Endothelial Dysfunction in COVID-19: Potential Mechanisms and Possible Therapeutic Options
Abstract
1. Introduction
2. Tropism of SARS-CoV-2 for Endothelium
3. Endothelial Dysfunction: Hypercoagulability and Inflammation
3.1. Hypercoagulability
3.2. Inflammation
4. Different Variants of SARS-CoV-2 and Their Impact on Endothelial Dysfunction
5. Pulmonary Manifestations and Pathophysiologic Mechanisms
6. Extrapulmonary Manifestations and Pathophysiology
6.1. Cardiac Manifestations
6.2. Renal Manifestations
6.3. Nervous System Involvement
6.4. Gastrointestinal Manifestations
6.5. Other Manifestations
7. Long Terms Consequences
8. Possible Therapeutic Options
8.1. Role of Heparin
8.2. Role of Corticosteroids
8.3. Role of RAAS Inhibitors
8.4. Role of Statins
8.5. Role of Antiviral Agents
8.6. Role of Monoclonal Antibodies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3CLpro | 3C-like protease |
| ACE2 | angiotensin-converting enzyme 2 |
| ADAM17 | ADAM metallopeptidase domain 17 |
| Ang | angiotensin |
| ARDS | acute respiratory distress syndrome |
| cGMP | cyclic guanosine monophosphate |
| COVID-19 | coronavirus disease 2019 |
| CRS | cytokine-release syndrome |
| DIC | disseminated intravascular coagulation |
| E | envelope |
| ECs | endothelial cells |
| ED | endothelial dysfunction |
| HIF-1α | hypoxia-inducible factor 1α |
| ICAM-1 | intracellular adhesion molecule 1 |
| ICU | intensive care unit |
| INFγ IV | Interferon-γ intravenous |
| LMWH | low-molecular-weight heparin |
| M | membrane-associated |
| MMP-2 | matrix metalloproteinase 2 |
| Mpro | major protease inhibitor |
| N | nucleocapsid |
| NETosis | neutrophil extracellular traps |
| NF-κB | nuclear factor κB |
| NO | nitric oxide |
| PAI-1 | plasminogen activator inhibitor 1 |
| PDGF | platelet-derived growth factor |
| PFT | pulmonary function test |
| POTS | postural tachycardia syndrome |
| RAAS | renin–angiotensin–aldosterone system |
| RBD | receptor-binding domain |
| ROS | reactive oxygen species |
| S | spike |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SDC1 | syndecan-1 |
| SpO2 | peripheral oxygen saturation |
| sVCAM1 | soluble vascular cell adhesion molecule 1 |
| TF | tissue factor |
| TLRs | Toll-like receptors |
| TMPRSS-2 | transmembrane protease serine protease-2 |
| TNF | tumor necrosis factor |
| uPA | urokinase plasminogen activator |
| URTI | upper respiratory tract infection |
| VEGF | vascular endothelial growth factor |
| VWF | von Willebrand factor |
| WHO | World Health Organization |
References
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ambrosino, P.; Calcaterra, I.; Molino, A.; Moretta, P.; Lupoli, R.; Spedicato, G.; Papa, A.; Motta, A.; Maniscalco, M.; Di Minno, M. Persistent Endothelial Dysfunction in Post-Acute COVID-19 Syndrome: A Case-Control Study. Biomedicines 2021, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.K.; Libby, P.; Ridker, P.M. COVID-19–A vascular disease. Trends Cardiovasc. Med. 2021, 31, 1–5. [Google Scholar] [CrossRef]
- Guervilly, C.; Burtey, S.; Sabatier, F.; Cauchois, R.; Lano, G.; Abdili, E.; Daviet, F.; Arnaud, L.; Brunet, P.; Hraiech, S.; et al. Circulating Endothelial Cells as a Marker of Endothelial Injury in Severe COVID-19. J. Infect. Dis. 2020, 222, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021, 18, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Fodor, A.; Tiperciuc, B.; Login, C.; Orasan, O.H.; Lazar, A.L.; Buchman, C.; Hanghicel, P.; Sitar-Taut, A.; Suharoschi, R.; Vulturar, R.; et al. Endothelial Dysfunction, Inflammation, and Oxidative Stress in COVID-19—Mechanisms and Therapeutic Targets. Oxidative Med. Cell. Longev. 2021, 2021, 8671713. [Google Scholar] [CrossRef]
- Corrons, J.L.V.; De Sanctis, V. The Multifacets of COVID-19 in Adult Patients: A Concise Clinical Review on Pulmonary and Extrapulmonary Manifestations for Healthcare Physicians: COVID-19 and Pulmonary and Extrapulmonary Manifestations. Acta Biomed. 2020, 91, e2020173. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.; Palomo, M.; Moreno-Castaño, A.B.; Fernández, S.; Torramadé-Moix, S.; Pascual, G.; Martinez-Sanchez, J.; Richardson, E.; Téllez, A.; Nicolas, J.M.; et al. Is the Endothelium the Missing Link in the Pathophysiology and Treatment of COVID-19 Complications? Cardiovasc. Drugs Ther. 2022, 36, 547–560. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [PubMed]
- Xu, J.; Xu, X.; Jiang, L.; Dua, K.; Hansbro, P.; Liu, G. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir. Res. 2020, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021, 296, 100306. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Ohtsu, H.; Suzuki, H.; Shirai, H.; Frank, G.D.; Eguchi, S. Angiotensin II signal transduction through the AT1 receptor: Novel insights into mechanisms and pathophysiology. Clin. Sci. 2007, 112, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Schroten, N.F.; Gaillard, C.A.J.M.; Van Veldhuisen, D.J.; Szymanski, M.K.; Hillege, H.L.; De Boer, R.A. New roles for renin and prorenin in heart failure and cardiorenal crosstalk. Hear. Fail. Rev. 2012, 17, 191–201. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Povlsen, A.L.; Grimm, D.; Wehland, M.; Infanger, M.; Krüger, M. The Vasoactive Mas Receptor in Essential Hypertension. J. Clin. Med. 2020, 9, 267. [Google Scholar] [CrossRef]
- Wu, C.-H.; Mohammadmoradi, S.; Chen, J.Z.; Sawada, H.; Daugherty, A.; Lu, H.S. Renin-Angiotensin System and Cardiovascular Functions. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e108–e116. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Tomaszewski, M.; Guzik, T.J. Binding of SARS-CoV-2 and angiotensin-converting enzyme 2: Clinical implications. Cardiovasc. Res. 2020, 116, e87–e89. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.A.V.; Millán, J.M.V.; Bonilla, E.F. Renin–angiotensin system: Basic and clinical aspects—A general perspective. Endocrinol. Diabetes Nutr. 2022, 69, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Nägele, M.P.; Haubner, B.; Tanner, F.C.; Ruschitzka, F.; Flammer, A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020, 314, 58–62. [Google Scholar] [CrossRef]
- Robles, J.P.; Zamora, M.; Adan-Castro, E.; Siqueiros-Marquez, L.; de la Escalera, G.M.; Clapp, C. The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin α5β1 and NF-κB signaling. J. Biol. Chem. 2022, 298, 101695. [Google Scholar] [CrossRef] [PubMed]
- Stahl, K.; Gronski, P.A.; Kiyan, Y.; Seeliger, B.; Bertram, A.; Pape, T.; Welte, T.; Hoeper, M.M.; Haller, H.; David, S. Injury to the Endothelial Glycocalyx in Critically Ill Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Del Turco, S.; Vianello, A.; Ragusa, R.; Caselli, C.; Basta, G. COVID-19 and cardiovascular consequences: Is the endothelial dysfunction the hardest challenge? Thromb. Res. 2020, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Benigni, A.; Casiraghi, F.; Ng, L.F.P.; Renia, L.; Remuzzi, G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021, 17, 46–64. [Google Scholar] [CrossRef]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef]
- Heitsch, H.; Brovkovych, S.; Malinski, T.; Wiemer, G. Angiotensin-(1-7)–Stimulated Nitric Oxide and Superoxide Release From Endothelial Cells. Hypertension 2001, 37, 72–76. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Bertolini, F.; Carriero, V.; Högman, M. Nitric oxide’s physiologic effects and potential as a therapeutic agent against COVID-19. J. Breath Res. 2020, 15, 014001. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.J.; Cañadas-Garre, M.; Cappa, R.C.; Maxwell, A.P.; McKnight, A.J. Genetic associations between genes in the renin-angiotensin-aldosterone system and renal disease: A systematic review and meta-analysis. BMJ Open 2019, 9, e026777. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, N.C.; Zhang, D.; Friedman, J.M.; Friedman, A.J. Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19. Nitric Oxide 2020, 103, 4–8. [Google Scholar] [CrossRef]
- Keyaerts, E.; Vijgen, L.; Chen, L.; Maes, P.; Hedenstierna, G.; Van Ranst, M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004, 8, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.H.; Tellier, C.; Michiels, C.; Ellertsen, I.; Dogné, J.-M.; Bäck, M. Effects of the dual TP receptor antagonist and thromboxane synthase inhibitor EV-077 on human endothelial and vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2013, 441, 393–398. [Google Scholar] [CrossRef]
- Gragnano, F.; Sperlongano, S.; Golia, E.; Natale, F.; Bianchi, R.; Crisci, M.; Fimiani, F.; Pariggiano, I.; Diana, V.; Carbone, A.; et al. The Role of von Willebrand Factor in Vascular Inflammation: From Pathogenesis to Targeted Therapy. Mediat. Inflamm. 2017, 2017, 5620314. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Ledderose, S.; Schulz, H.; et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated with Respiratory Failure and Coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef]
- Fitch-Tewfik, J.L.; Flaumenhaft, R. Platelet Granule Exocytosis: A Comparison with Chromaffin Cells. Front. Endocrinol. 2013, 4, 77. [Google Scholar] [CrossRef]
- Sut, C.; Tariket, S.; Aubron, C.; Aloui, C.; Hamzeh-Cognasse, H.; Berthelot, P.; Laradi, S.; Greinacher, A.; Garraud, O.; Cognasse, F. The Non-Hemostatic Aspects of Transfused Platelets. Front. Med. 2018, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J.; Cornwell, M.; Myndzar, K.; Rolling, C.C.; Xia, Y.; Drenkova, K.; Biebuyck, A.; Fields, A.T.; Tawil, M.; Luttrell-Williams, E.; et al. Platelets amplify endotheliopathy in COVID-19. Sci. Adv. 2021, 7, eabh2434. [Google Scholar] [CrossRef]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet gene expression and function in patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Azevedo-Quintanilha, I.G.; Palhinha, L.; Teixeira, L.; Barreto, E.A.; Pão, C.R.R.; Righy, C.; Franco, S.; Souza, T.M.L.; Kurtz, P.; et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 2020, 136, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Canzano, P.; Brambilla, M.; Porro, B.; Cosentino, N.; Tortorici, E.; Vicini, S.; Poggio, P.; Cascella, A.; Pengo, M.F.; Veglia, F.; et al. Platelet and Endothelial Activation as Potential Mechanisms Behind the Thrombotic Complications of COVID-19 Patients. JACC Basic Transl. Sci. 2021, 6, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 201, 1299–1300. [Google Scholar] [CrossRef]
- Spronk, H.M.; De Jong, A.M.; Crijns, H.J.; Schotten, U.; Van Gelder, I.C.; TenCate, H. Pleiotropic effects of factor Xa and thrombin: What to expect from novel anticoagulants. Cardiovasc. Res. 2014, 101, 344–351. [Google Scholar] [CrossRef]
- Norooznezhad, A.H.; Mansouri, K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19). Microvasc. Res. 2021, 137, 104188. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; Gomez, M.G.; García, M.A.P.; Carrasco, J.L.; Madrid, J.F.; Álvarez-Argüelles, H.; Díaz-Flores, L. Participation of Intussusceptive Angiogenesis in the Morphogenesis of Lobular Capillary Hemangioma. Sci. Rep. 2020, 10, 4987. [Google Scholar] [CrossRef]
- Jahani, M.; Rezazadeh, D.; Mohammadi, P.; Abdolmaleki, A.; Norooznezhad, A.; Mansouri, K. Regenerative Medicine and Angiogenesis; Challenges and Opportunities. Adv. Pharm. Bull. 2020, 10, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.L. Hypoxia–a key regulatory factor in tumour growth. Nat. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Ackermann, M.; Mentzer, S.J.; Kolb, M.; Jonigk, D. Inflammation and intussusceptive angiogenesis in COVID-19: Everything in and out of flow. Eur. Respir. J. 2020, 56, 2003147. [Google Scholar] [CrossRef]
- D’Alonzo, D.; De Fenza, M.; Pavone, V. COVID-19 and pneumonia: A role for the uPA/uPAR system. Drug Discov. Today 2020, 25, 1528–1534. [Google Scholar] [CrossRef]
- Chen, D.; Wu, X.; Yang, J.; Yu, L. Serum plasminogen activator urokinase receptor predicts elevated risk of acute respiratory distress syndrome in patients with sepsis and is positively associated with disease severity, inflammation and mortality. Exp. Ther. Med. 2019, 18, 2984–2992. [Google Scholar] [CrossRef]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e0016321. [Google Scholar] [CrossRef] [PubMed]
- Schultze, J.L.; Aschenbrenner, A.C. COVID-19 and the human innate immune system. Cell 2021, 184, 1671–1692. [Google Scholar] [CrossRef] [PubMed]
- Pons, S.; Arnaud, M.; Loiselle, M.; Arrii, E.; Azoulay, E.; Zafrani, L. Immune Consequences of Endothelial Cells’ Activation and Dysfunction During Sepsis. Crit. Care Clin. 2020, 36, 401–413. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992.e3–1000.e3. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Hariri, L.; Hardin, C.C. Covid-19, Angiogenesis, and ARDS Endotypes. N. Engl. J. Med. 2020, 383, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Atlanta GUSDoHaHS. Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions Centers for Disease Control and Prevention; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021.
- Moghaddar, M.; Radman, R.; Macreadie, I. Severity, Pathogenicity and Transmissibility of Delta and Lambda Variants of SARS-CoV-2, Toxicity of Spike Protein and Possibilities for Future Prevention of COVID-19. Microorganisms 2021, 9, 2167. [Google Scholar] [CrossRef]
- Ahmad, A.; Fawaz, M.A.M.; Aisha, A. A comparative overview of SARS-CoV-2 and its variants of concern. Infez. Med. 2022, 30, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Mungmunpuntipantip, R.; Wiwanitkit, V. Change in binding affinity with ACE2 receptor in beta, delta and omicron SARS CoV2 variants. Int. J. Physiol. Pathophysiol. Pharmacol. 2022, 14, 124–128. [Google Scholar] [PubMed]
- Ulloa, A.C.; Buchan, S.A.; Daneman, N.; Brown, K.A. Estimates of SARS-CoV-2 Omicron Variant Severity in Ontario, Canada. JAMA 2022, 327, 1286–1288. [Google Scholar] [CrossRef]
- Sheikh, A.; Kerr, S.; Woolhouse, M.; McMenamin, J.; Robertson, C.; Simpson, C.R.; Millington, T.; Shi, T.; Agrawal, U.; Hameed, S.S.; et al. Severity of omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): A national cohort study with nested test-negative design. Lancet Infect. Dis. 2022, 22, 959–966. [Google Scholar] [CrossRef]
- Bojkova, D.; Widera, M.; Ciesek, S.; Wass, M.N.; Michaelis, M.; Cinatl, J. Reduced interferon antagonism but similar drug sensitivity in Omicron variant compared to Delta variant of SARS-CoV-2 isolates. Cell Res. 2022, 32, 319–321. [Google Scholar] [CrossRef]
- McMahan, K.; Giffin, V.; Tostanoski, L.H.; Chung, B.; Siamatu, M.; Suthar, M.S.; Halfmann, P.; Kawaoka, Y.; Piedra-Mora, C.; Jain, N.; et al. Reduced pathogenicity of the SARS-CoV-2 omicron variant in hamsters. Med 2022, 3, 262.e4–268.e4. [Google Scholar] [CrossRef]
- Peacock, T.P.; Brown, J.C.; Zhou, J.; Thakur, N.; Newman, J.; Kugathasan, R.; Sukhova, K.; Kaforou, M.; Bailey, D.; Barclay, W.S. The SARS-CoV-2 variant, Omicron, shows rapid replication in human primary nasal epithelial cultures and efficiently uses the endosomal route of entry. BioRxiv 2022, preprint. [Google Scholar] [CrossRef]
- Bouzid, D.; Visseaux, B.; Kassasseya, C.; Daoud, A.; Fémy, F.; Hermand, C.; Truchot, J.; Beaune, S.; Javaud, N.; Peyrony, O.; et al. Comparison of Patients Infected with Delta Versus Omicron COVID-19 Variants Presenting to Paris Emergency Departments: A Retrospective Cohort Study. Ann. Intern. Med. 2022, 175, 831–837. [Google Scholar] [CrossRef]
- Corriero, A.; Ribezzi, M.; Mele, F.; Angrisani, C.; Romaniello, F.; Daleno, A.; Loconsole, D.; Centrone, F.; Chironna, M.; Brienza, N. COVID-19 Variants in Critically Ill Patients: A Comparison of the Delta and Omicron Variant Profiles. Infect. Dis. Rep. 2022, 14, 492–500. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, P.; Wei, Y.; Yue, H.; Wang, Y.; Hu, M.; Zhang, S.; Cao, T.; Yang, C.; Li, M.; et al. Histopathologic Changes and SARS-CoV-2 Immunostaining in the Lung of a Patient With COVID-19. Ann. Intern. Med. 2020, 172, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Wu, D.; Wang, X.; Xi, D.; Chen, T.; Chen, G.; Wang, H.; Lu, H.; Wang, M.; Zhu, L.; et al. The mechanism underlying extrapulmonary complications of the coronavirus disease 2019 and its therapeutic implication. Signal Transduct. Target. Ther. 2022, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, X.; Qu, J. Coronavirus disease 2019 (COVID-19): A clinical update. Front. Med. 2020, 14, 126–135. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Ottestad, W.; Seim, M.; Mæhlen, J.O. Covid-19 med stille hypoksemi. Tidsskr. Den Nor. Legeforening 2020, 140. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Korompoki, E.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020, 20, 493–506. [Google Scholar] [CrossRef]
- Channappanavar, R.; Zhao, J.; Perlman, S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014, 59, 118–128. [Google Scholar] [CrossRef]
- Huang, S.H. What We Know So Far (As of March 26, 2020) About COVID-19—An MRT Point of View. J. Med. Imaging Radiat. Sci. 2020, 51, 200–203. [Google Scholar] [CrossRef]
- Xu, B.; Fan, C.-Y.; Wang, A.-L.; Zou, Y.-L.; Yu, Y.-H.; He, C.; Xia, W.-G.; Zhang, J.-X.; Miao, Q. Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. J. Infect. 2020, 81, e51–e60. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, S.; Chen, Z. Advances in COVID-19: The virus, the pathogenesis, and evidence-based control and therapeutic strategies. Front. Med. 2020, 14, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.-Y. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, S.-Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Jingdong, M.A.; Guan, J.; Wang, M.; Song, J.; Tian, D.; Li, P. Manifestations of digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: A single-center, descriptive study. Chin. J. Dig. 2020, 40, E005. Available online: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-2296 (accessed on 17 August 2021).
- Shinu, P.; Morsy, M.A.; Deb, P.K.; Nair, A.B.; Goyal, M.; Shah, J.; Kotta, S. SARS CoV-2 Organotropism Associated Pathogenic Relationship of Gut-Brain Axis and Illness. Front. Mol. Biosci. 2020, 7, 606779. [Google Scholar] [CrossRef]
- Mak, J.W.Y.; Chan, F.K.L.; Ng, S.C. Probiotics and COVID-19: One size does not fit all. Lancet Gastroenterol. Hepatol. 2020, 5, 644–645. [Google Scholar] [CrossRef]
- Wander, P.; Epstein, M.; Bernstein, D. COVID-19 Presenting as Acute Hepatitis. Am. J. Gastroenterol. 2020, 115, 941–942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, L.; Wang, F.-S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef]
- Tao, Y.Y.; Tang, L.V.; Hu, Y. Treatments in the COVID-19 pandemic: An update on clinical trials. Expert Opin. Emerg. Drugs 2020, 25, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wong, S.H.; Lui, R.N.; Sung, J.J. Covid-19 and the digestive system. J. Gastroenterol. Hepatol. 2020, 35, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Steardo, L.; Zorec, R.; Verkhratsky, A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020, 229, e13473. [Google Scholar] [CrossRef]
- Tan, Y.-K.; Goh, C.; Leow, A.S.T.; Tambyah, P.A.; Ang, A.; Yap, E.-S.; Tu, T.-M.; Sharma, V.K.; Yeo, L.L.L.; Chan, B.P.L.; et al. COVID-19 and ischemic stroke: A systematic review and meta-summary of the literature. J. Thromb. Thrombolysis 2020, 50, 587–595. [Google Scholar] [CrossRef]
- Elrobaa, I.H.; New, K.J. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front. Public Health 2021, 9, 711616. [Google Scholar] [CrossRef] [PubMed]
- Beeching, N.F.T.; Fowler, R. Coronavirus Disease 2019 (COVID-19) BMJ Best Practice. 2020. Available online: https://bestpractice.bmj.com/topics/en-gb/3000168/emergingtxs (accessed on 11 April 2020).
- Douglas, K.A.; Douglas, V.P.; Moschos, M.M. Ocular Manifestations of COVID-19 (SARS-CoV-2): A Critical Review of Current Literature. Vivo 2020, 34, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Vindegaard, N.; Benros, M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain, Behav. Immun. 2020, 89, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.-H.; Feng, G.; Liu, W.; Targher, G.; Byrne, C.D.; Zheng, M. Extrapulmonary complications of COVID-19: A multisystem disease? J. Med. Virol. 2021, 93, 323–335. [Google Scholar] [CrossRef]
- Sharma, A.; Garcia, G.; Arumugaswami, V.; Svendsen, C.N. Human iPSC-Derived Cardiomyocytes are Susceptible to SARS-CoV-2 Infection. Preprint. bioRxiv 2020, 2020. [Google Scholar] [CrossRef]
- Boukhris, M.; Hillani, A.; Moroni, F.; Annabi, M.S.; Addad, F.; Ribeiro, M.H.; Mansour, S.; Zhao, X.; Ybarra, L.F.; Abbate, A.; et al. Cardiovascular Implications of the COVID-19 Pandemic: A Global Perspective. Can. J. Cardiol. 2020, 36, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Chen, T.; Mui, D.; Ferrari, V.; Jagasia, D.; Scherrer-Crosbie, M.; Chen, Y.; Han, Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart 2020, 106, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Aggarwal, G.; Cheruiyot, I.; Aggarwal, S.; Wong, J.; Lippi, G.; Lavie, C.J.; Henry, B.M.; Sanchis-Gomar, F. Association of Cardiovascular Disease with Coronavirus Disease 2019 (COVID-19) Severity: A Meta-Analysis. Curr. Probl. Cardiol. 2020, 45, 100617. [Google Scholar] [CrossRef]
- Maitz, T.; Parfianowicz, D.; Vojtek, A.; Rajeswaran, Y.; Vyas, A.V.; Gupta, R. COVID-19 Cardiovascular Connection: A Review of Cardiac Manifestations in COVID-19 Infection and Treatment Modalities. Curr. Probl. Cardiol. 2022, 101186. [Google Scholar] [CrossRef]
- Pranata, R.; Huang, I.; Raharjo, S.B. Incidence and impact of cardiac arrhythmias in coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Indian Pacing Electrophysiol. J. 2020, 20, 193–198. [Google Scholar] [CrossRef]
- Middeldorp, S.; Coppens, M.; Van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.A.; Bouman, C.C.S.; Beenen, L.F.M.; Kootte, R.S.; Heijmans, J.; et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, K.; Zuo, P.; Liu, Y.; Zhang, M.; Xie, S.; Zhang, H.; Chen, X.; Liu, C. Early decrease in blood platelet count is associated with poor prognosis in COVID-19 patients—Indications for predictive, preventive, and personalized medical approach. EPMA J. 2020, 11, 139–145. [Google Scholar] [CrossRef]
- Bao, C.; Tao, X.; Cui, W.; Yi, B.; Pan, T.; Young, K.H.; Qian, W. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Exp. Hematol. Oncol. 2020, 9, 16. [Google Scholar] [CrossRef]
- Malas, M.B.; Naazie, I.N.; Elsayed, N.; Mathlouthi, A.; Marmor, R.; Clary, B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. eClinicalMedicine 2020, 29–30, 100639. [Google Scholar] [CrossRef]
- Naicker, S.; Yang, C.-W.; Hwang, S.-J.; Liu, B.-C.; Chen, J.-H.; Jha, V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020, 97, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Laukkanen, J.A. Renal complications in COVID-19: A systematic review and meta-analysis. Ann. Med. 2020, 52, 345–353. [Google Scholar] [CrossRef]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D.; on behalf of the Northwell COVID-19 Research Consortium and the Northwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Wang, R.; Feng, Z.; Zhang, J.; Yang, H.; Tan, Y.; Wang, H.; Wang, C.; Liu, L.; et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat. Commun. 2021, 12, 2506. [Google Scholar] [CrossRef] [PubMed]
- Callard, F.; Perego, E. How and why patients made Long Covid. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef]
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann. Intern. Med. 2021, 174, 576–578. [Google Scholar] [CrossRef]
- Mumtaz, A.; Sheikh, A.A.E.; Khan, A.M.; Khalid, S.N.; Khan, J.; Nasrullah, A.; Sagheer, S.; Sheikh, A.B. COVID-19 Vaccine and Long COVID: A Scoping Review. Life 2022, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus; WHO: Geneva, Switzerland, 6 October 2021; Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 1 May 2022).
- Ahamed, J.; Laurence, J. Long COVID endotheliopathy: Hypothesized mechanisms and potential therapeutic approaches. J. Clin. Investig. 2022, 132, e161167. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Scherlinger, M.; Felten, R.; Gallais, F.; Nazon, C.; Chatelus, E.; Pijnenburg, L.; Mengin, A.; Gras, A.; Vidailhet, P.; Arnould-Michel, R.; et al. Refining “Long-COVID” by a Prospective Multimodal Evaluation of Patients with Long-Term Symptoms Attributed to SARS-CoV-2 Infection. Infect. Dis. Ther. 2021, 10, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Koc, H.C.; Xiao, J.; Liu, W.; Li, Y.; Chen, G. Long COVID and its Management. Int. J. Biol. Sci. 2022, 18, 4768–4780. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Mandal, S.; Barnett, J.; Brill, E.S.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, E.T.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef]
- Wichmann, D. Autopsy Findings and Venous Thromboembolism in Patients with COVID-19. Ann. Intern. Med. 2020, 173, 1030. [Google Scholar] [CrossRef]
- Raj, S.R.; Arnold, A.C.; Barboi, A.; Claydon, V.E.; Limberg, J.K.; Lucci, V.-E.M.; Numan, M.; Peltier, A.; Snapper, H.; Vernino, S.; et al. Long-COVID postural tachycardia syndrome: An American Autonomic Society statement. Clin. Auton. Res. 2021, 31, 365–368. [Google Scholar] [CrossRef]
- Sun, B.; Tang, N.; Peluso, M.J.; Iyer, N.S.; Torres, L.; Donatelli, J.L.; Munter, S.E.; Nixon, C.C.; Rutishauser, R.L.; Rodriguez-Barraquer, I.; et al. Characterization and Biomarker Analyses of Post-COVID-19 Complications and Neurological Manifestations. Cells 2021, 10, 386. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Belen-Apak, F.; Sarialioglu, F. The old but new: Can unfractioned heparin and low molecular weight heparins inhibit proteolytic activation and cellular internalization of SARS-CoV2 by inhibition of host cell proteases? Med. Hypotheses 2020, 142, 109743. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Arnold, K.; Pawlinski, R.; Key, N.S. Using heparin molecules to manage COVID-2019. Res. Pr. Thromb. Haemost. 2020, 4, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Gupta, A.; Jimenez, D.; Burton, J.R.; Der Nigoghossian, C.; Chuich, T.; Nouri, S.N.; Dreyfus, I.; Driggin, E.; et al. Pharmacological Agents Targeting Thromboinflammation in COVID-19: Review and Implications for Future Research. Thromb. Haemost. 2020, 120, 1004–1024. [Google Scholar] [CrossRef]
- Poterucha, T.J.; Libby, P.; Goldhaber, S.Z. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb. Haemost. 2017, 117, 437–444. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Sadeghipour, P.; Talasaz, A.H.; Rashidi, F.; Sharif-Kashani, B.; Beigmohammadi, M.T.; Farrokhpour, M.; Sezavar, S.H.; Payandemehr, P.; Dabbagh, A.; INSPIRATION Investigators. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 2021, 325, 1620–1630. [Google Scholar] [CrossRef]
- Sholzberg, M.; Tang, G.H.; Rahhal, H.; AlHamzah, M.; Kreuziger, L.B.; Áinle, F.N.; Alomran, F.; Alayed, K.; Alsheef, M.; AlSumait, F.; et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021, 375, n2400. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; Diab, W.; Wang, J.; Khanijo, S.; Mignatti, A.; Gianos, E.; Cohen, M.; Sharifova, G.; et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19. JAMA Intern. Med. 2022, 181, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Cryer, M.J.; Farhan, S.; Kaufmann, C.C.; Jäger, B.; Garg, A.; Krishnan, P.; Mehran, R.; Huber, K. Prothrombotic Milieu, Thrombotic Events and Prophylactic Anticoagulation in Hospitalized COVID-19 Positive Patients: A Review. Clin. Appl. Thromb. 2022, 28, 10760296221074353. [Google Scholar] [CrossRef]
- Sholzberg, M.; Tang, G.H.; Negri, E.; Rahhal, H.; Kreuziger, L.B.; Pompilio, C.E.; James, P.; Fralick, M.; AlHamzah, M.; Alomran, F.; et al. Coagulopathy of hospitalised COVID-19: A Pragmatic Randomised Controlled Trial of Therapeutic Anticoagulation versus Standard Care as a Rapid Response to the COVID-19 Pandemic (RAPID COVID COAG–RAPID Trial): A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943, Erratum in JAMA Intern. Med. 2020, 180, 1031. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Mandourah, Y.; Al-Hameed, F.; Sindi, A.A.; Almekhlafi, G.A.; Hussein, M.A.; Jose, J.; Pinto, R.; Al-Omari, A.; Kharaba, A.; et al. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 757–767. [Google Scholar] [CrossRef]
- Lansbury, L.; Rodrigo, C.; Leonardi-Bee, J.; Nguyen-Van-Tam, J.; Lim, W.S. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst. Rev. 2019, 2, CD010406. [Google Scholar] [CrossRef] [PubMed]
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic Review of Treatment Effects. PLoS Med. 2006, 3, e343. [Google Scholar] [CrossRef] [PubMed]
- Huerta, C.; Johansson, S.; Wallander, M.-A.; Rodríguez, L.A.G. Risk Factors and Short-term Mortality of Venous Thromboembolism Diagnosed in the Primary Care Setting in the United Kingdom. Arch. Intern. Med. 2007, 167, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection is Suspected: Interim Guidance. 28 January 2020. Available online: https://apps.who.int/iris/handle/10665/330893 (accessed on 9 March 2020).
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Hong, S.; Wang, H.; Zhang, Z.; Qiao, L. The roles of methylprednisolone treatment in patients with COVID-19: A systematic review and meta-analysis. Steroids 2022, 183, 109022. [Google Scholar] [CrossRef]
- Lopes, R.D.; Macedo, A.V.S.; de Barros, E.S.P.G.M.; Moll-Bernardes, R.J.; Dos Santos, T.M.; Mazza, L.; Feldman, A.; D’Andrea Saba Arruda, G.; de Albuquerque, D.C.; Camiletti, A.S.; et al. Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Young, D.; Coupland, C.; Channon, K.M.; Tan, P.S.; Harrison, A.D.; Rowan, K.; Aveyard, P.; Pavord, I.D.; Watkinson, P.J. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart 2020, 106, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Cai, Y.; Zhang, K.; Zhou, L.; Zhang, Y.; Zhang, X.; Li, Q.; Li, W.; Yang, S.; Zhao, X.; et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: A retrospective observational study. Eur. Hear. J. 2020, 41, 2058–2066. [Google Scholar] [CrossRef]
- Angeli, F.; Verdecchia, P.; Balestrino, A.; Bruschi, C.; Ceriana, P.; Chiovato, L.; Vecchia, L.A.D.; Fanfulla, F.; La Rovere, M.T.; Perego, F.; et al. Renin Angiotensin System Blockers and Risk of Mortality in Hypertensive Patients Hospitalized for COVID-19: An Italian Registry. J. Cardiovasc. Dev. Dis. 2022, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Hermida, N.; Balligand, J.-L. Low-Density Lipoprotein-Cholesterol-Induced Endothelial Dysfunction and Oxidative Stress: The Role of Statins. Antioxid. Redox Signal. 2014, 20, 1216–1237. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Arocutipa, C.; Melgar-Talavera, B.; Alvarado-Yarasca, Á.; Saravia-Bartra, M.M.; Cazorla, P.; Belzusarri, I.; Hernandez, A.V. Statins reduce mortality in patients with COVID-19: An updated meta-analysis of 147 824 patients. Int. J. Infect. Dis. 2021, 110, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-S.; Lin, P.-C.; Chen, Y.-S.; Pan, T.-C.; Tang, P.-L. The use of statins was associated with reduced COVID-19 mortality: A systematic review and meta-analysis. Ann. Med. 2021, 53, 874–884. [Google Scholar] [CrossRef] [PubMed]
- INSPIRATION-S Investigators. Atorvastatin versus placebo in patients with covid-19 in intensive care: Randomized controlled trial. BMJ 2022, 376, e068407. [Google Scholar] [CrossRef]
- Lee, K.C.H.; Sewa, D.W.; Phua, G.C. Potential role of statins in COVID-19. Int. J. Infect. Dis. 2020, 96, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Preliminary report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Ader, F.; Bouscambert-Duchamp, M.; Hites, M.; Peiffer-Smadja, N.; Poissy, J.; Belhadi, D.; Diallo, A.; Lê, M.-P.; Peytavin, G.; Staub, T.; et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2022, 22, 209–221. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium; Pan, H.; Peto, R. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef]
- Streinu-Cercel, A.; Săndulescu, O.; Preotescu, L.-L.; Kim, J.Y.; Kim, Y.-S.; Cheon, S.; Jang, Y.R.; Lee, S.J.; Kim, S.H.; Chang, I.; et al. Efficacy and Safety of Regdanvimab (CT-P59): A Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial in Outpatients with Mild-to-Moderate Coronavirus Disease 2019. Open Forum Infect. Dis. 2022, 9, ofac053. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Casal, M.C.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of Covid-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
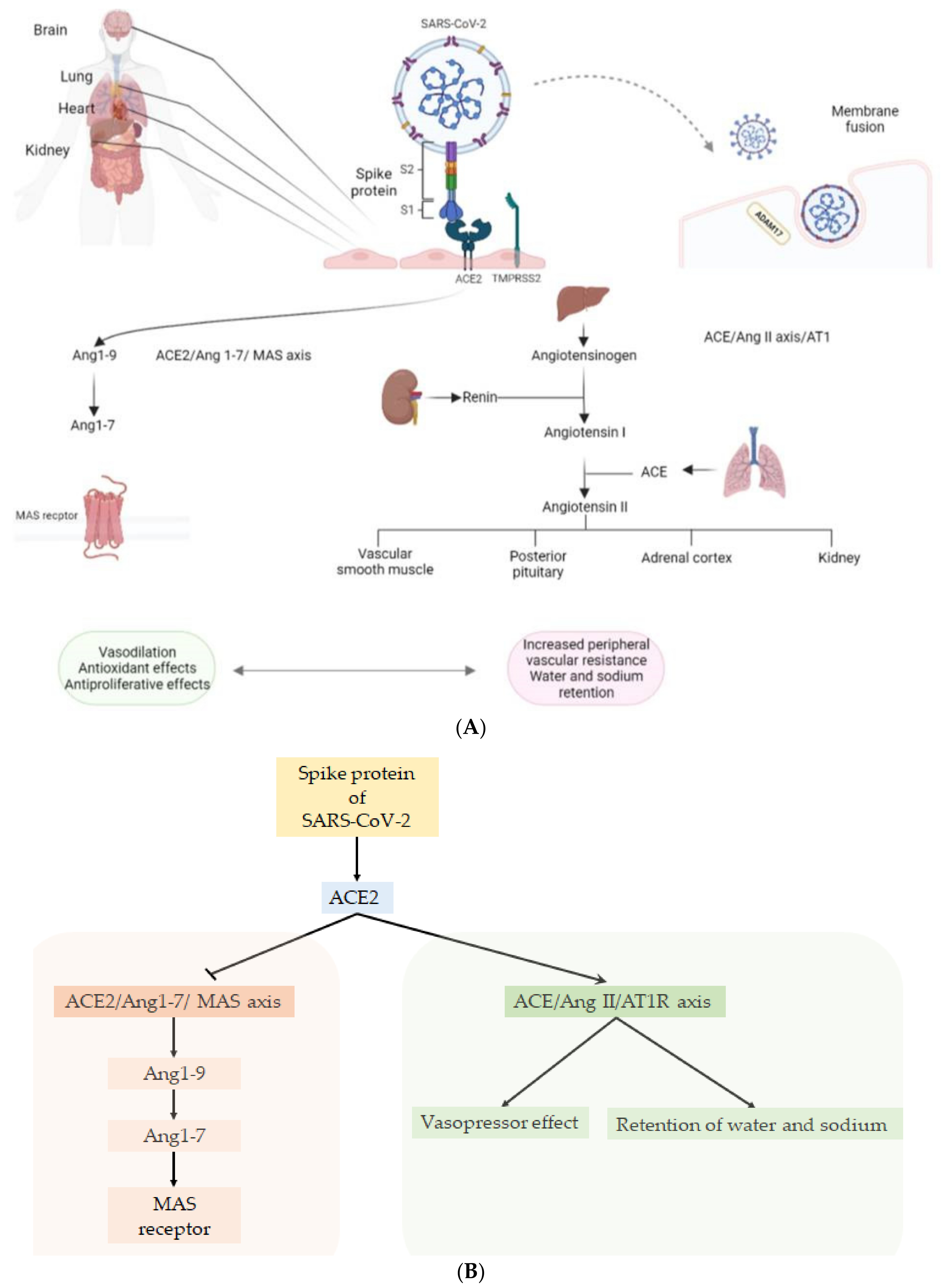
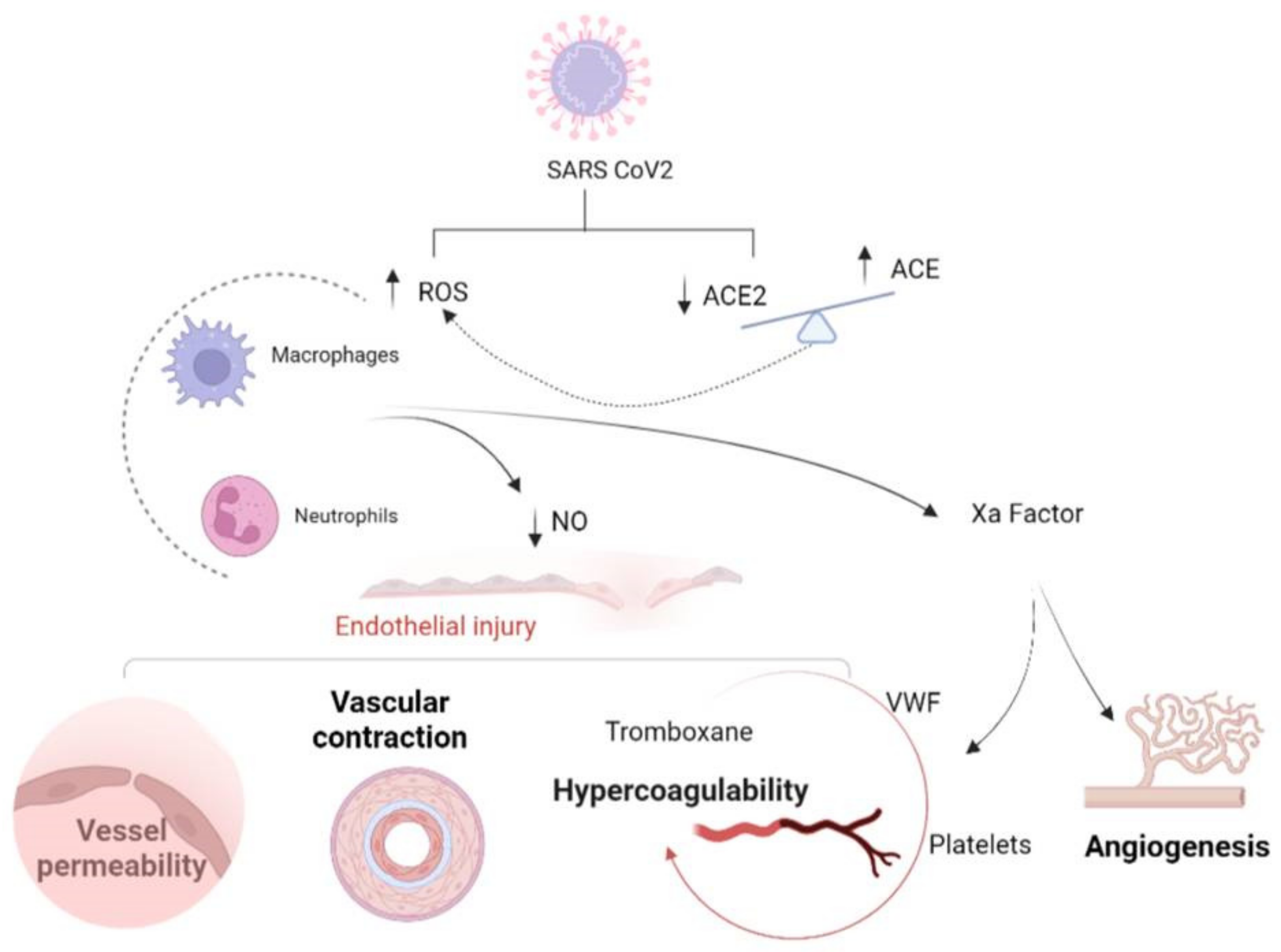
| Organ or System Involved | Clinical Presentation/Disease | Laboratory/Exams | Pathophysiology |
|---|---|---|---|
| Lung [74,75,76,77,78,79,80,81,82,83,84,85,86,87] | Cough (mostly dry), dyspnea, fatigue, sore throat, rhinorrhea, sneezing and, in severe cases, pneumonia, respiratory failure or acute respiratory distress syndrome (ARDS) | Moderate/severe hypoxemia, ground-glass opacities in chest X-rays | Alveolar injury, interstitial inflammation, cytokine storm, immune suppression, diffuse pulmonary, intravascular coagulopathy, silent hypoxia |
| Gastrointestinal [88,89,90,91,92,93,94,95,96] | Nausea, vomiting, diarrhea, heartburn, loss of appetite, abdominal pain andbloating | Elevated liver enzymes andbilirubin | Direct action of virus on the ACE2 receptors, drugs hepatotoxicity, pneumonia-associated hypoxia |
| Brain [97,98,99,100,101] | Hyposmia–anosmia, hypogeusia–ageusia, visual disturbance, fatigue,somnolence, headaches, nausea and vomiting, dizziness, myalgia, ataxia, encephalopathy, cerebrovascular disease (large vessel strokes), seizures, meningoencephalitis, neuropathy, Guillain–Barrésyndrome, neurogenic ARDS, coma | Elevated creatine kinase withmyalgia, brain MRI showing hyperintensities in regions with infarction or encephalitis, SARS-CoV-2 detection in cerebrospinal fluid or brain tissues in some patients | Virus’s direct effects mediated by the ACE2 receptors, the ascent by olfactory nerve axons, ischemic stroke due to viral hypercoagulability, hypoxia, cytokine storm |
| Mental/Psychiatric [102,103] | Post-traumatic stress disorder, depression, anxiety, insomnia, anger, fear, exacerbation ofneurological or psychiatric disorders | Elevated plasma calcium andphosphorus (indicative of stress) | Isolation and quarantine, the effect of COVID- 19 in the brain tissue |
| Heart [104,105,106,107,108,109,110] | Chest pain, heart attack, myocardial injury, arrhythmias, cardiogenic shock and even sudden death | Elevated cardiac enzymes, abnormal EKG (prolonged QTc intervals, elevated ST), cardiac-specific troponin and brain natriuretic peptide | The effect of SARS-CoV-2 on the ACE2 receptors, adrenergic activation, electrolytic imbalances, fluid excess, side effects of treatments, cytokine-release syndrome, hypoxia |
| Blood vessels [111,112,113,114] | Pulmonary embolism, disseminated intravascular coagulation, deep vein thrombosis, large vessel stroke, systemic arterial and venous thromboembolism | Elevated D-dimer, interleukin-6, other cytokines, ferritin and lactate dehydrogenase, prolonged PT/PTT | Direct effect of SARS-CoV-2, Platelet hyperactivity and increased thrombus formation |
| Kidney [115,116,117,118,119,120,121] | Renal failure, tubular necrosis | Proteinuria, hematuria | Direct viral injury, Cytokine storm, Disorder of renin–angiotensin– aldosterone system (RAAS) homeostasis |
| Therapeutic Approach | Mechanism | Conclusions from the Literature |
|---|---|---|
| Heparin | -antithrombotic activity [137] -antiviral activity [138] -inhibits the entry of SARS-CoV-2 into human cells [139] | The INSPIRATION study showed that there are no significant differences between the use of intermediate-dose vs. standard-dose prophylactic anticoagulants among patients with COVID-19 in terms of reduction invenous or arterial thrombosis [143]. The RAPID study demonstrated a non-significant trend toward a reduction in the number of fatal events [144]. The HEP-COVID study [145] showed a 32% reduction in the number of venous or arterial thromboembolic events and all-cause mortality. Data suggest improved efficacy and safety of the use of weight-optimized prophylactic dosing in non-severe hospitalized patients [146]. |
| Corticosteroids | -anti-inflammatory activity [139] -reduction in the levels of procoagulant factors such as fibrinogen and VWF [148] | The RECOVERY trial demonstrated that using dexamethasone reduces 28-day mortality only in patients with severe disease [155]. The benefits are greater in cases of severe disease, in which short-term treatment with low-dose methylprednisolone is indicated [157]. |
| RAAS Inhibitors | -upregulate ACE2 expression that converts AngII to Ang and increases NO release with an anti-inflammatory and anticoagulant role [158] | A cohort study showed that RAAS inhibitors reduce the risk of COVID-19 [159]. There is no evidence for their role to ameliorate ED. |
| Statins | -reduce low-density lipoprotein cholesterol and oxidative stress [162] | The INSPIRATION study demonstrated the beneficial role of statins by decreasing the inflammatory response [166]. |
| Antiviral Agents | Mechanism | Conclusions from the Literature |
|---|---|---|
| Remdesivir | Blocks the replication of viral RNA [168] | Clinical benefit was obtained only in subjects on standard oxygen therapy, while no clinical benefit was observed in subjects onhigh-flow oxygen therapy or on mechanical ventilation or ECMO [170]. The early therapy (from 3 to 5 days) with remdesivir reduced hospitalization and deaths compared with theplacebo in patients with a high risk for COVID-19 progression [174]. |
| PF-07321332/ritonavir | PF-07321332 is a peptidomimetic major protease inhibitor (Mpro) of SARS-CoV-2 and ritonavir is an inhibitor of the metabolism of PF-07321332 [175] | Treatment within 5 days ofthe start of symptoms, in subjects who were non-hospitalized and with at least one risk factor for progression to severe COVID-19, reduced hospitalization for COVID-19, worsening or deaths from any cause within 28 days[175]. |
| Molnupiravir | Reduces multiplication of SARS-CoV-2 [176] | Early treatment (within 5 days of the start of symptoms) with molnupiravir showed reduced hospitalization and deaths in unvaccinated adults with COVID-19 [176]. |
| Monoclonal Antibodies | Mechanism | Conclusions from the Literature |
|---|---|---|
| Casirivimab/imdevimab | Two neutralizing immunoglobulin gamma 1 (IgG1) human monoclonal antibodiesagainst the spike protein SARS-CoV-2 [177]. | A single intravenous or subcutaneous dose within 3 days of the start of SARS-CoV-2 infection reduced hospitalizations, deaths and viral load in non-hospitalized adults with COVID-19; the treatment improved survival and reduced the risk of worsening or death in hospitalized patients with severe COVID-19 [177]. |
| Regdanvimab | Blocks the interaction regions between SARS-CoV-2 S protein RBD and ACE2 [178]. | A single infusion showed a trend toward a minor decrease in time to negative conversion of RT-qPCR results and reduced the need for hospitalization and oxygen therapy in patients with mild-to-moderate COVID-19 [178]. |
| Sotrovimab | Neutralizes SARS-CoV-2 directly targeting the SARS-CoV-2 spike glycoprotein [179]. | A single infusion within 5 days of the onset of symptoms reduced hospitalizations, deathsand admissions to ICU in high-risk non-hospitalized patients with COVID-19 [179]. |
| Tixagevimab-cilgavimab | Bind epitopes of the SARS-CoV-2 spike-protein receptor-binding domain to neutralize the virus [180]. | A single dose reduced infection and severe disease in people who had an increased risk of an inadequate response to COVID-19 vaccination, an increased risk of exposure to SARS-CoV-2 or both. It was ineffective against Omicron BA.1, whereas it was effective against BA.2 [180]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelle, M.C.; Zaffina, I.; Lucà, S.; Forte, V.; Trapanese, V.; Melina, M.; Giofrè, F.; Arturi, F. Endothelial Dysfunction in COVID-19: Potential Mechanisms and Possible Therapeutic Options. Life 2022, 12, 1605. https://doi.org/10.3390/life12101605
Pelle MC, Zaffina I, Lucà S, Forte V, Trapanese V, Melina M, Giofrè F, Arturi F. Endothelial Dysfunction in COVID-19: Potential Mechanisms and Possible Therapeutic Options. Life. 2022; 12(10):1605. https://doi.org/10.3390/life12101605
Chicago/Turabian StylePelle, Maria Chiara, Isabella Zaffina, Stefania Lucà, Valentina Forte, Vincenzo Trapanese, Melania Melina, Federica Giofrè, and Franco Arturi. 2022. "Endothelial Dysfunction in COVID-19: Potential Mechanisms and Possible Therapeutic Options" Life 12, no. 10: 1605. https://doi.org/10.3390/life12101605
APA StylePelle, M. C., Zaffina, I., Lucà, S., Forte, V., Trapanese, V., Melina, M., Giofrè, F., & Arturi, F. (2022). Endothelial Dysfunction in COVID-19: Potential Mechanisms and Possible Therapeutic Options. Life, 12(10), 1605. https://doi.org/10.3390/life12101605







