Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants
Abstract
1. Introduction
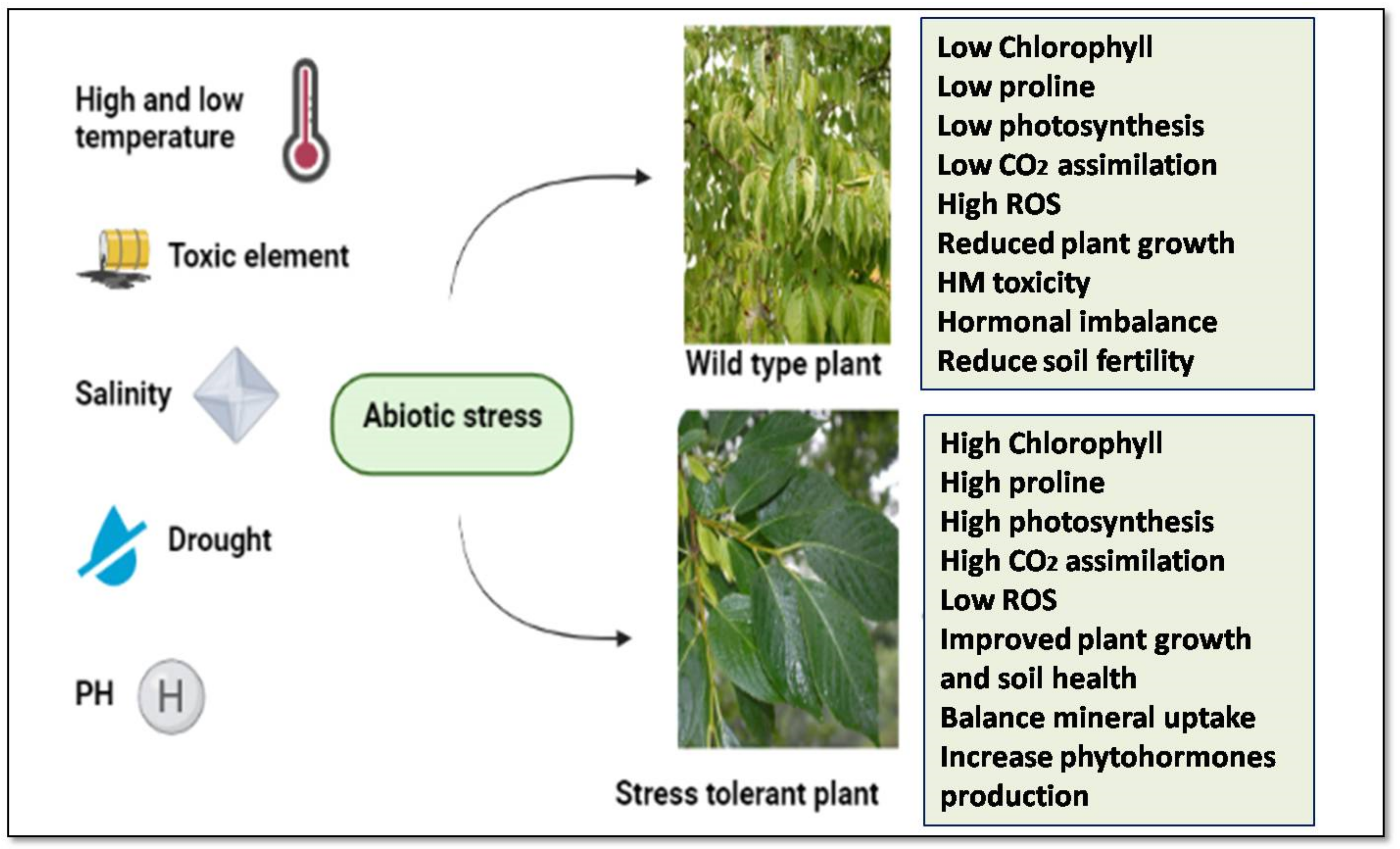
2. Abiotic Stresses and Crop Plants
2.1. Salt Stress
2.2. Drought Stress
2.3. Cold Stress
2.4. Heat Stress
2.5. Heavy Metals Stress
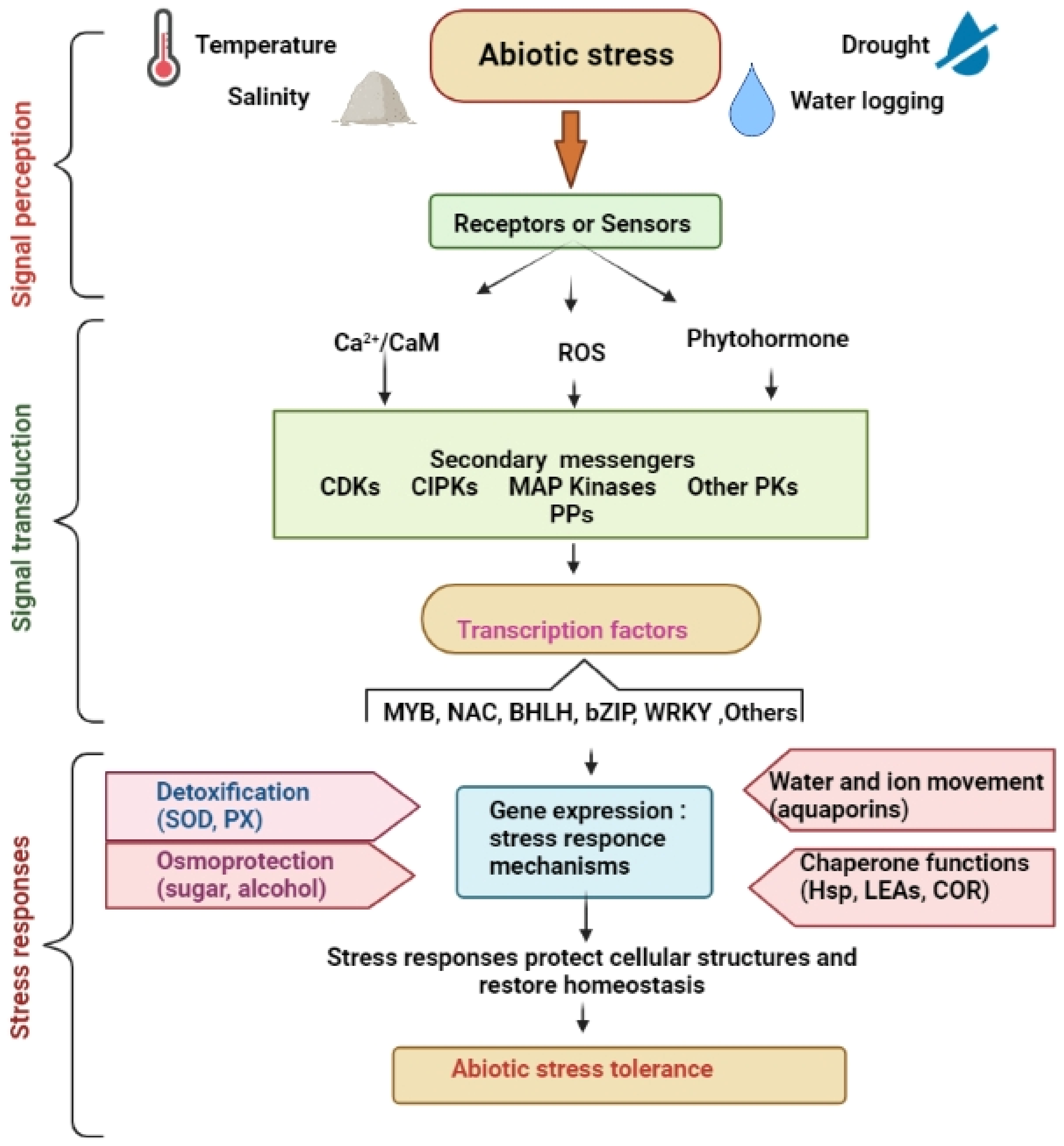
3. Sensing and Responding Mechanisms of Plants during Abiotic Stress Conditions
3.1. Gene Regulation at the Transcriptional Level
3.2. Gene Regulation at Posttranscriptional Level
3.3. Gene Regulation at the Posttranslational Level
4. Crucial Signal Transduction Mechanism for Abiotic Stress
4.1. Oxidative or Osmotic Stress Signaling in Plants
4.2. Ca2+-Dependent Activation of LEA Genes
4.3. Calcium Ion-Dependent SOS Signaling
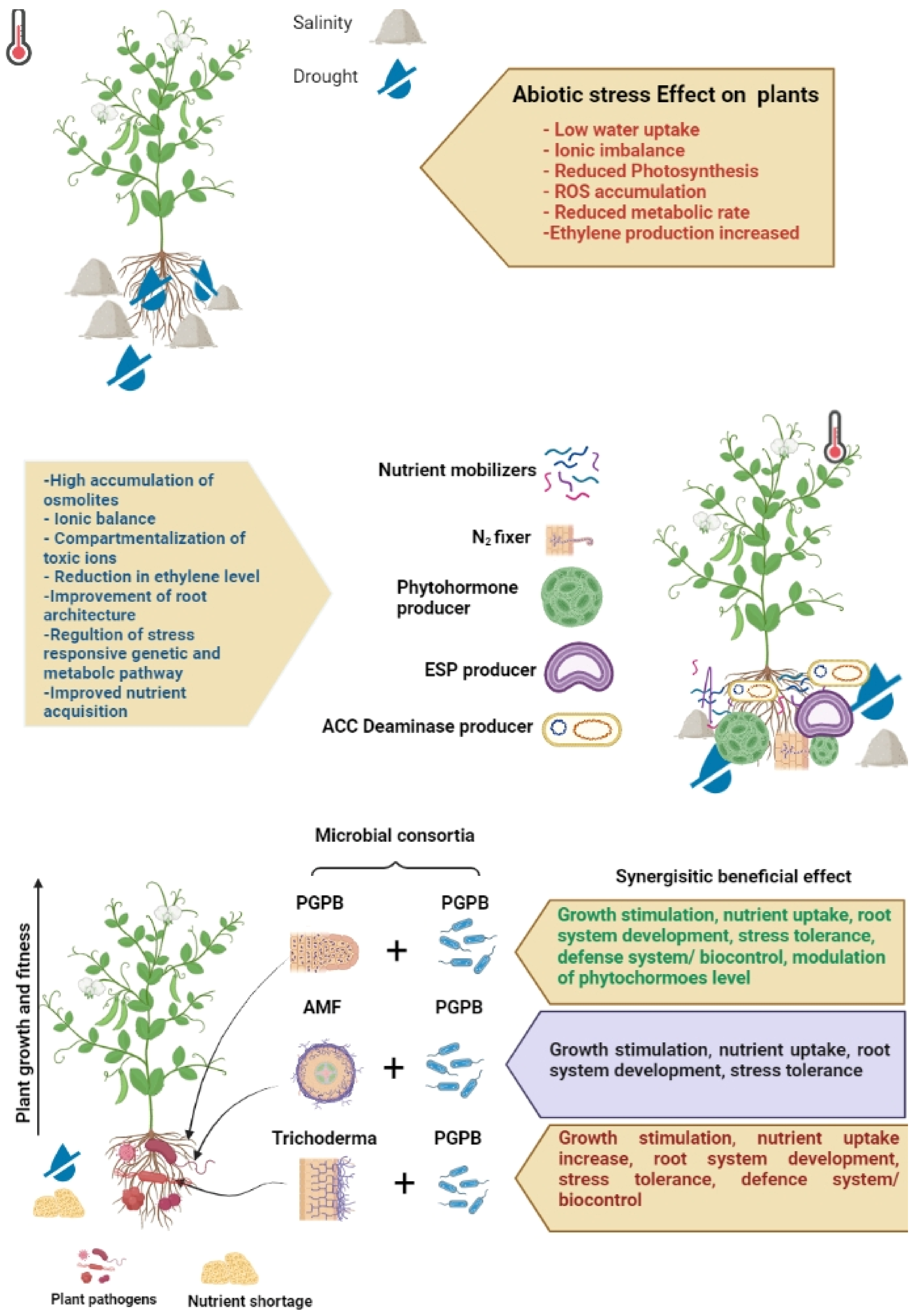
5. Functions of the Microbiome in Abiotic Stress Management
5.1. Arbuscular Mycorrhizal Fungi (AMF)
| Type of Microbes | Abiotic Stress Type | Plant and Tolerance Mechanism | References |
|---|---|---|---|
| Pseudomonas putida P45 | Drought | Sunflower (Helianthus annuus) showed EPS production and enhanced soil aggregation | [141] |
| Pseudomonas | Drought | Pea (Pisum sativum) plants reduced the production of ethylene | [142] |
| Azospirillium sp. | Drought | Wheat (Triticum aestivum) has better water relations | [143] |
| AM fungi | Drought and salinity | Sorghum (Sorghum bicolor) showed better water relations | [144] |
| Scytonema | Coastal salinity | Rice (Oryza sativa) with extracellular products and gibberellic acid | [145] |
| Burkholderia phytofirmans | Cold | Grapevine (Vitis vinifera) with ACC-deaminase synthesis | [146] |
| Burkholderia sp. and Methylobacterium oryzae | Cd and Ni toxicity | Tomato (Solanum lycopersicum) with lower uptake along with translocation of heavy metals | [147] |
| Pseudomonas fluorescences | Salinity | Groundnut (Arachis hypogaea) with ACC-deaminase synthesis | [148] |
| Rhizobium tropici; P. polymyxa | Drought | Common bean (Phaseolus vulgaris) with change in hormonal composition and stomatal conductance | [149] |
| Glomus intraradices and Pseudomonas mendocina | Drought | Lettuce (Lactuca sativa) has antioxidant status improved | [150] |
| Pseudomonas strain AMK-P6 | Heat | Sorghum (Sorghum bicolor) with better biochemical status due to activation of heat shock proteins | [151] |
| Glomus sp. and Bacillus megaterium | Drought | Trifolium (Trifolium repens) with proline and IAA production | [152] |
| Paraphaeosphaeria quadriseptata | Drought | Arabidopsis (Arabidopsis thaliana) has HSP-heat shock protein induction | [153] |
| Bacillus subtilis | Salinity | Arabidopsis (Arabidopsis thaliana) has reduced root Na+ import by reduced transcriptional expression of AtHTK1 (a high-affinity KC transporter) genes | [153] |
| Pseudomonas putida | Salinity | Cotton (Gossypium hirusutum) stopped the salinity-associated accumulation of ABA in seedlings | [154] |
| Glomus etunicatum and Glomus clarum | Salinity | Wheat (Triticum aestivum), Chilli (Capsicum annum) and mung bean (Vigna radiata) have increased KC concentration in root and reduced NaC in shoots and root | [155] |
| PGPRs | Heat | Clover (Trifolium repens) plants with greater nitrogen fixation | [156] |
| Bacillus licheformis | Drought | Capsicum annum with expression and activation of stress-related proteins and genes | [157] |
| Bacillus thuringiensis | Drought | Wheat (Triticum aestivum) showed the organic compound production | [158] |
| Pantoea dispersa and Azospirillium brailense | Salinity | Capsicum annuum has an increase in photosynthesis rates well as stomatal conductance | [159] |
| Burkholderia phytofirmans and Enterobacter sp. | Drought | Maize (Zea mays) showed an increased rate of shoot and root biomass | [160] |
| Pseudomonas koreensis strain | Salinity | Soyabean (Glycine max) has increase KC level and decreased Na+ level | [161] |
| Enterobacter intermedius | Zn toxicity | White mustard (Sinapis alba) with ACC deaminase, IAA IAA, hydrocyanic acid, and solubilization of phosphate | [162] |
| Serratia sp. and Bacillus cereus | Drought | Cucumber (Cucumis sativa) showed the production of genes responsible for the synthesis of proline, an antioxidant enzyme, and monodehydroascorbate | [163] Wang et al.; 2012 |
| Photobacterium spp. | Mercury toxicity | Common reed (Phragmites australis) showed activity of IAA and mercury reductase | [164] |
| Rhizobium leguminosarum and Pseudominas brassicacerum | Zinc toxicity | Mustard (Brassica juncea) with metal chelating molecules | [165] |
| Rhizobium | Salinity | Asian rice (Oryza sativa) with RAB 18 salt stress-associated gene expression | [166] |
| PB 50 strain of B. megaterium | Drought | Rice (Oryza sativa) showed better plant growth under osmotic stress, plants protected via stomatal closure with enhanced soluble sugar, carotenoid content and protein content | [167] |
| Bacillus albus and Bacillus cereus | Drought | Maize (Zea mays) seeds have a higher germination rate and increased seedling length with reduced toxic effects | [168] |
| Gluconacetobacter diazotrophics (Pal5) | Drought | Rice (Oryza sativa L.) shows gor, P5CR, BADH and cat genes expression with increase glycine betaine and proline content | [169] |
| Penicillium sp. and Calcoaceticus | Drought | Foxtail millet (Setaria italica) with increased glycine betaine and proline content, sugars and chlorophyll a and b with the decrease in lipid oxidation | [170] |
| Streptomyces pactum and actinomyces | Drought | Wheat (T. aestivum) reduces stress via an enhancement in sugar levels and antioxidant enzymes | [171] |
| B. amyloliquefaciens; Pseudomonas putida | Drought | Chick pea (Cicer arietinum) with better photosynthesis, chlorophyll content, biomass and osmolyte content | [172] |
| PGPR consortium | Salinit | Common bean (Phaseolus vulgaris) with available iron content in the soil increased | [173,174] |
| B. gladioli; P. aeruginosa | Cd toxicity | Tomato (Solanum lycopersicum) with higher expression of metal transporter genes | [175] |
| Mesorhizobium; Rhizobium | Salinity | Chickpea (Cicer arietinum) with enhanced nitrogen fixation | [176] |
| Bacillus megaterium | Osmotic | Maize (Zea mays) with higher expression of 2 ZmPIP isoforms in roots | [177] |
5.2. Actinomycetes
5.3. PGPR/Plant Growth-Promoting Bacteria
6. Nanoparticles’ Application in Combating Abiotic Stress in Plants
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umar, O.B.; Ranti, L.A.; Abdulbaki, A.S.; Bola, A.L.; Abdulhamid, A.K.; Biola, M.R.; Victor, K.O. Stresses in Plants: Biotic and Abiotic. In Current Trends in Wheat Research; Ansari, M., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Wania, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Mei, H.; Cheng-Qiang, H.; Nai-Zheng, D. Abiotic Stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 7, 1771. [Google Scholar] [CrossRef]
- Jogaiah, S.; Govind, S.R.; Tran, L.S.P. Systems biology-based approaches toward understanding drought tolerance in food crops. Crit. Rev. Biotechnol. 2013, 33, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Sihag, S.; Brar, B.; Joshi, U.N. Salicylic acid induces amelioration of chromium toxicity and affects antioxidant enzyme activity in Sorghum bicolor L. Int. J. Phytoremediation 2019, 21, 293–304. [Google Scholar] [CrossRef]
- Fedoron, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.; Fischhon, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically rethinking agriculture for the 21st century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef]
- Kundzewicz, Z.; Ulbrich, U.; Brucher, T.; Graczyk, D.; Kruger, A.; Leckebusch, G.C. Summer floods in Central Europe—Climate change track? Nat. Hazards 2005, 36, 165–189. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163–176. [Google Scholar] [CrossRef]
- Mathivanan, S. Abiotic Stress-Induced Molecular and Physiological Changes and Adaptive Mechanisms in Plants. In Abiotic Stress in Plants; Fahad, S., Saud, S., Chen, Y., Wu, C., Wang, D., Eds.; IntechOpen: London UK, 2021. [Google Scholar] [CrossRef]
- Ruelland, E.; Vaultier, M.N.; Zachowski, A.; Hurry, V. Cold signalling and cold acclimation in plants. Adv. Bot. Res. 2009, 49, 35–150. [Google Scholar]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef]
- Rajkowitsch, L.; Chen, D.; Stampfl, S.; Semrad, K.; Waldsich, C.; Mayer, O.; Jantsch, M.F.; Konrat, R.; Bläsi, U.; Schroeder, R. RNA chaperones, RNA annealers and RNA helicases. RNA Biol. 2007, 4, 118–130. [Google Scholar] [CrossRef]
- Hadiarto, T.; Tran, L.S.P. Progress studies of drought-responsive genes in rice. Plant Cell Rep. 2011, 30, 297–310. [Google Scholar] [CrossRef]
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Melo, F.V.; Oliveira, M.M.; Saibo, N.J.; Lourenço, T.F. Modulation of abiotic stress responses in rice by E3-ubiquitin ligases: A promising way to develop stress-tolerant crops. Front. Plant Sci. 2021, 12, 368. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR signaling cascade and its regulation in plants responding to cold stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef]
- Eremina, M.; Rozhon, W.; Poppenberger, B. Hormonal control of cold stress responses in plants. Cell. Mol. Life Sci. 2016, 73, 797–810. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When Defense Pathways Collide. The Response of Arabidopsis to a Combination of Drought and Heat Stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, H.; Shi, C.; Xia, G.; Lai, Z. Transcriptome analysis of the role of autophagy in plant response to heat stress. PLoS ONE 2021, 16, e0247783. [Google Scholar] [CrossRef]
- Pan, T.; Sun, X.; Liu, Y.; Li, H.; Deng, G.; Lin, H.; Wang, S. Heat stress alters genome-wide profiles of circular RNAs in Arabidopsis. Plant Mol. Biol. 2018, 96, 217–229. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Spatio-temporal heterogeneity in Arabidopsis thaliana leaves under drought stress. Plant Biol. 2012, 14, 118–128. [Google Scholar] [CrossRef]
- Dubey, A.K.; Kumar, N.; Kumar, A.; Ansari, M.A.; Ranjan, R.; Gautam, A.; Sahu, N.; Pandey, V.; Behera, S.K.; Mallick, S.; et al. Over-expression of CarMT gene modulates the physiological performance and antioxidant defense system to provide tolerance against drought stress in Arabidopsis thaliana L. Ecotoxicol. Environ. Saf. 2019, 171, 54–65. [Google Scholar] [CrossRef]
- Shen, J.; Xing, T.; Yuan, H.; Liu, Z.; Jin, Z.; Zhang, L.; Pei, Y. Hydrogen sulfide improves drought tolerance in Arabidopsis thaliana by microRNA expressions. PLoS ONE 2013, 8, e77047. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, Z.; Ma, Q.; Sun, L.; Zhang, L.; Liu, Z.; Liu, D.; Hao, X.; Pei, Y. Hydrogen sulfide mediates ion fluxes inducing stomatal closure in response to drought stress in Arabidopsis thaliana. Plant Soil 2017, 419, 141–152. [Google Scholar] [CrossRef]
- Panta, G.; Rieger, M.; Rowland, L. Effect of cold and drought stress on blueberry dehydrin accumulation. J. Hortic. Sci. Biotechnol. 2001, 76, 549–556. [Google Scholar]
- Ergin, S.; Kesici, M.; Gulen, H. Changes in H2O2 and peroxidase activities in strawberry plants under heat stress. Harran TarımveGıdaBilimleri Derg. 2012, 16, 25–35. [Google Scholar]
- Gulen, H.; Eris, A. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 2004, 166, 739–744. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, S.D.; Jang, C.S. Oryza sativa heat-induced RING finger protein 1 (OsHIRP1) positively regulates plant response to heat stress. Plant Mol. Biol. 2019, 99, 545–559. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, W.; Zhao, X.; Zhu, L.; Fu, B.; Li, Z. DNA methylation alterations of rice in response to cold stress. Plant Omics J. 2011, 4, 364–369. [Google Scholar]
- De Azevedo Neto, A.D.; Prisco, J.T.; Eneas-Filho, J.; de Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Kilasi, N.L.; Singh, J.; Vallejos, C.E.; Ye, C.; Jagadish, S.K.; Kusolwa, P.; Rathinasabapathi, B. Heat stress tolerance in rice (Oryza sativa L.): Identification of quantitative trait loci and candidate genes for seedling growth under heat stress. Front. Plant Sci. 2018, 9, 1578. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.; Quiles, M.J. The effects of cold stress on photosynthesis in Hibiscus plants. PLoS ONE 2015, 10, e0137472. [Google Scholar] [CrossRef] [PubMed]
- Munoz, R.; Quiles, M.J. Water deficit and heat affect the tolerance to high illumination in hibiscus plants. Int. J. Mol. Sci. Mar. 2013, 7, 5432–5444. [Google Scholar] [CrossRef]
- Ni, L.; Wang, Z.; Liu, X.; Wu, S.; Hua, J.; Yin, Y.; Li, H.; Gu, C. Transcriptome Analysis of Salt Stress in Hibiscus hamabo Sieb. et Zucc Based on Pacbio Full-Length Transcriptome Sequencing. Int. J. Mol. Sci. 2022, 23, 138. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.; Djalovic, I.; Siddique, K.H. Effect of cold stress on photosynthetic traits, carbohydrates, morphology, and anatomy in nine cultivars of Stevia rebaudiana. Front. Plant Sci. 2018, 9, 1430. [Google Scholar] [CrossRef]
- Debnath, M.; Ashwath, N.; Midmore, D.J. Physiological and morphological responses to abiotic stresses in two cultivars of Stevia rebaudiana (Bert.) Bertoni. S. Afr. J. Bot. 2019, 123, 124–132. [Google Scholar] [CrossRef]
- Karimi, M.; Ahmadi, A.; Hashemi, J.; Abbasi, A.; Tavarini, S.; Pompeiano, A.; Angelini, L.G. The positive role of steviol glycosides in stevia (Stevia rebaudianaBertoni) under drought stress condition. Plant Biosyst. 2016, 150, 1323–1331. [Google Scholar] [CrossRef]
- Pandey, M.; Chikara, S.K. Effect of Salinity and Drought Stress on Growth Parameters, Glycoside Content and Expression Level of Vital Genes in Steviol Glycosides Biosynthesis Pathway of Stevia rebaudiana (Bertoni). Int. J. Genet. 2015, 7, 153–160. [Google Scholar]
- Li, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Cold priming drives the sub-cellular antioxidant systems to protect photosynthetic electron transport against subsequent low temperature stress in winter wheat. Plant Physiol. Biochem. 2014, 82, 34–43. [Google Scholar] [CrossRef]
- Ni, Z.; Li, H.; Zhao, Y.; Peng, H.; Hu, Z.; Xin, M.; Sun, Q. Genetic improvement of heat tolerance in wheat: Recent progress in understanding the underlying molecular mechanisms. Crop J. 2018, 6, 32–41. [Google Scholar] [CrossRef]
- Schmidt, J.; Tricker, P.J.; Eckermann, P.; Kalambettu, P.; Garcia, M.; Fleury, D. Novel alleles for combined drought and heat stress tolerance in wheat. Front. Plant Sci. 2020, 10, 1800. [Google Scholar] [CrossRef]
- Hussain, N.; Ghaffar, A.; Zafar, Z.U.; Javed, M.; Shah, K.H.; Noreen, S.; Manzoor, H.; Iqbal, M.; Hassan, I.F.Z.; Bano, H.; et al. Identification of novel source of salt tolerance in local bread wheat germplasm using morpho-physiological and biochemical attributes. Sci. Rep. 2021, 11, 10854. [Google Scholar] [CrossRef]
- Omrani, S.; Arzani, A.; Esmaeilzadeh Moghaddam, M.; Mahlooji, M. Genetic analysis of salinity tolerance in wheat (Triticum aestivum L.). PLoS ONE 2022, 17, e0265520. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, W.; Fang, L.; Sun, X.; Su, L.; Liang, Z.; Wang, N.; Londo, J.P.; Li, S.; Xin, H. Genome-wide identification of WRKY family genes and their response to cold stress in Vitis Vinifera. BMC Plant Biol. 2014, 14, 103. [Google Scholar] [CrossRef]
- Yu, W.; Wang, L.; Zhao, R.; Sheng, J.; Zhang, S.; Li, R.; Shen, L. Knockout of SlMAPK3 enhances tolerance to heat stress involving ROS homeostasis in tomato plants. BMC Plant Biol. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Niron, H.; Barlas, N.; Salih, B.; Türet, M. Comparative Transcriptome, Metabolome, and Ionome Analysis of Two Contrasting Common Bean Genotypes in Saline Conditions. Front. Plant Sci. 2020, 11, 2007. [Google Scholar] [CrossRef]
- Stoeva, N.; Kaymakanova, M. Effect of salt stress on the growth and photosynthesis rate of bean plants (Phaseolus vulgaris L.). J. Cent. Eur. Agric. 2008, 9, 385–391. [Google Scholar]
- Singh, M.; Khan, M.M.A.; Uddin, M.; Naeem, M.; Qureshi, M.I. Proliferating effect of radiolytically depolymerized carrageenan on physiological attributes, plant water relation parameters, essential oil production and active constituents of Cymbopogon flexuosus Steud. under drought stress. PLoS ONE 2017, 12, e0180129. [Google Scholar] [CrossRef]
- Shalata, A.; Tal, M. The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol. Plant. 1998, 104, 169–174. [Google Scholar] [CrossRef]
- Maggio, A.; Raimondi, G.; Martino, A.; De Pascale, S. Salt stress response in tomato beyond the salinity tolerance threshold. Environ. Exp. Bot. 2007, 59, 276–282. [Google Scholar] [CrossRef]
- Schubert, S.; Serraj, R.; Plies-Balzer, E.; Mengel, K. Effect of drought stress on growth, sugar concentrations and amino acid accumulation in N2-fixing alfalfa (Medicago sativa). J. Plant Physiol. 1995, 146, 541–546. [Google Scholar] [CrossRef]
- Khan, F.; Hakeem, K.R. Cell signaling during drought and salt stress. In Plant Signaling: Understanding the Molecular Crosstalk; Springer: New Delhi, India, 2014; pp. 227–239. [Google Scholar]
- Abhinandan, K.; Skori, L.; Stanic, M.; Hickerson, N.M.; Jamshed, M.; Samuel, M.A. Abiotic stress signaling in wheat—An inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front. Plant Sci. 2018, 9, 734. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture—Climate Change, Agriculture and Food Security; Food and Agricultural Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar] [CrossRef]
- Maas, E.V.; Honman, G.J. Crop salt tolerance—Current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Yildiz, C.; Acikgoz, H. A kernel extreme learning machine-based neural network to forecast very short-term power output of an on-grid photovoltaic power plant. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 43, 395–412. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Li, X.N.; Pu, H.C.; Liu, F.L.; Zhou, Q.; Cai, J.; Dai, T.B.; Cao, W.; Jiang, D. Winter wheat photosynthesis and grain yield responses to spring freeze. Agron. J. 2015, 107, 1002–1010. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought enacts on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, D.F.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 45–756. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, C.; Li, H.; Ouyang, B.; Wang, T.; Zhang, J.; Wang, X.; Ye, Z. Overexpression of ShDHN, a dehydrin gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses in tomato. Plant Sci. 2015, 231, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Dowgert, M.F.; Steponkus, P.L. Behavior of the plasma membrane of isolated protoplasts during a freeze-thaw cycle. Plant Physiol. 1984, 75, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Pearce, R.S. Plant freezing and damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Q.; Wang, S.; Hong, Y.; Wang, Z. Rice and cold stress: Methods for its evaluation and summary of cold tolerance-related quantitative trait loci. Rice 2014, 7, 1–12. [Google Scholar] [CrossRef]
- Liliane, T.N.; Charles, M.S. Factors affecting yield of crops. In Agronomy-Climate Change Food Security; Amanullah, Ed.; Intech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Xiao, J.; Bao, F. Effects of chilling on the structure, function and development of chloroplasts. Front. Plant Sci. 2018, 22, 1715. [Google Scholar] [CrossRef]
- Mamun, E.A.; Alfred, S.; Cantrill, L.C.; Overall, R.L.; Sutton, B.G. Effects of chilling on male gametophyte development in rice. Cell Biol. Int. 2006, 30(7), 583–591. [Google Scholar] [CrossRef]
- Fahad, S.; Chen, Y.; Saud, S.; Wang, K.; Xiong, D.; Chen, C.; Wu, C.; Shah, F.; Nie, L.; Huang, J. Ultraviolet radiation effect on photosynthetic pigments, biochemical attributes, antioxidant enzyme activity and hormonal contents of wheat. J. Food Agric. Environ. 2013, 11, 1635–1641. [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 18, 169–181. [Google Scholar] [CrossRef]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid response of plants to temperature changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef]
- Rauwane, M.; Ntushelo, K. Understanding biotic stress and hormone signalling in cassava (Manhot esculenta): Potential for using hyphenated analytical techniques. Appl. Sci. 2020, 20, 8152. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Mani, A.; Sankaranarayanan, K. Heavy Metal and Mineral Element-Induced Abiotic Stress in Rice Plant. In Rice Crop—Current Developments; Shah, F., Khan, Z.H., Iqbal, A., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Hewedy, O.A.; Abdel Lateif, K.S.; Seleiman, M.F.; Shami, A.; Albarakaty, F.M.; M El-Meihy, R. Phylogenetic diversity of Trichoderma strains and their antagonistic potential against soil-borne pathogens under stress conditions. Biology 2020, 9, 189. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Plieth, C.; Hansen, U.P.; Knight, H.; Knight, M.R. Temperature sensing by plants: The primary characteristics of signal perception and calcium response. Plant J. 1999, 18, 491–497. [Google Scholar] [CrossRef]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 1996, 8, 489–503. [Google Scholar]
- Knight, M.R.; Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytologist. 2012, 195, 737–751. [Google Scholar] [CrossRef]
- Tyystjarvi, E. Photoinhibition of photosystem II. Int. Rev. Cell Mol. Biol. 2013, 300, 243–303. [Google Scholar]
- Xiong, L.; Zhu, J.K. Abiotic stress signal transduction in plants: Molecular and genetic perspectives. Physiol. Plant. 2001, 112, 152–166. [Google Scholar] [CrossRef]
- Sha Valli Khan, P.S.; Nagamallaiah, G.V.; Dhanunjay Rao, M.; Sergeant, K.; Hausman, J.F. Abioticstress tolerance in plants insights from proteomics. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Ed.; Elsevier Academic Press: London, UK, 2014; Volume 2, pp. 23–68. [Google Scholar]
- Skalak, J.; Nicolas, K.L.; Vankova, R.; Hejatko, J. Signal integration in plant abiotic stress responses via multistep phosphorelay signaling. Front. Plant Sci. 2021, 12, 644823. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K.; et al. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 15, 6. [Google Scholar] [CrossRef]
- Juven-Gershon, T.; Hsu, J.Y.; Theisen, J.W.; Kadonaga, J.T. The RNA polymerase II core promoter—The gateway to transcription. Curr. Opin. Cell Biol. 2008, 20, 253–259. [Google Scholar] [CrossRef]
- Hu, H.; Dai, M.; Yao, J.; Xiao, B.; Li, X.; Zhang, Q.; Xiong, L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 12987–12992. [Google Scholar] [CrossRef]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY transcription factors: Molecular regulation and stress responses in plants. Front. Plant Sci. 2016, 3, 760. [Google Scholar] [CrossRef]
- Wu, X.; Shiroto, Y.; Kishitani, S.; Ito, Y.; Toriyama, K. Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep. 2009, 28, 21–30. [Google Scholar] [CrossRef]
- Singh, D.; Laxmi, A. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015, 6, 895. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively. Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.; Bargues, M.; Terol, J.; Perez-Alonso, M.; Salinas, J. The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression is regulated by low temperature but not by abscisic acid or dehydration. Plant Physiol. 1999, 119, 463–470. [Google Scholar] [CrossRef]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Floris, M.; Mahgoub, H.; Lanet, E.; Robaglia, C.; Menand, B. Posttranscriptional regulation of gene expression in plants during abiotic stress. Int. J. Mol. Sci. 2009, 10, 3168–3185. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kapoor, A.; Zhu, J.; Zhu, J.K. Stabilized1, a stress-upregulated nuclear protein, is required for pre-mRNA splicing, mRNA turnover, and stress tolerance in Arabidopsis. Plant Cell 2006, 18, 1736–1749. [Google Scholar] [PubMed]
- Guan, Q.; Wu, J.; Zhang, Y.; Jiang, C.; Liu, R.; Chai, C.; Zhu, J.A. Dead box RNA helicase is critical for pre-mRNA splicing, cold-responsive gene regulation, and cold tolerance in Arabidopsis. Plant Cell 2013, 25, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Calixto, C.P.G.; Guo, W.B.; James, A.B.; Tzioutziou, N.A.; Entizne, J.C.; Panter, P.E.; Knight, H.; Nimmo, H.G.; Zhang, R.; Brown, J.W. Rapid and dynamic alternative splicing impacts the Arabidopsis cold response transcriptome. Plant Cell 2018, 30, 1424–1444. [Google Scholar]
- Sunkar, R.; Kapoor, A.; Zhu, J.K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 2006, 18, 2051–2065. [Google Scholar]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar]
- Yoshida, R.; Umezawa, T.; Mizoguchi, T.; Takahashi, S.; Takahashi, F.; Shinozaki, K. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J. Biologic. Chem. 2006, 281, 5310–5318. [Google Scholar] [CrossRef]
- Kim, J.M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 2015, 6, 114. [Google Scholar] [CrossRef]
- To, T.K.; Nakaminami, K.; Kim, J.M.; Morosawa, T.; Ishida, J.; Tanaka, M.; Yokoyama, S.; Shinozaki, K.; Seki, M. Arabidopsis HDA6 is required for freezing tolerance. Biochem. Biophys. Res. Commun. 2011, 406, 414–419. [Google Scholar] [CrossRef]
- Roy, D.; Paul, A.; Roy, A.; Ghosh, R.; Ganguly, P.; Chaudhuri, S. Deferential acetylation of histone H3 at the regulatory region of OsDREB1b promoter facilitates chromatin remodelling and transcription activation during cold stress. PLoS ONE 2004, 9, e100343. [Google Scholar]
- Hu, Y.; Zhang, L.; Zhao, L.; Li, J.; He, S.B.; Zhou, K.; Yang, F.; Huang, M.; Jiang, L.; Li, L. Trichostatin A selectively suppresses the cold-induced transcription of the ZmDREB1 gene in maize. PLoS ONE 2011, 6, e22132. [Google Scholar] [CrossRef]
- He, X.J.; Hsu, Y.F.; Zhu, S.H.; Liu, H.L.; Pontes, O.; Zhu, J.H.; Cui, X.; Wang, C.S.; Zhu, J.K. A conserved transcriptional regulator is required for RNA-directed DNA methylation and plant development. Genes Dev. 2009, 23, 2717–2722. [Google Scholar] [CrossRef]
- Chan, Z.L.; Wang, Y.P.; Cao, M.J.; Gong, Y.H.; Mu, Z.X.; Wang, H.Q.; Hu, Y.; Deng, X.; He, X.J.; Zhu, J.K. RDM4 modulates cold stress resistance in Arabidopsis partially through the CBF-mediated pathway. New Phytol. 2016, 209, 1527–1539. [Google Scholar] [CrossRef]
- Sunkar, R.; Bartels, D.; Kirch, H.H. Overexpression of a stress-inducible dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J. 2003, 35, 452–464. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, 165–183. [Google Scholar] [CrossRef]
- Serrano, R.; Gaxiola, R.; Rios, G.; Forment, J.; Vicente, O.; Ros, R. Salt stress proteins identified by a functional approach in yeast. Monatshefte Chem./Chem. Mon. 2003, 134, 1445–1464. [Google Scholar] [CrossRef]
- Fahad, S.; Nie, L.; Chen, Y.; Wu, C.; Xiong, D.; Saud, S.; Hongyan, L.; Cui, K.; Huang, J. Crop plant hormones and environmental stress. Sustain. Agric. Rev. 2015, 15, 371–400. [Google Scholar]
- Garay-Arroyo, A.; Colmenero-Flores, J.M.; Garciarrubio, A.; Covarrubias, A.A. Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J. Biol. Chem. 2000, 275, 5668–5674. [Google Scholar] [CrossRef]
- Fahad, S.; Bano, A. Effect of salicylic acid on physiological and biochemical characterization of maize grown in the saline area. Pak. J. Bot. 2012, 44, 1433–1438. [Google Scholar]
- Eimer, M. Transgenic drought- and salt-tolerant plants. Genet. Eng. Newsl. 2004, 15, 1–14. [Google Scholar]
- Serrano, R.; Montesinos, C. Molecular bases of desiccation tolerance in plant cells and potential applications in food dehydration. Food Sci. Technol. Int. 2003, 9, 157–161. [Google Scholar] [CrossRef]
- Quintero, F.J.; Ohta, M.; Shi, H.; Zhu, J.K.; Pardo, J.M. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Natl. Acad. Sci. USA 2002, 25, 9061–9066. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef]
- Sandrini, M.; Nerva, L.; Sillo, F.; Balestrini, R.; Chitarra, W.; Zampieri, E. Abiotic stress and belowground microbiome: The potential of omics approaches. Int. J. Mol. Sci. 2022, 23, 1091. [Google Scholar] [CrossRef]
- Antoine, S.; Heriche, M.; Boussageon, R.; Noceto, P.A.; van Tuinen, D.; Wipf, D.; Courty, P.E. A historical perspective on mycorrhizal mutualism emphasizing arbuscular mycorrhizas and their emerging challenges. Mycorrhiza 2021, 31, 637–653. [Google Scholar]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Boopathi, T.; Manivannan, P. Comprehensive assessment of ameliorative effects of AMF in alleviating abiotic stress in tomato plants. J. Fungi 2021, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Kosova, K.; Vitamvas, P.; Urban, M.O.; Prasil, I.T. Plant proteome responses to salinity Stress-Comparison of glycophytes and halophytes. Funct. Plant Biol. 2013, 40, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Peng, F.; Tedeschi, A.; Xue, X.; Wang, T.; Liao, J.; Zhang, W.; Huang, C. Do halophytes and glycophytes differ in their interactions with arbuscular mycorrhizal fungi under salt stress? A meta-analysis. Bot. Stud. 2020, 61, 13. [Google Scholar] [CrossRef]
- Auge, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: A meta-analysis. Front. Plant Sci. 2014, 5, 562. [Google Scholar]
- Evelin, H.; Giri, B.; Kapoor, R. Ultrastructural evidence for AMF mediated salt stress mitigation in Trigonella foenum-graecum. Mycorrhiza 2013, 23, 71–86. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Eroglu, C.G.; Cabral, C.; Ravnskov, S.; Bak Topbjerg, H.; Wollenweber, B. Arbuscular mycorrhiza influences carbon-use efficiency and grain yield of wheat grown under pre- and post-anthesis salinity stress. Plant Biol. 2020, 22, 863–871. [Google Scholar] [CrossRef]
- Sharma, K.; Gupta, S.; Thokchom, S.D.; Jangir, P.; Kapoor, R. Arbuscular mycorrhiza-mediated regulation of polyamines and aquaporins during abiotic stress: Deep insights on the recondite players. Front. Plant Sci. 2021, 12, 1072. [Google Scholar] [CrossRef]
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Di Serio, C.; Maserti, B.; Guerrieri, E.; et al. The association with two different arbuscular mycorrhizal fungi differently affects water stress tolerance in tomato. Front. Plant Sci. 2018, 9, 1480. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef]
- Rivero, J.; Alvarez, D.; Flors, V.; Azcon-Aguilar, C.; Pozo, M.J. Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol. 2018, 220, 1322–1336. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.K.Z.; Minakshi, G.; Reddy, G.; Venkateswarlu, B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Arshad, M.; Sharoona, B.; Mahmood, T. Inoculation with Pseudomonas spp. containing ACC deaminase partially eliminate the effects of drought stress on growth, yield and ripening of pea (Pisum sativum L.). Pedosphere 2008, 18, 611–620. [Google Scholar] [CrossRef]
- Creus, C.M.; Sueldo, R.J.; Barassi, C.A. Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field. Can. J. Bot. 2004, 82, 273–281. [Google Scholar] [CrossRef]
- Cho, E.K.; Hong, C.B. Over-expression of tobacco NtHSP70-1 contributes to drought-stress tolerance in plants. Plant Cell Rep. 2006, 25, 349–358. [Google Scholar] [CrossRef]
- Rodriguez, A.A.; Stella, A.M.; Storni, M.M.; Zulpa, G.; Zaccaro, M.C. Effect of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2006, 2, 7. [Google Scholar] [CrossRef]
- Barka, E.A.; Nowak, J.; Clement, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium Burkholderiaphytofirmans strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Sa, T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 2007, 69, 220–228. [Google Scholar] [CrossRef]
- SaravanaMartins, S.J.; Rocha, G.A.; De Melo, H.C.; De Castro Georg, R.; Ulhoa, C.J.; De Campos Dianese, É.; Oshiquiri, L.H.; da Cunha, M.G.; da Rocha, M.R.; de Araújo, L.G.; et al. Plant-associated bacteria mitigate drought stress in soybean. Environ. Sci. Pollut. Res. 2018, 25, 13676–13686. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Burity, H.A.; Martinez, C.R.; Chanway, C.P. Alleviation of drought stress in common bean (Phaseolus vulgaris L.) by coinoculation with Paenibacilluspolymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008, 40, 182–188. [Google Scholar] [CrossRef]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Plant growth promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water stressed plants. Funct. Plant. Biol. 2008, 35, 141–151. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Kishore, N.; Rao, L.V.; Venkateswarlu, B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fert. Soils 2009, 46, 45–55. [Google Scholar] [CrossRef]
- Marulanda, A.; Porcel, R.; Barea, J.M.; Azcon, R. Drought tolerance and antioxidant activities in lavender plants colonized by native drought tolerant or drought sensitive Glomus species. Microb. Ecol. 2007, 54, 543–552. [Google Scholar] [CrossRef]
- McLellan, C.A.; Turbyville, T.J.; Wijeratne, K.; Kerschen, A.; Vierling, E.; Queitsch, C.; Whiteshell, L.; Gunatilaka, A.A.L. A rhizosphere fungus enhances Arabidopsis thermotolerance through production of an HSP90 inhibitor. Plant Physiol. 2007, 145, 174–182. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.S.; Sun, Y.; Dowd, S.E.; Shi, H.; Paré, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef]
- Yao, L.X.; Wu, Z.S.; Zheng, Y.Y.; Kaleem, I.; Li, C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Daei, G.; Ardekani, M.R.; Rejali, F.; Teimuri, S.; Miransari, M. Alleviation of salinity stress on wheat yield, yield components, and nutrient uptake using arbuscular mycorrhizal fungi under field conditions. J. Plant Physiol. 2009, 66, 617–625. [Google Scholar] [CrossRef]
- Lazzarotto, P.; Calanca, P.; Semenov, M.; Fuhrer, J. Transient responses to increasing CO2 and climate change in an unfertilized grass clover sward. Clim. Res. 2010, 41, 221–232. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, S.D. Induction of drought stress resistance by multifunctional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- Timmusk, S.; El-Daim, I.A.A.; Copolovici, L.; Tanilas, T.; Kannaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed]
- del Amor, F.; Cuadra-Crespo, P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2012, 39, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderiaphytofirmans PsJN and Enterobacter sp FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Kasotia, A.; Varma, A.; Choudhary, D.K. Pseudomonas-mediated mitigation of salt stress and growth promotion in Glycine max. Agric. Res. 2015, 4, 31–41. [Google Scholar] [CrossRef]
- Plociniczak, T.; Sinkkonen, A.; Romantschuk, M.; Piotrowska-seget, Z. Characterization of Enterobacter intermedius MH8b and its use for the enhancement of heavy metal uptake by Sinapsis alba L. Appl. Soil Ecol. 2013, 63, 1–7. [Google Scholar] [CrossRef]
- Wang, C.; Yang, W.; Wang, C.; Gu, C.; Niu, D.; Liu, H.X.; Wang, Y.P.; Guo, J.H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth promoting rhizobacterium strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef]
- Mathew, D.C.; Ho, Y.N.; Gicana, R.G.; Mathew, G.M.; Chien, M.C.; Huang, C.C. A rhizosphere-associated symbiont, Photobacterium spp. strain MELD1, and its targeted synergistic activity for phytoprotection against mercury. PLoS ONE 2015, 10, e0121178. [Google Scholar] [CrossRef]
- Adediran, G.A.; Ngwenya, B.T.; Mosselmans, J.F.W.; Heal, K.V. Bacteria–zinc co-localization implicates enhanced synthesis of cysteine-rich peptides in zinc detoxification when Brassica juncea is inoculated with accumulation in N2-fixing alfalfa (Medicago sativa). J. Plant. Physiol. 2016, 146, 541–546. [Google Scholar]
- Jha, Y.; Sablok, G.; Subbarao, N.; Sudhakar, R.; Fazil, M.H.U.T.; Subramanian, R.B.; Squartini, A.; Kumar, S. Bacterial-induced expression of RAB18 protein in Orzya sativa salinity stress and insights into molecular interaction with GTP ligand. J. Mol. Recognit. 2014, 27, 521–527. [Google Scholar] [CrossRef]
- Arun, K.D.; Sabarinathan, K.G.; Gomathy, M.; Kannan, R.; Balachandar, D. Mitigation of drought stress in rice crop with plant growth-promoting abiotic stress-tolerant rice phyllosphere bacteria. J. Basic Microbiol. 2020, 60, 768–786. [Google Scholar] [CrossRef]
- Ashry, N.M.; Alaidaroos, B.A.; Mohamed, S.A.; Badr, O.A.; El-Saadony, M.T.; Esmael, A. Utilization of drought-tolerant bacterial strains isolated from harsh soils as a plant growth-promoting rhizobacteria (PGPR). Saudi J. Biol. Sci. 2022, 29, 1760–1769. [Google Scholar] [CrossRef]
- Filgueiras, L.; Silva, R.; Almeida, I.; Vidal, M.; Baldani, J.I.; Meneses, C.H.S.G. Gluconacetobacterdiazotrophicus mitigates drought stress in Oryza sativa L. Plant Soil 2020, 451, 57–73. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Sheikh, I.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Amelioration of drought stress in Foxtail millet (Setariaitalica L.) by P-solubilizing drought-tolerant microbes with multifarious plant growth promoting attributes. Environ. Sustain. 2020, 3, 23–34. [Google Scholar] [CrossRef]
- Li, H.; Guo, Q.; Jing, Y.; Liu, Z.; Zheng, Z.; Sun, Y.; Xue, Q.; Lai, H. Application of Streptomyces pactum Act12 enhances drought resistance in wheat. J. Plant Growth Regul. 2020, 39, 122–132. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2016, 11, e1071004. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Silva, H.; Cunha, A. Siderophore-producing rhizobacteria as a promising tool for empowering plants to cope with iron limitation in saline soils: A review. Pedosphere 2019, 29, 409–420. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 2019, 9, 5855. [Google Scholar] [CrossRef]
- Chaudhary, D.; Sindhu, S.S. Amelioration of salt stress in chickpea (Cicer arietinum L.) by coinculation of ACC deaminase-containing rhizospheric bacteria with Mesorhizobium strains. Legume Res. 2017, 40, 80–86. [Google Scholar] [CrossRef]
- Marulanda, A.; Azcón, R.; Chaumont, F.; Ruiz-Lozano, J.M.; Aroca, R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 2010, 232, 533–543. [Google Scholar] [CrossRef]
- Balestrini, R.; Brunetti, C.; Chitarra, W.; Nerva, L. Photosynthetic traits and nitrogen uptake in crops: Which is the role of arbuscular mycorrhizal fungi? Plants 2020, 9, 1105. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wu, Q.S.; Kuca, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 23, 50–57. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Fiorilli, V.; Vallino, M.; Biselli, C.; Faccio, A.; Bagnaresi, P.; Bonfante, P. Host and non-host roots in rice: Cellular and molecular approaches reveal differential responses to arbuscular mycorrhizal fungi. Front. Plant Sci. 2015, 6, 636. [Google Scholar] [CrossRef]
- Fiorilli, V.; Catoni, M.; Miozzi, L.; Novero, M.; Accotto, G.P.; Lanfranco, L. Global and cell-type gene expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus. New Phytol. 2009, 184, 975–987. [Google Scholar] [CrossRef]
- Balestrini, R.; Salvioli, A.; Dal Molin, A.; Novero, M.; Gabelli, G.; Papparelli, E.; Marroni, F.; Bonfante, P. Impact of an arbuscular mycorrhizal fungus versus a mixed microbial inoculum on the transcriptome reprogramming of grapevine roots. Mycorrhiza 2017, 7, 417–430. [Google Scholar] [CrossRef]
- Fiorilli, V.; Vannini, C.; Ortolani, F.; Garcia-Seco, D.; Chiapello, M.; Novero, M.; Domingo, G.; Terzi, V.; Morcia, C.; Bagnaresi, P.; et al. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci. Rep. 2018, 8, 9625. [Google Scholar] [CrossRef]
- Balestrini, R.; Rosso, L.C.; Veronico, P.; Melillo, M.T.; De Luca, F.; Fanelli, E.; Colagiero, M.; Salvioli, A.; Ciancio, A.; Pentimone, I. Transcriptomic responses to water deficit and nematode infection in mycorrhizal tomato roots. Front. Microbiol. 2019, 10, 1807. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Maheswari, M.; Srinivasarao, C. 6 Actinomycetes as mitigators of climate change and abiotic stress. In Plant Growth Promoting Actinobacteria; Subramaniam, G., Arumugam, S., Rajendran, V., Eds.; Springer: Singapore, 2016; pp. 203–212. [Google Scholar]
- Yandigeri, M.S.; Meena, K.K.; Singh, D.; Malviya, N.; Singh, D.P.; Solanki, M.K.; Yadav, A.K.; Arora, D.K. Drought-tolerant endophytic Actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul. 2012, 68, 411–420. [Google Scholar] [CrossRef]
- Singh, R.P.; Pandey, D.M.; Jha, P.N.; Ma, Y. ACC deaminase producing rhizobacterium Enterobacter cloacae ZNP-4 enhance abiotic stress tolerance in wheat plant. PLoS ONE 2022, 17, e0267127. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.M.; El-Sabbagh, S.M.; El-Shouny, W.A.; Ebrahim, M.K.H. Physiological response of Zea mays to NaCl stress with respect to Azotobacter chroococcum and Streptomyces niveus. Pak. J. Biol. Sci. 2003, 6, 2073–2080. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Damodharan, K.; Yang, S.H.; Suh, J.W. Streptomyces sp. Strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J. Appl. Microbiol. 2014, 117, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, N.; Alikhani, H.A.; Pourbabaei, A.A.; Besharati, H. Effects of two new siderophore-producing rhizobacteria on growth and iron content of maize and canola plants. J. Plant Nutr. 2017, 40, 736–746. [Google Scholar] [CrossRef]
- Hasegawa, S.; Meguro, A.; Toyoda, K.; Nishimura, T.; Kunoh, H. Drought tolerance of tissue-cultured seedlings of mountain laurel (Kalmia latifolia L.) induced by an endophytic actinomycete II. Acceleration of callose accumulation and lignification. Actinomycetologica 2005, 19, 13–17. [Google Scholar] [CrossRef][Green Version]
- Johnston-Monje, D.; Lundberg, D.S.; Lazarovits, G.; Reis, V.M.; Raizada, M.N. Bacterial populations in juvenile maize rhizospheres originate from both seed and soil. Plant Soil 2016, 405, 337–355. [Google Scholar] [CrossRef]
- Genitsaris, S.; Stefanidou, N.; Leontidou, K.; Matsi, T.; Karamanoli, K.; Mellidou, I. Bacterial communities in the rhizosphere and phyllosphere of halophytes and drought-tolerant plants in mediterranean ecosystems. Microorganisms 2020, 8, 1708. [Google Scholar] [CrossRef]
- Ullah, M.A.; Mahmood, I.A.; Ali, A.; Nawaz, Q.; Sultan, T.; Zaman, B.U. Effect of inoculation methods of biozote-max (plant growth promoting rhizobacteria-pgpr) on growth and yield of rice under naturally salt-affected soil. Res. Plant Biol. 2017, 7, 24–26. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR mediated alterations in root traits: Way toward sustainable crop production. Front. Sustain. Food Syst. 2021, 4, 618230. [Google Scholar] [CrossRef]
- Vanamala, P.; Sultana, U.; Sindhura, P.; Gul, M.Z. Plant Growth-Promoting Rhizobacteria (PGPR): A Unique Strategy for Sustainable Agriculture. In Handbook of Research on Microbial Remediation and Microbial Biotechnology for Sustainable Soil; IGI Global: Hershey, PA, USA, 2021; pp. 332–357. [Google Scholar]
- Ansari, F.A.; Ahmad, I. Alleviating drought stress of crops through PGPR: Mechanism and application. In Microbial Interventions in Agriculture and Environment; Singh, D.P., Gupta, V.K., Prabha, R., Eds.; Springer: Singapore, 2019; pp. 341–358. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Shahid, M.A.; Nasim, W.; Babar, A. Interaction between PGPR and PGR for water conservation and plant growth attributes under drought condition. Biologia 2018, 73, 1083–1098. [Google Scholar] [CrossRef]
- Sinha, D.; Mukherjee, S.; Mahapatra, D. Multifaceted potential of Plant Growth Promoting Rhizobacteria (PGPR): An overview. In Handbook of Research on Microbial Remediation and Microbial Biotechnology for Sustainable Soil; Ahmad Malik, J., Ed.; IGI Global: Hershey, PA, USA, 2021; pp. 205–268. [Google Scholar] [CrossRef]
- Monteiro, G.; Nogueira, G.; Neto, C.; Nascimento, V.; Freitas, J. Promotion of Nitrogen Assimilation by Plant Growth-Promoting Rhizobacteria. In Nitrogen in Agriculture-Physiological, Agricultural and Ecological Aspects; Intech Open: London, UK, 2021. [Google Scholar]
- Jochum, M.D.; Mcwilliams, K.L.; Borrego, E.J.; Kolomiets, M.V.; Niu, G.; Pierson, E.A.; Jo, Y.K. Bioprospecting plant growth-promoting rhizobacteria that mitigate drought stress in grasses. Front. Microbiol. 2019, 10, 2106. [Google Scholar] [CrossRef]
- Bano, Q.; Ilyas, N.; Bano, A.; Zafar, N.; Akram, A.; Hassan, F. Effect of Azospirillum inoculation on maize (Zea mays L.) under drought stress. Pak. J. Bot. 2013, 45, 13–20. [Google Scholar]
- Kour, D.; Rana, K.L.; Sheikh, I.; Kumar, V.; Yadav, A.N.; Dhaliwal, H.S.; Saxena, A.K. Alleviation of drought stress and plant growth promotion by Pseudomonas libanensis EU-LWNA-33, a drought-adaptive phosphorus-solubilizing bacterium. Proc. Natl. Acad. Sci. India B Biol. Sci. 2020, 90, 785–795. [Google Scholar] [CrossRef]
- Zerrouk, I.Z.; Benchabane, M.; Khelifi, L.; Yokawa, K.; Ludwig-Muller, J.; Baluska, F. Pseudomonas strain isolated from date-palm rhizospheres improves root growth and promotes root formation in maize exposed to salt and aluminum stress. J. Plant Physiol. 2016, 191, 111–119. [Google Scholar] [CrossRef]
- Santos-Medellin, C.; Edwards, J.; Liechty, Z.; Nguyen, B.; Sundaresan, V. Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. MBio 2017, 8, e00764-17. [Google Scholar] [CrossRef]
- Priyanka, J.P.; Goral, R.T.; Rupal, K.S.; Saraf, M. Rhizospheric microflora: A natural alleviator of drought stress in agricultural crops. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Sayyed, R.Z., Ed.; Springer: Singapore, 2019; pp. 103–115. [Google Scholar] [CrossRef]
- Chiappero, J.; Del Rosario Cappellari, L.; Alderete, L.G.S.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Industr. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, I.C.; Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci. 2018, 64, 574–587. [Google Scholar] [CrossRef]
- Hanif, S.; Saleem, M.F.; Sarwar, M.; Irshad, M.; Shakoor, A.; Wahid, M.A.; Khan, H.Z. Biochemically triggered heat and drought stress tolerance in rice by proline application. J Plant Growth Regul. 2021, 40, 305–312. [Google Scholar] [CrossRef]
- Rana, R.A.; Siddiqui, M.N.; Skalicky, M.; Brestic, M.; Hossain, A.; Kayesh, E.; Popov, M.; Hejnak, V.; Gupta, D.R.; Mahmud, N.U.; et al. Prospects of nanotechnology in improving the productivity and quality of horticultural crops. Horticulturae 2021, 7, 332. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; Zia ur Rehman, M.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; Rehman, M.Z.; ur Qayyum, M.F.; Wang, H.; Rinklebe, J. Responses of wheat (Triticum aestivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol. Environ. Saf. 2019, 173, 156–164. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Ali, S.; Hafeez, M.; Khalid, S.; ur Rehman, M.Z.; Hussain, A.; Hussain, K.; Chatha, S.A.S.; Rizwan, M. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 2020, 238, 124681. [Google Scholar] [CrossRef]
- Gao, M.; Zhou, J.; Liu, H.; Zhang, W.; Hu, Y.; Liang, J.; Zhou, J. Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci. Total Environ. 2018, 631–632, 1100–1108. [Google Scholar] [CrossRef]
- Rehman, M.Z.; Rizwan, M.; Hussain, A.; Saqib, M.; Ali, S.; Sohail, M.I.; Shafiq, M.; Hafeez, F. Alleviation of cadmium (cd) toxicity and minimizing its uptake in wheat (Triticum aestivum) by using organic carbon sources in cd-spiked soil. Environ. Pollut. 2018, 241, 557–565. [Google Scholar] [CrossRef]
- Ashkavand, P.; Tabari, M.; Zarafshar, M.; Tomaskova, I.; Struve, D. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. For. Res. Pap. 2018, 76, 350–359. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Mohammadi, H.; Kariman, K. Nanosilicon-based recovery of barley (Hordeum vulgare) plants subjected to drought stress. Environ. Sci. Nano 2020, 7, 443–461. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
- Behboudi, F.; Tahmasebi-Sarvestani, Z.; Kassaee, M.Z.; Modarres-Sanavy, S.A.M.; Sorooshzadeh, A.; Mokhtassi-Bidgoli, A. Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. J. Plant Nutr. 2019, 42, 1439–1451. [Google Scholar] [CrossRef]
- Das, A.; Das, B. Nanotechnology a potential tool to mitigate abiotic stress in crop plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Sun, D.; Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.; Cahill, D.M. Delivery of abscisic acid to plants using glutathione responsive mesoporous silica nanoparticles. J. Nanosci. Nanotechnol. 2017, 18, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese nanoparticles control salinity-modulated molecular responses in Capsicum annuum L. through priming: A sustainable approach for agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T. Synergistic effect of zinc oxide nanoparticles and heat stress on the alleviation of transcriptional gene silencing in Arabidopsis thaliana. Bull. Environ. Contam. Toxicol. 2020, 104, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Asthir, B.; Kaur, G.; Kalia, A.; Sharma, A. Zinc oxide and titanium dioxide nanoparticles influence heat stress tolerance mediated by antioxidant defense system in wheat. Cereal Res. Commun. 2021, 49, 1–12. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Selem, E.; Desoky, E.S.M.; Fouda, S.E.; El-Tahan, A.M.; et al. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021, 28, 4461–4471. [Google Scholar] [CrossRef]
- Iqbal, M.; Raja, N.I.; Mashwani ZU, R.; Hussain, M.; Ejaz, M.; Yasmeen, F. Effect of silver nanoparticles on growth of wheat under heat stress. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 387–395. [Google Scholar] [CrossRef]
| Abiotic Stress Type | Mechanism and Key Parameters Studied | Plants/Crops | References |
|---|---|---|---|
| Cold stress | The CBF (C repeat binding factor) transcriptional cascade, CBF expression and CBF-independent regulons mediate the transcriptional regulation and pre-mRNA processing, export, and degradation involved in post-regulatory mechanisms | Arabidopsis | [18] |
| Low-temperature stress | Alter hormonal expression | [19] | |
| Heat and drought stress | Enhancing the accumulation of carbohydrates | [20] | |
| Heat stress | Autophagy plays a vital role in cellular homeostasis, metabolism, and other processes | [21] | |
| Heat stress | ceRNA networks are mediated by the differentially expressed circRNA, by the influence of various important genes, and participate in response to hydrogen peroxide, heat stress, and phytochrome signaling pathway | [22] | |
| Water stress | Lipid peroxidation decreases with scavenging reactive oxygen species and higher excitation energy dissolution due to photochemical quenching with reduced excitation pressure | [23] | |
| Drought stress | The physiological activities and antioxidant protective systems modulate CarMT gene overexpression | [24] | |
| Drought stress | H2S endogenous production rate increases and a noteworthy transcriptional reorganization of pertinent miRNAs | [25] | |
| Drought stress | A transmembrane potassium ions efflux as well as calcium and chloride ions influxes are induced due to endogenous hydrogen sulfides | [26] | |
| Cold and drought stress | Dehydrins concentrated in roots and stems | Blueberry | [27] |
| Heat stress | Lower accumulation of H2O2 and damage to cells | Strawberry | [28,29] |
| Salinity stress | Plant response is positively regulated due to OsH1RP1-ring finger protein 1 | Maize | [30] |
| Cold stress | Changes in DNA methylation | Rice | [31] |
| Heat stress | Lipid peroxidation as well as antioxidant enzymes in roots and leaves | [32] | |
| Drought stress | E3-ubiquitin breakdown | [16,33] | |
| Heat stress | Candidate genes as well as quantitative trait loci | [34] | |
| Cold stress | Linear electron transport chain is downregulated and PSII is repressed, as represented by the lowering in the PSII photochemistry efficiency along with electron transport efficiency | Hibiscus | [35] |
| Water deficit and heat stress | Contribution of ferredoxin-mediated cyclic pathway and chlororespiration | [36] | |
| Salinity stress | Simultaneous expression of variable expressed genes | [37] | |
| Cold stress | Enhances epidermal cell density, stomatal density and index, width of xylem vessel and phloem tissue and sclerenchyma | Candyleaf | [38] |
| Salt stress | Accumulation of biomass, ions concentration in tissue and steviolglycosides | [39] | |
| Drought stress | The use of steviol glycosides enhances the harvest index | [40] | |
| Salinity and drought stress | Sodium chloride serves as an activator, and mannitol works for the downregulation of genes involved in the steviol glycosides synthesis pathways that alter the steviol glycosides production | [41] | |
| Cold stress | Photosynthetic electron transport chain protection by the sub-cellular antioxidant system | Wheat | [42] |
| Heat and drought stress | Signaling of phytohormone and epigenetic control | [43,44] | |
| Salinity stress | Maintenance of osmoprotectants, photosynthetic activity and sodium/potassium ions ratio | [45,46] | |
| Cold stress | WRKY gene expression | Grapevine | [47] |
| Heat stress | HSPs genes, along with antioxidant enzyme expression | Tomato | [48] |
| Salinity stress | Reduced the accumulation of different ions such as sodium, magnesium, and zinc in leaves and roots | Commonbean | [49] |
| Drought stress | Enhances plant metabolism with water relation parameters, antioxidant enzyme water relation parameters, activities of antioxidant enzymes and yield per plant increases | Lemon grass | [50,51] |
| Salinity stress | Antioxidants in leaves and lipid peroxidation | Tomato | [52] |
| Salinity stress | Biomass production as well as stomatal conductance | [53] | |
| Drought stress | Sugar and amino acid content accumulation | Alfalfa | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saharan, B.S.; Brar, B.; Duhan, J.S.; Kumar, R.; Marwaha, S.; Rajput, V.D.; Minkina, T. Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants. Life 2022, 12, 1634. https://doi.org/10.3390/life12101634
Saharan BS, Brar B, Duhan JS, Kumar R, Marwaha S, Rajput VD, Minkina T. Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants. Life. 2022; 12(10):1634. https://doi.org/10.3390/life12101634
Chicago/Turabian StyleSaharan, Baljeet Singh, Basanti Brar, Joginder Singh Duhan, Ravinder Kumar, Sumnil Marwaha, Vishnu D. Rajput, and Tatiana Minkina. 2022. "Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants" Life 12, no. 10: 1634. https://doi.org/10.3390/life12101634
APA StyleSaharan, B. S., Brar, B., Duhan, J. S., Kumar, R., Marwaha, S., Rajput, V. D., & Minkina, T. (2022). Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants. Life, 12(10), 1634. https://doi.org/10.3390/life12101634










