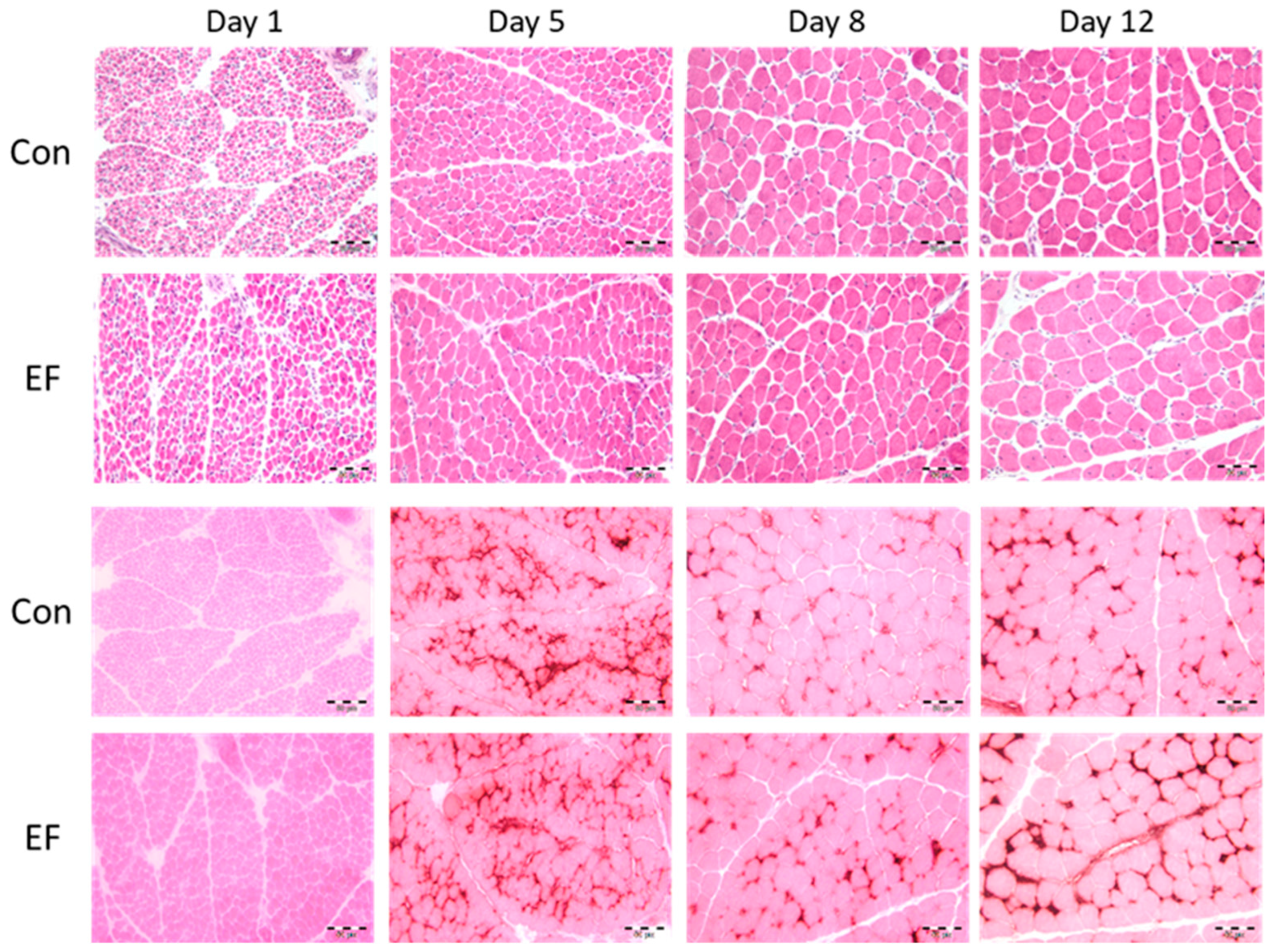
2.3. Histomorphological Analysis
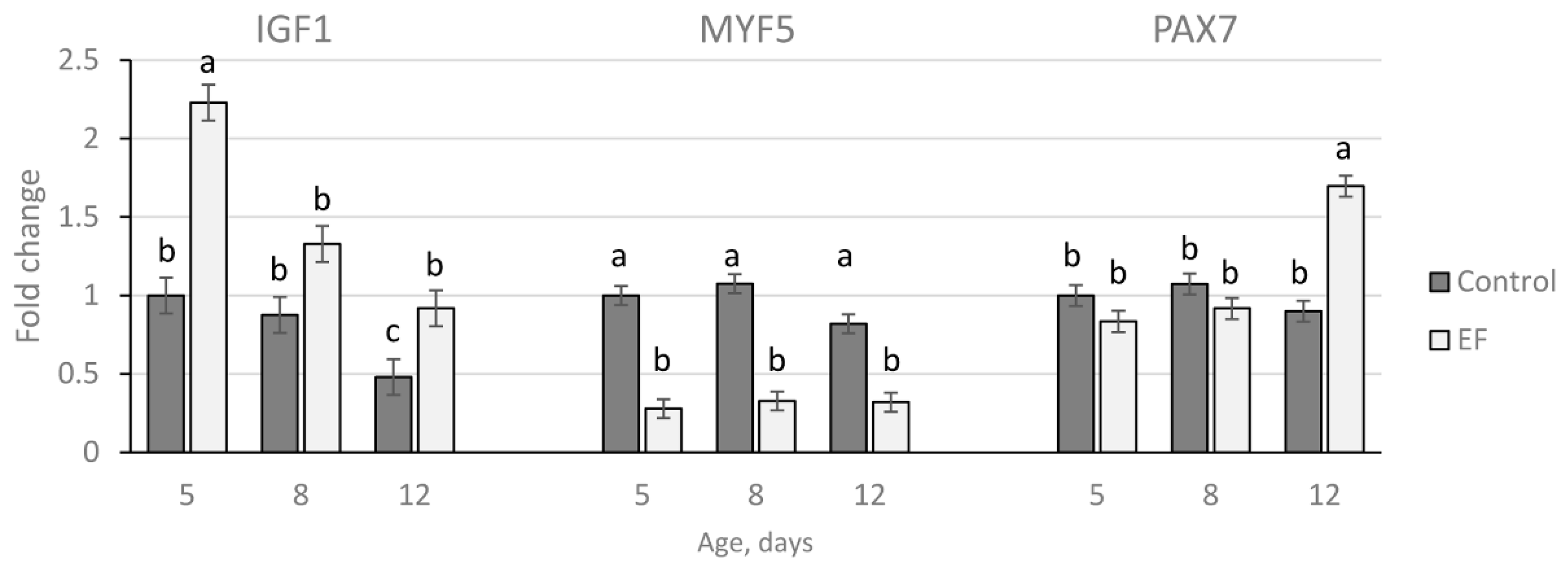
2.4. Gene Expression Analysis
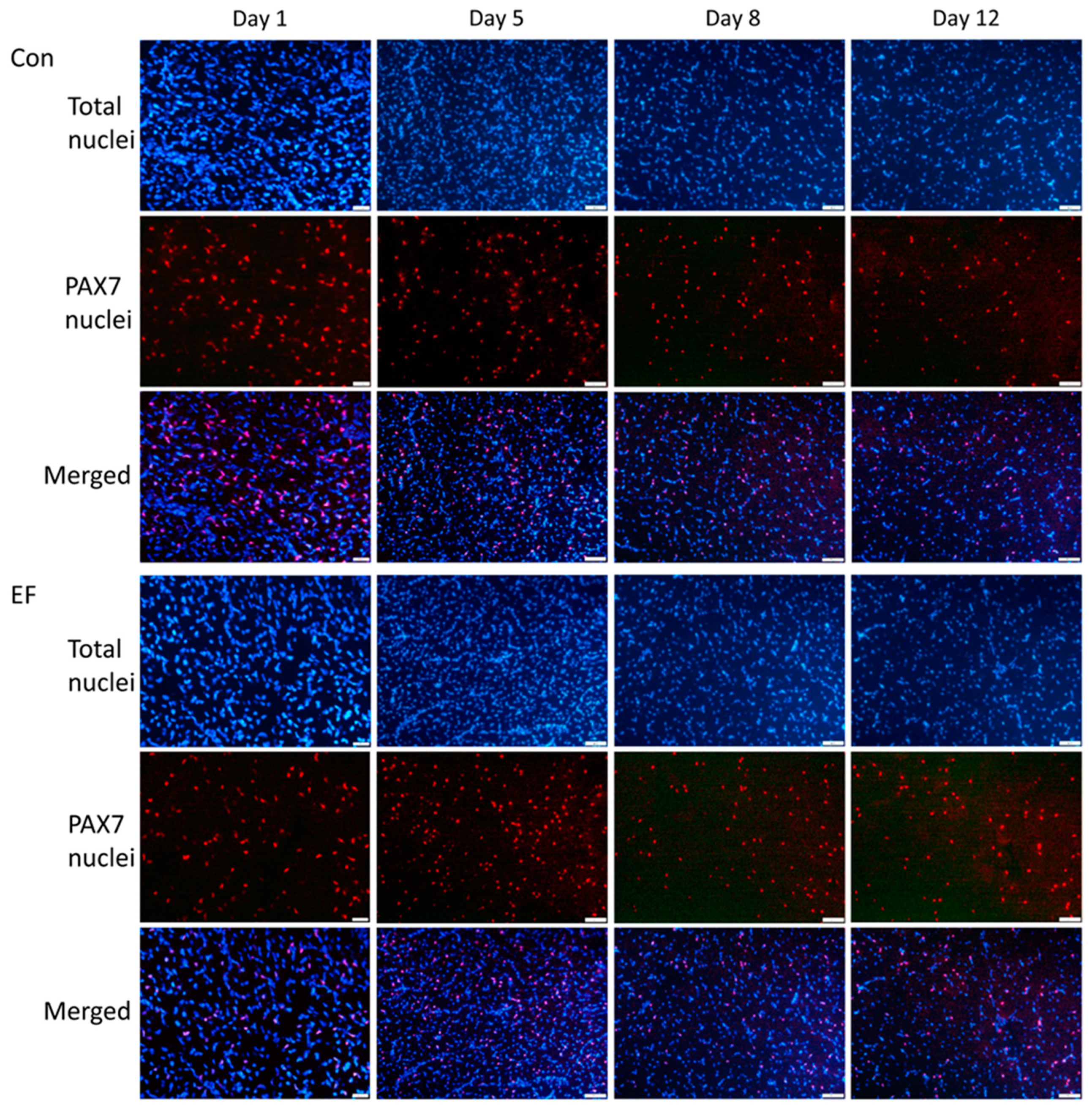
2.5. Immunohistochemistry and Image Analysis
2.6. Statistical Analysis
3. Results
3.1. Weight Development
3.2. DNA, RNA, Protein and Muscle Enzymes
3.3. Muscle Structure
3.4. Gene Expression
3.5. PAX7 Protein Abundance in Pectoralis Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marangoni, F.; Corsello, G.; Criselli, C.; Ferrara, N.; Ghiselli, A.; Luccin, L.; Poli, A. Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing; an Italien consensus document. Food Nutr. Res. 2015, 59, 27606. [Google Scholar] [CrossRef]
- Scanes, C.G. The global importance of poultry. Poult. Sci. 2007, 86, 1057–1058. [Google Scholar] [CrossRef]
- Wyszyńska, A.K.; Godlewska, R. Lactic Acid Bacteria—A Promising Tool for Controlling Chicken Campylobacter Infection. Front. Microbiol. 2021, 12, 703441. [Google Scholar] [CrossRef]
- Crhanova, M.; Hradecka, H.; Faldynova, M.; Matulova, M.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar Enteritidis infection. Infect. Immun. 2011, 79, 2755–2763. [Google Scholar] [CrossRef]
- Biloni, A.; Quintana, C.F.; Menconi, A.; Kallapura, G.; Latorre, J.; Pixley, C.; Layton, S.; Dalmagro, M.; Hernandez-Velasco, X.; Wolfenden, A.; et al. Evaluation of effects of EarlyBird associated with FloraMax-B11 on Salmonella Enteritidis, intestinal morphology, and performance of broiler chickens. Poult. Sci. 2013, 92, 2337–2346. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Abdel Hamid, F.M.; El-Gohary, F.A.; Risha, E.F. Incorporation efficacy comparison of probiotic and antibiotic on growth performance, some immunological and biochemical parameters in Salmonella Enteritidis challenged chicks. Life Sci. J. 2013, 10, 3550–3558. [Google Scholar]
- Dina, M.W.; El-Hamd, S.; Ahmed Hams, M. Effect of probiotic on Salmonella Enteritidis infection on broiler chickens. Egypt. J. Chem. Environ. Health 2016, 2, 298–314. [Google Scholar]
- Mehdi, Y.; Létourneau-Montminy, M.-P.; Gaucher, Y.C.; Suresh, G.; Rouissi, T.; Kaur Brar, S.; Cote, C.; Ramirez, A.A. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef]
- Iheukwumere, I.H.; Uneze, B.C.; Ejike, C.E. Efficacy of some selected antimicrobial substances in prevention of enteric bacterial infection in broiler chicks. J. Biol. Agric. Healthc. 2017, 7, 58–66. [Google Scholar]
- Miles, R.D.; Butcher, G.D.; Henry, P.R.; Littell, R.C. Effect of antibiotic growth promotors on broiler performance, intestinal growth parameters, and quantitative morphology. Poult. Sci. 2006, 85, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Kilonzo-Nthenge, A.; Nahashon, S.N.; Chen, F.; Adefope, N. Prevalence and antimicrobial resistance of pathogenic bacteria in chicken and guinea fowl. Poult. Sci. 2008, 87, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.F.; Smith, T.J.; Nachmann, K.E. Restrictions on antimicrobial use in food animal production: An international regulatory and economic survey. Glob. Health 2013, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Téllez, G.; Lauková, A.; Latorre, J.D.; Hernandez-Velasco, X.; Hargis, B.M.; Callaway, T. Food-producing animals and their health in relation to human health. Microb. Ecol. Health Dis. 2015, 26, 25876. [Google Scholar] [CrossRef]
- Zommiti, M.; Cambronel, M.; Maillot, O.; Barreau, M.; Sebei, K.; Feuilloley, M.; Ferchichi, M.; Connil, N. Evaluation of probiotic properties and safety of Enterococcus faecium isolated from artisanal Tunisian meat “Dried Ossban”. Front. Microbiol. 2018, 9, 1685. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361S–364S. [Google Scholar] [CrossRef]
- Van der Wielen, P.W.J.J.; Lipman, L.J.A.; van Knapen, F.; Biesterveld, S. Competitive exclusion of salmonella enterica serovar Enteritidis by lactobacillus crispatus and clostridium lactifermentans in a sequencing fed-batch culture. Appl. Environ. Microbiol. 2002, 68, 555–559. [Google Scholar] [CrossRef]
- Audisio, M.; Oliver, G.; Apella, M.C. Antagonistic effect of Enterococcus faecium J96 against human and poultry pathogenic Salmonella spp. J. Food Protect. 1999, 62, 751–755. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Abdel-Raheen, S.; Böhm, J. Effects of dietary inclusion of probiotic and symbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009, 88, 49–55. [Google Scholar] [CrossRef]
- De Lima Almeida Paz, I.C.; de Lima Almeida, I.G.; de La Vega, L.T.; Milbrandt, E.L.; Rodrigues Borges, M.; Coelho Chaves, G.H.; dos Ouros, C.C.; da Silva, M.I.L.; Caldara, F.R.; Filho, R.L. Productivity and well-being of broiler chickens supplemented with probiotic. J. Appl. Poult. Res. 2019, 28, 930–942. [Google Scholar] [CrossRef]
- Niewold, T.A. The nonantibiotic anti-inflammatory effect of antimicrobial growth promotors, the real mode of action? A hypothesis. Poult. Sci. 2007, 86, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Levkut, M.; Pistl, J.; Lauková, A.; Revajová, V.; Herich, R.; Ševčíková, Z.; Strompfová, V.; Szabóová, R.; Kokinčáková, T. Antimicrobial activity of Enterococcus faecium EF55 against Salmonella Enteritidis in chicks. Acta Vet. Hung. 2009, 57, 13–24. [Google Scholar] [CrossRef]
- Herich, R.; Kokinčáková, T.; Lauková, A.; Levkutová, M. Effect of preventive application of Enterococcus faecium EF55 on intestinal mucosa during salmonellosis in chickens. Czech J. Anim. Sci. 2010, 55, 42–47. [Google Scholar] [CrossRef]
- Lauková, A.; Chrastinová, L.; Pogány Simonová, M.; Strompfová, V.; Plachá, I.; Čobanová, K.; Formelová, Z.; Chrenková, M.; Ondruška, L. Enterococcus faecium AL 41: Its enterocin M and their beneficial use in rabbits husbandry. Probiotics Antimicrob. 2012, 4, 243–249. [Google Scholar] [CrossRef]
- Lauková, A.; Mareková, M.; Štyriak, I. Inhibitory effect of different enterocins against fecal bacterial isolates. Berl. Muench. Tierärztl. Wochenschr. 2003, 116, 37–40. [Google Scholar]
- Levkut, M.; Revajová, V.; Lauková, A.; Ševčíková, Z.; Spiŝáková, V.; Faixová, Z.; Levkutová, M.; Strompfová, V.; Pistl, J.; Levkut, M. Leucocytic responses and intestinal mucin dynamics of broilers protected with Enterococcus faecium EF55 and challenged with Salmonella Enteritidis. Res. Vet. Sci. 2012, 93, 195–201. [Google Scholar] [CrossRef]
- Karaffová, V.; Bobíková, K.; Husáková, E.; Levkut, M.; Herich, R.; Ravajová, V.; levkutová, M.; Levkut, M. Interaction of TGF-ß4 and IL-17 with IgA secretion in the intestine of chickens fed with E. faecium AL41 and challenged with S. Enteritidis. Res. Vet. Sci. 2015, 100, 75–79. [Google Scholar] [CrossRef]
- Samli, E.S.; Senkoylu, N.; Koc, F.; Kanter, M.; Agma, A. Effects of Enterococcus faecium and dried whey on broiler performance, gut histomorphology and intestinal microbiota. Arch. Anim. Nutr. 2007, 61, 42–49. [Google Scholar] [CrossRef]
- Berri, C.; Godet, E.; Hattab, N.H.; Duclos, M.J. Growth and differentiation of the chicken Pectoralis major muscle: Effect of genotype and early nutrition. Arch. Anim. Breed. 2006, 49, 31–32. [Google Scholar]
- Tixier-Boichard, M. From the jungle fowl to highly performing chickens: Are we reaching limits? World’s Poult. Sci. J. 2020, 76, 2–17. [Google Scholar] [CrossRef]
- Smith, J.H. Relation of body size to muscle cell size and number in the chicken. Poult. Sci. 1963, 42, 283–290. [Google Scholar] [CrossRef]
- Mozdziak, P.E.; Schultz, E.; Cassens, R.G. Myonuclear accretion is a major determinant of avian skeletal muscle growth. Am. J. Physiol. 1997, 272, C565–C571. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.J.; Molette, C.; Guernec, A.; Remignon, H.; Berri, C. Cellular aspects of breast muscle development in chickens with high and low growth rate. Arch. Anim. Breed. 2006, 49, 147–151. [Google Scholar]
- Jacquemin, V.; Butler-Browne, G.S.; Furling, D.; Mouly, V. IL-13 mediates the recruitment of reserve cells for fusion during IGF-1-induced hypertrophy of human myotubes. J. Cell Sci. 2007, 120, 670–681. [Google Scholar] [CrossRef]
- Geiger, A.E.; Daughtry, M.R.; Gow, C.M.; Siegel, P.B.; Shi, H.; Gerrard, D.E. Long-term selection of chickens for body weight alters muscle satellite cell behaviors. Poult. Sci. 2018, 97, 2557–2567. [Google Scholar] [CrossRef]
- Moore, D.T.; Ferket, P.R.; Mozdziak, P.E. Muscle development in the late embryonic and early post-hatch poult. Int. J. Poult. Sci. 2005, 4, 138–142. [Google Scholar] [CrossRef]
- Velleman, S.G.; Coy, C.S.; Emmerson, D.A. Effect of the timing of posthatch feed restrictions on broiler breast muscle development and muscle transcriptional regulatory factor gene expression. Poult. Sci. 2014, 93, 1484–1494. [Google Scholar] [CrossRef]
- Halevy, O.; Gayra, A.; Barak, M.; Uni, Z.; Sklan, D. Early posthatch starvation decreases satellite cell proliferation and skeletal muscle growth in chicks. J. Nutr. 2000, 130, 858–864. [Google Scholar] [CrossRef]
- Olguin, H.C.; Olwin, B.B. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: A potential mechanism for self-renewal. Dev. Biol. 2004, 275, 375–388. [Google Scholar] [CrossRef]
- Halevy, O.; Piestun, Y.; Allouh, M.Z.; Rosser, B.W.C.; Rinkevich, Y.; Reshef, R.; Rozenboim, I.; Wleklinski-Lee, M.; Yablonka-Reuveni, Z. The pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev. Dyn. 2004, 231, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Rudnicki, M.A. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev. Biol. 2000, 218, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Gayraud-Morel, B.; Chrétien, F.; Jory, A.; Sambasivan, R.; Negroni, E.; Flamant, P.; Soubigou, G.; Coppé, J.-Y.; Di Santo, J.; Cumano, A.; et al. MYF5 haploinsufficiency reveals distinct cell fate potentials for adult skeletal muscle stem cells. J. Cell Sci. 2012, 125, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Yablonka-Reuveni, Z.; Paterson, B.M. MyoD and myogenin expression patterns in cultures of fetal and adult chicken myoblasts. J. Histochem. Cytochem. 2001, 49, 455–462. [Google Scholar] [CrossRef]
- Zammit, P.S.; Relaix, F.; Nagata, Y.; Pérez Ruiz, A.; Collins, C.A.; Partridge, T.A.; Beauchamp, J.R. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 2006, 119, 1824–1832. [Google Scholar] [CrossRef]
- Halevy, O.; Hodik, V.; Mett, A. The effects of growth hormone on avian skeletal muscle satellite cell proliferation and differentiation. Gen. Comp. Endocrinol. 1996, 101, 43–52. [Google Scholar] [CrossRef]
- Florini, J.R.; Ewton, D.Z.; Coolican, S.A. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr. Rev. 1996, 17, 481–517. [Google Scholar] [CrossRef]
- Clark, D.L.; Walter, K.G.; Velleman, S.G. Incubation temperature and time of hatch impact broiler muscle growth and morphology. Poult. Sci. 2017, 96, 4085–4095. [Google Scholar] [CrossRef]
- Zitnan, R.; Albrecht, E.; Kalbe, C.; Miersch, C.; Revajova, V.; Levkut Jr., M.; Röntgen, M. Muscle characteristics in chicks challenged with Salmonella Enteritidis and the effect of preventive application of the probiotic Enterococcus faecium. Poult. Sci. 2019, 98, 2014–2025. [Google Scholar] [CrossRef]
- Letnická, A.; Karaffová, V.; Levkut, M.; Revajová, V.; Herich, R. Influence of oral application of Enterococcus faecium AL41 on TGF-β4 and IL-17 expression and immunocompetent cell distribution in chickens challenged with Campylobacter jejuni. Acta Vet. Hung. 2017, 65, 317–326. [Google Scholar] [CrossRef]
- Karaffová, V.; Tóthová, C.; Szabóová, R.; Revajová, V.; Lauková, A.; Ševcíková, Z.; Herich, R.; Žitnan, R.; Levkut, M.; Levkut, M.; et al. The effect of Enterococcus faecium AL41 on the acute phase proteins and selected mucosal immune molecules in broiler chickens. Life 2022, 12, 598. [Google Scholar] [CrossRef] [PubMed]
- Cobb-Vantress Inc. Cobb500 Slow Feather Breeder Management Supplement; Cobb-Vantress: Siloam Springs, AR, USA, 2020. [Google Scholar]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Lösel, D.; Franke, A.; Kalbe, C. Comparison of different skeletal muscles from growing domestic pigs and wild boars. Arch. Anim. Breed. 2013, 56, 766–777. [Google Scholar] [CrossRef]
- Romeis, B. Mikroskopische Technik; Urban & Schwarzenberg: Munich, Germany, 1989. [Google Scholar]
- Spannhof, L. Einführung in die Praxis der Histochemie; VEB Gustav-Fischer-Verlag: Jena, Germany, 1967. [Google Scholar]
- Karaffová, V.; Bobíková, K.; Levkut, M.; Revajová, V.; Ševčíková, Z.; Levkut, M. The influence of Farmatan® and Flimabend® on the mucosal immunity of broiler chicken. Poult. Sci. 2019, 98, 1161–1166. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, C.; Li, K.; Gui, G.; Zhang, G.; Yang, H. Association of growth rate with hormone levels and myogenic gene expression profile in broilers. J. Anim. Sci. Biotechnol. 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.; Chen, C.; Waters, E.; West, F.D.; Kim, W.K. Isolation and differentiation of mesenchymal stem cells from broiler chicken compact bones. Front. Physiol. 2019, 9, 1892. [Google Scholar] [CrossRef] [PubMed]
- De Boever, S.; Vangestel, C.; De Backer, P.; Croubels, S.; Sys, S.U. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet. Immunol. Immunopathol. 2008, 122, 312–317. [Google Scholar] [CrossRef]
- Schmidt, E.E.; Schibler, U. Cell size regulation, a mechanism that controls cellular RNA accumulation: Consequences on regulation of the ubiquitous transcription factors Oct1 and NF-Y, and liver-enriched transcription factor DBP. J. Cell Biol. 1995, 128, 467–483. [Google Scholar] [CrossRef]
- Haddad, F.; Baldwin, K.M.; Tesch, P.A. Pretranslational markers of contractile protein expression in human skeletal muscle: Effect of limb unloading plus resistance exercise. J. Appl. Physiol. 2005, 98, 46–52. [Google Scholar] [CrossRef]
- Rehfeldt, C.; Renne, U.; Sawitzky, M.; Binder, G.; Hoeflich, A. Increased fat mass, decreased myofiber size, and a shift to glycolytic muscle metabolism in adolescent male transgenic mice overexpressing IGFBP-2. Am. J. Physiol. Endocrinol. Metabol. 2010, 299, E287–E298. [Google Scholar] [CrossRef]
- Biressi, S.; Molinaro, M.; Cossa, G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev. Biol. 2007, 308, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Bottinelli, R.; Reggiani, C. Human skeletal muscle fibres, molecular and functional diversity. Prog. Biophys. Mol. Biol. 2000, 73, 195–262. [Google Scholar] [CrossRef]
- Verdiglione, R.; Cassandro, M. Characterization of muscle fiber type in the pectoralis major muscle of slow-growing local and commercial chicken strains. Poult. Sci. 2013, 92, 2433–2437. [Google Scholar] [CrossRef] [PubMed]
- Kocamis, H.; McFarland, D.C.; Killefer, J. Temporal expression of growth factor genes during myogenesis of satellite cells derived from the biceps femoris and pectoralis major muscles of the chicken. J. Cell. Physiol. 2001, 186, 146–152. [Google Scholar] [CrossRef]
- Doherty, M.K.; McLean, L.; Hayter, J.R.; Pratt, J.M.; Robertson, D.H.; El-Shafei, A.; Gaskell, S.J.; Beynon, R.J. The proteome of chicken skeletal muscle: Changes in soluble protein expression during growth in a layer strain. Proteomics 2004, 4, 2082–2093. [Google Scholar] [CrossRef]
- Schmid, C.; Steiner, T.; Froesch, E.R. Preferential enhancement of myoblast differentiation by insulin-like growth factors (IGF I and IGF II) in primary cultures of chicken embryonic cells. FEBS Lett. 1983, 16, 117–121. [Google Scholar] [CrossRef]
- Duclos, M.J.; Wilkie, R.S.; Goddard, C. Stimulation of DNA synthesis in chicken muscle satellite cells by insulin and insulin-like growth factors: Evidence for exclusive mediation by a type-I insulin-like growth factor receptor. J. Endocrinol. 1991, 128, 35–42. [Google Scholar] [CrossRef]
- Vandenburgh, H.H.; Karlisch, P.; Shansky, J.; Feldstein, R. Insulin and IGF-I induce pronounced hypertrophy of skeletal myofibers in tissue culture. Am. J. Physiol. Cell Physiol. 1991, 29, C475–C484. [Google Scholar] [CrossRef]
- Florini, J.R.; Ewton, D.Z.; Roof, S.L. Insulin-like growth factor-I stimulates terminal myogenic differentiation by induction of myogenin gene expression. Mol. Endocrinol. 1991, 5, 718–724. [Google Scholar] [CrossRef]
- Musaro, A.; Rosenthal, N. Maturation of the myogenic program is induced by postmitotic expression of insulin-like growth factor I. Mol. Cell. Biol. 1999, 19, 3115–3124. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors MYF5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell. Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Beauchamp, J.R.; Heslop, L.; Yu, D.S.W.; Tajbakhsh, S.; Kelly, R.G.; Wernig, A.; Buckingham, M.E.; Partridge, T.A.; Zammit, P.S. Expression of CD34 and of MYF5 defines the majority pf quiescent adult skeletal muscle satellite cells. J. Cell Biol. 2000, 151, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- McKinnell, I.W.; Ishibashi, J.; Le Grand, F.; Punch, V.G.J.; Addicks, G.C.; Greenblatt, J.F.; Dilworth, F.J.; Rudnicki, M.A. Pac7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat. Cell Biol. 2008, 10, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Abu Khalil, R.; Le Grand, F.; Pallafacchina, G.; Valable, S.; Authier, F.-J.; Rudnicki, M.A.; Gheradi, R.K.; Germain, S.; Chretien, F.; Sotiropoulos, A.; et al. Autocrine and paracrine angiopoietin 1/Tie1-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell 2009, 5, 298–309. [Google Scholar] [CrossRef]
- Lepper, C.; Conway, S.J.; Fan, C.M. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 2009, 460, 627–631. [Google Scholar] [CrossRef]
- Olguin, H.C.; Yang, Z.; Tapscott, S.J.; Stephen, J.; Olwin, B.B. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J. Cell Biol. 2007, 177, 769–779. [Google Scholar] [CrossRef]
- Hoving-Bolink, A.H.; Kranen, R.W.; Klont, R.E.; Gerritsen, C.L.M.; de Greef, K.H. Fibre area and capillary supply in broiler breast muscle in relation to productivity and ascites. Meat Sci. 2000, 66, 397–402. [Google Scholar] [CrossRef]
- Coultas, L.; Chawengsaksophak, K.; Rossant, J. Endothelial cells and VEGF in vascular development. Nature 2005, 438, 937–945. [Google Scholar] [CrossRef]
- Christov, C.; Chrétien, F.; Abou-Khalil, R.; Bassez, G.; Vallet, G.; Authier, F.-J.; Bassaglia, Y.; Shinin, V.; Tajbakhsh, S.; Chazaud, B.; et al. Muscle satellite cells and endothelial cells: Close neighbors and privileged partners. Mol. Biol. Cell. 2007, 18, 1397–1409. [Google Scholar] [CrossRef]
- Bryan, B.A.; Walshe, T.E.; Mitchell, D.C.; Havumaki, J.S.; Saint-Geniez, M.; Maharaj, A.S.; Maldonado, A.E.; D’Amore, P.A. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol. Biol. Cell 2008, 19, 994–1006. [Google Scholar] [CrossRef]
- Radaelli, G.; Piccirilo, A.; Birolo, M.; Bertotto, D.; Gratta, F.; Ballarina, C.; Vascellari, M.; Xiccato, G.; Trocino, A. Effect of age on the occurrence of muscle fiber degeneration associated with myopathies in broiler chickens submitted to feed restriction. Poult. Sci. 2017, 96, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Papah, M.B.; Brannick, E.M.; Schmidt, C.J.; Abasht, B. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of wooden breast disease in modern broiler checkens. Avian Pathol. 2017, 46, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Sihvo, H.-K.; Airas, N.; Lindén, J.; Puolanne, E. Pectoral vessel density and early ultrastructural changes in broiler chicken wooden breast myopathy. J. Comp. Path. 2018, 161, 1–10. [Google Scholar] [CrossRef] [PubMed]

| Primer | Sequence 5′–3′ | Annealing Temperature/Time | References |
|---|---|---|---|
| IGF1 Fw | GAGCTGGTTGATGCTCTTCAGTT | 60 °C/1 min | Xiao et al., 2017 [58] |
| IGF1 Rev | CCAGCCTCCTCAGGTCACAACT | ||
| MYF5 Fw | CAGAGACTCCCCAAAGTGGAGAT | 60 °C/1 min | |
| MYF5 Rev | GTCCCGGCAGGTGATAGTAGTTC | ||
| PAX7 Fw | AGGCTGACTTCTCCATCTCTCCT | 60 °C/1 min | Adhikari et al., 2019 [59] |
| PAX7 Rev | TGTAACTGGTGGTGCTGTAGGTG | ||
| GAPDH Fw | CCTGCATCTGCCCATTT | 59 °C/30 s | De Boever et al., 2008 [60] |
| GAPDH Rev | GGCACGCCATCACTATC |
| Control | EF | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day of Age | 1 (n = 6) | 5 (n = 10) | 8 (n = 10) | 12 (n = 10) | 1 (n = 6) | 5 (n = 10) | 8 (n = 10) | 12 (n = 10) | SE d 1 | SE d 5–12 | Age | Group | Age × Group |
| Daily gain, g | 14.0 c | 22.8 a | 17.3 b | 14.9 c | 24.1 a | 18.4 b | 0.39 | <0.001 | <0.001 | 0.919 | |||
| Weights, g | |||||||||||||
| Slaughter weight | 43.2 d | 112.7 c | 224.4 b | 250.5 a,B | 43.8 d | 116.8 c | 233.1 b | 261.4 a,A | 4.0 | 3.1 | <0.001 | 0.012 | 0.456 |
| Pectoralis muscle | 1.74 d | 8.26 c | 35.66 b | 58.54 a,B | 1.64 d | 8.19 c | 37.21 b | 62.63 a,A | 1.39 | 1.07 | <0.001 | 0.100 | 0.212 |
| Liver | 2.36 c | 7.47 b,A | 11.32 a | 12.46 a,A | 1.69 c | 4.50 b,B | 10.67 a | 11.21 a,B | 0.54 | 0.42 | <0.001 | <0.001 | 0.030 |
| Stomach | 5.01 b | 4.77 b,B | 12.34 a | 11.31 a | 4.32 b | 6.53 b,A | 11.14 a | 11.64 a | 0.60 | 0.46 | <0.001 | 0.901 | 0.014 |
| Intestine | 5.62 c | 9.88 b | 21.61 a | 24.46 a | 4.90 c | 9.28 b | 22.13 a | 22.39 a | 1.04 | 0.80 | <0.001 | 0.246 | 0.464 |
| Control | EF | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day of Age | 1 (n = 6) | 5 (n = 10) | 8 (n = 10) | 12 (n = 10) | 1 (n = 6) | 5 (n = 10) | 8 (n = 10) | 12 (n = 10) | SE d 1 | SE d 5–12 | Age | Group | Age × Group |
| Total DNA, mg | 0.48 d | 3.03 c | 7.72 b | 11.38 a | 0.37 d | 2.95 c | 7.58 b | 12.36 a | 0.34 | 0.27 | <0.001 | 0.435 | 0.123 |
| Total RNA, mg | 2.54 d | 20.42 c | 48.56 b | 59.78 a,B | 2.16 d | 19.80 c | 44.34 b | 67.04 a,A | 1.96 | 1.52 | <0.001 | 0.660 | 0.003 |
| Total protein, mg | 80 c | 501 c | 2551 b | 4620 a | 78 c | 482 c | 2753 b | 4961 a | 131 | 102 | <0.001 | 0.098 | 0.276 |
| DNA/RNA | 0.188 a,A | 0.148 b | 0.159 b,B | 0.191 a | 0.170 b,B | 0.149 c | 0.171 b,A | 0.184 a | 0.003 | 0.003 | <0.001 | 0.138 | <0.001 |
| RNA/DNA | 5.32 c,B | 6.76 a | 6.28 b,A | 5.25 c | 5.93 b,A | 6.74 a | 5.83 bc,B | 5.44 c | 0.11 | 0.09 | <0.001 | 0.249 | <0.001 |
| DNA, µg/g | 273 b,A | 366 a | 217 c | 195 d | 226 b,B | 360 a | 204 c | 197 c | 5 | 4 | <0.001 | <0.001 | <0.001 |
| RNA, µg/g | 1453 b | 2474 a | 1362 b,A | 1024 c | 1340 b | 2423 a | 1192 bc,B | 1071 c | 44 | 34 | <0.001 | 0.008 | 0.020 |
| Protein, mg/g | 45.9 d | 60.7 c | 71.6 b | 79.0 a | 47.3 c | 58.8 c | 74.0 b | 79.1 a | 1.6 | 1.2 | <0.001 | 0.588 | 0.327 |
| DNA/protein, µg/mg | 5.98 a,A | 6.04 a | 3.03 b | 2.46 c | 4.79 b,B | 6.13 a | 2.76 c | 2.50 c | 0.12 | 0.09 | <0.001 | <0.001 | <0.001 |
| RNA/protein, µg/mg | 31.8 b | 40.8 a | 19.1 c,A | 12.9 d | 28.4 b | 41.3 a | 16.1 c,B | 13.6 c | 0.8 | 0.6 | <0.001 | 0.009 | 0.004 |
| Protein/RNA, mg/µg | 0.032 c | 0.025 c | 0.053 b,B | 0.077 a | 0.036 c | 0.024 d | 0.063 b,A | 0.074 a | 0.002 | 0.001 | <0.001 | 0.019 | <0.001 |
| Total CK, IU | 1591 c | 13,347 c | 65,827 b | 116,724 a | 1612c | 12,557 c | 67,609 b | 126,644 a | 3553 | 2752 | <0.001 | 0.198 | 0.218 |
| CK, IU/g | 919 c | 1622 b | 1845 ab | 1994 a | 968 c | 1540 b | 1822 a | 2026 a | 70 | 54 | <0.001 | 0.882 | 0.668 |
| CK/protein, IU/mg | 20.2 b | 26.8 a | 25.7a | 25.2a | 20.4b | 26.3a | 24.6 a | 25.7 a | 1.0 | 0.8 | <0.001 | 0.703 | 0.751 |
| ICDH, IU/g | 3.12 a | 2.28 b | 1.41 c | 1.25 c | 2.96 a | 2.31 b | 1.39 c | 1.13 c | 0.09 | 0.07 | <0.001 | 0.218 | 0.533 |
| LDH, IU/g | 43 d | 330 c | 751 b | 929 a | 54 d | 315 c | 765 b | 892 a | 29 | 22 | <0.001 | 0.694 | 0.658 |
| ICDH/protein, IU/mg | 0.068 a | 0.038 b | 0.020 c | 0.016 c | 0.062 a | 0.039 b | 0.019 c | 0.014 c | 0.001 | 0.001 | <0.001 | 0.070 | 0.040 |
| LDH/protein, IU/mg | 0.93 d | 5.42 c | 10.5 b | 11.74 a | 1.14 c | 5.36 b | 10.34 a | 11.28 a | 0.32 | 0.25 | <0.001 | 0.536 | 0.695 |
| LDH/ICDH | 14 d | 146 c | 540 b | 748 a | 19 d | 138 c | 554 b | 796 a | 29 | 23 | <0.001 | 0.394 | 0.660 |
| Control | EF | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day of Age | 1 (n = 6) | 5 (n = 10) | 8 (n = 10) | 12 (n = 10) | 1 (n = 6) | 5 (n = 9) | 8 (n = 10) | 12 (n = 10) | SE d 1 | SE d 5–12 | Age | Group | Age × Group |
| Nuclei/mm2 | 7.622 c,A | 2.689 b,B | 1.648 a | 1.198 a | 6.515 c,B | 3.356 b,A | 1.803 a | 1.142 a | 257 | 199 | <0.001 | 0.580 | 0.004 |
| FCSA, µm2 | 54.5 d | 227.7 c | 577.5 b | 816.1 a,B | 67.9 d | 220.6 c | 586.4 b | 1107.8 a,A | 48.9 | 37.9 | <0.001 | 0.011 | <0.001 |
| Nuclei/fiber | 0.40 d | 0.57 c,B | 0.92 b,B | 0.95 a,B | 0.43 c | 0.71 b,A | 1.04 a,A | 1.23 a,A | 0.05 | 0.04 | <0.001 | <0.001 | 0.021 |
| CCSA, µm2 | 3.59 c | 10.02 b | 13.31 a,B | 4.77 c | 11.78 b | 22.44 a,A | 0.88 | <0.001 | <0.001 | <0.001 | |||
| Capillary area, % | 2.29 | 2.21 B | 1.92 B | 2.41 | 3.01 A | 2.89 A | 0.22 | 0.461 | 0.001 | 0.141 | |||
| Capillaries/mm2 | 6496 a,A | 2162 b | 1476 b | 5246 a,B | 2616 b | 1324 c | 222 | <0.001 | 0.091 | 0.002 | |||
| Capillaries/fiber | 1.50 A | 1.30 | 1.29 | 1.01 B | 1.39 | 1.45 | 0.13 | 0.656 | 0.453 | 0.033 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albrecht, E.; Zitnan, R.; Karaffova, V.; Revajova, V.; Čechová, M.; Levkut Jr., M.; Röntgen, M. Effects of the Probiotic Enterococcus faecium on Muscle Characteristics of Chickens. Life 2022, 12, 1695. https://doi.org/10.3390/life12111695
Albrecht E, Zitnan R, Karaffova V, Revajova V, Čechová M, Levkut Jr. M, Röntgen M. Effects of the Probiotic Enterococcus faecium on Muscle Characteristics of Chickens. Life. 2022; 12(11):1695. https://doi.org/10.3390/life12111695
Chicago/Turabian StyleAlbrecht, Elke, Rudolf Zitnan, Viera Karaffova, Viera Revajova, Michaela Čechová, Martin Levkut Jr., and Monika Röntgen. 2022. "Effects of the Probiotic Enterococcus faecium on Muscle Characteristics of Chickens" Life 12, no. 11: 1695. https://doi.org/10.3390/life12111695
APA StyleAlbrecht, E., Zitnan, R., Karaffova, V., Revajova, V., Čechová, M., Levkut Jr., M., & Röntgen, M. (2022). Effects of the Probiotic Enterococcus faecium on Muscle Characteristics of Chickens. Life, 12(11), 1695. https://doi.org/10.3390/life12111695







