Increased Scabies Incidence at the Beginning of the 21st Century: What Do Reports from Europe and the World Show?
Abstract
1. Introduction
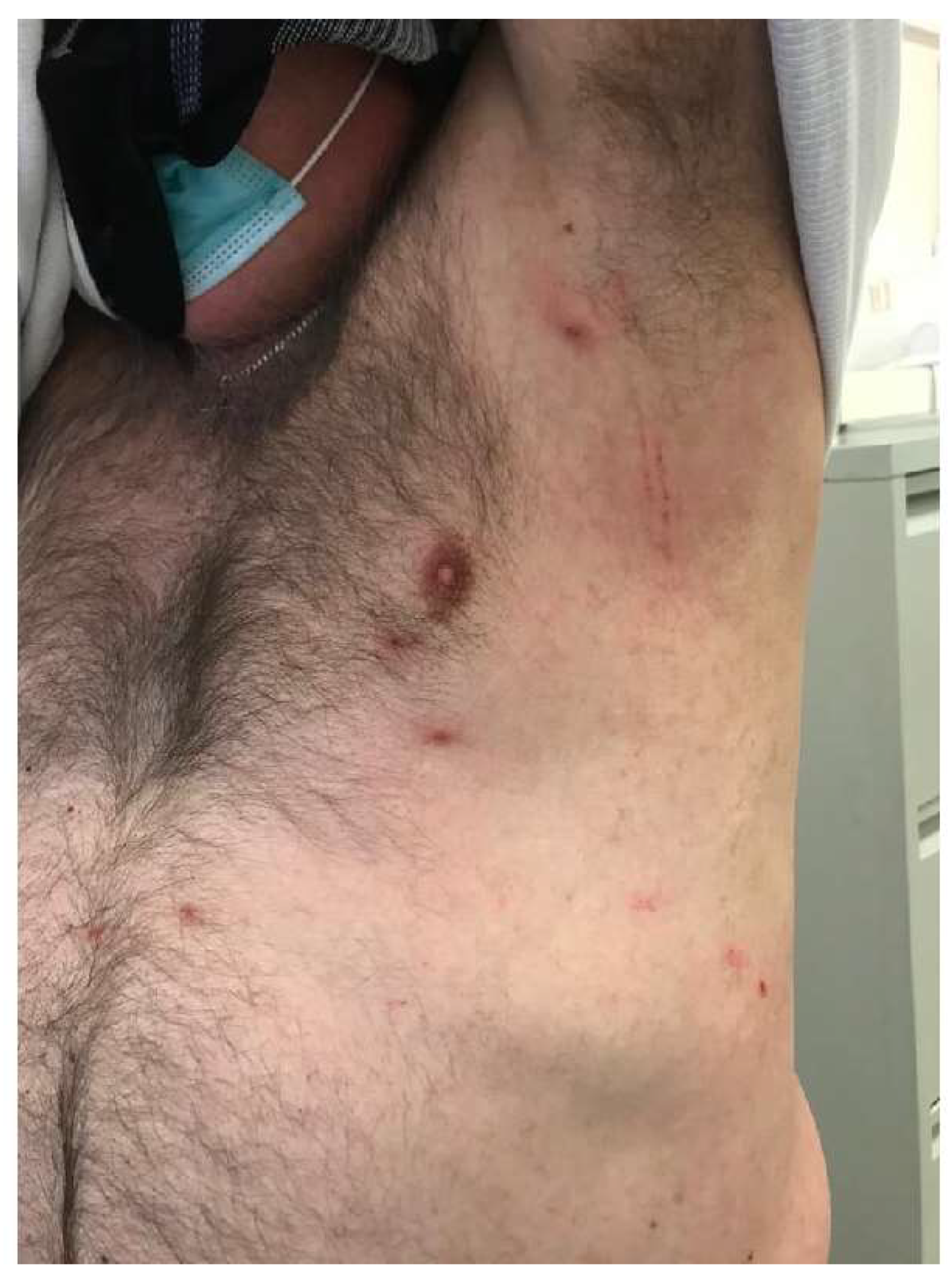
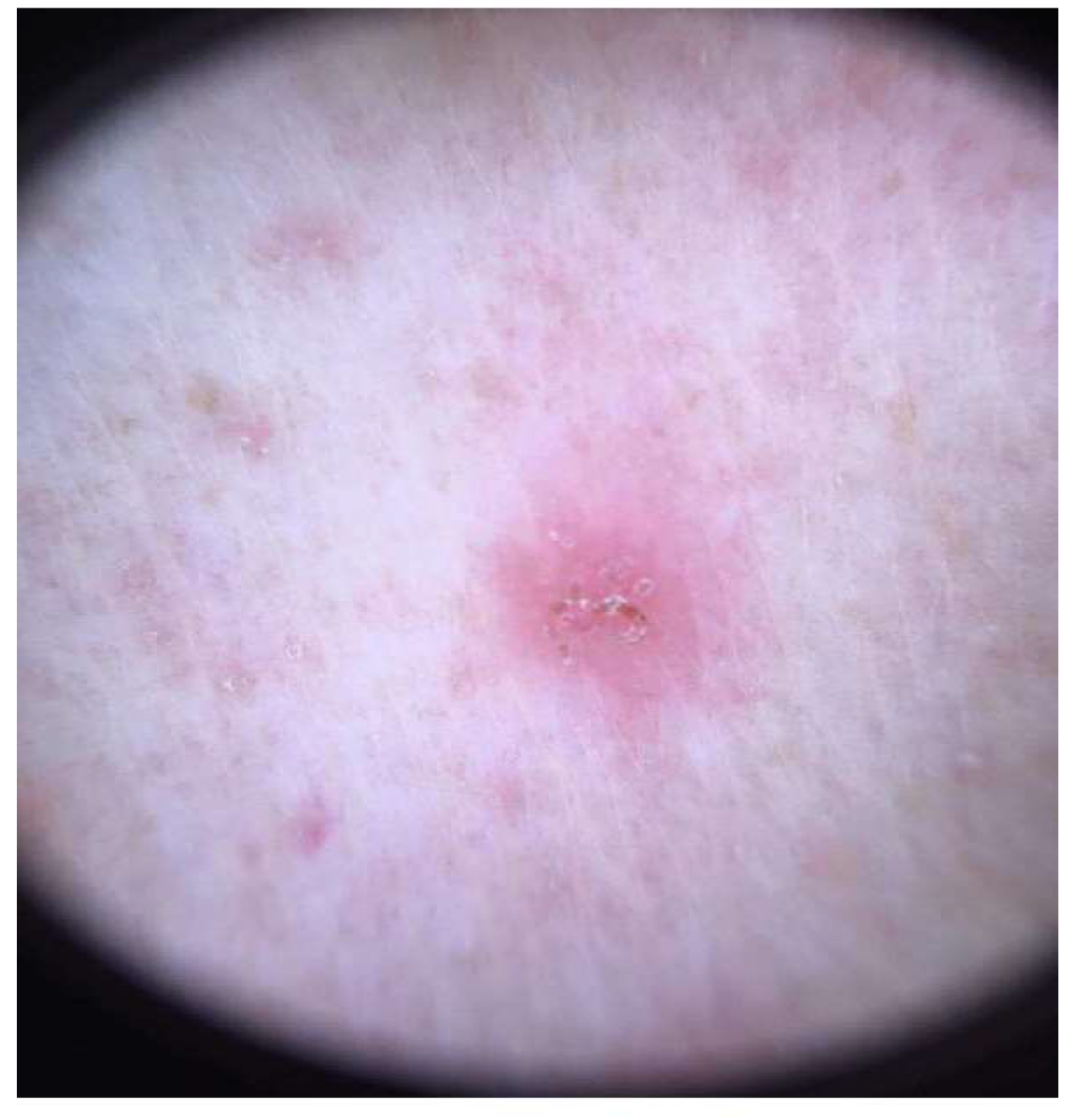
2. Clinical Features and Management of Scabies
3. General Epidemiological Aspects of Scabies
4. Trends in Scabies Incidence/Prevalence Recorded during the Last Two Decades
5. Measures for Improvement of Negative Trends
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sunderkötter, C.; Aebischer, A.; Neufeld, M.; Löser, C.; Kreuter, A.; Bialek, R.; Hamm, H.; Feldmeier, H. Increase of scabies in Germany and development of resistant mites? Evidence and consequences. J. Dtsch. Dermatol. Ges. 2019, 17, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Amato, E.; Dansie, L.S.; Grøneng, G.M.; Blix, H.S.; Bentele, H.; Veneti, L.; Stefanoff, P.; MacDonald, E.; Blystad, H.H.; Soleng, A. Increase of scabies infestations, Norway, 2006 to 2018. Eurosurveillance 2019, 24, 190020. [Google Scholar] [CrossRef]
- Akaslan, T.Ç.; Mert, Ö.; Su Küçük, Ö. Scabies increase during the COVID-19 pandemic: Should we change our treatment strategy during the pandemic? Ann. Parasitol. 2022, 68, 35–38. [Google Scholar]
- Lugović-Mihić, L. The increase in Croatia’s scabies incidence: How did refugees and traveling contribute? Travel Med. Infect. Dis. 2019, 29, 74. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, B.; Hooiveld, M.; Marks, S.; Snijdewind, I.; van den Kerkhof, H.; Wintermans, B.; Bom, B.; Schimmer, B.; Fanoy, E. Increasing incidence of reported scabies infestations in the Netherlands, 2011–2021. PLoS ONE 2022, 17, e0268865. [Google Scholar] [CrossRef] [PubMed]
- Lugović-Mihić, L.; Aždajić, M.D.; Filipović, S.K.; Bukvić, I.; Prkačin, I.; Grbić, D.Š.; Ličina, M.L.K. An Increasing Scabies Incidence in Croatia: A Call for Coordinated Action among Dermatologists, Physicians and Epidemiologists. Slov. J. Public Health 2020, 59, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Garbe, C.; Girbig, G.; Strömer, K.; Kirsten, N. Epidemiologie der Skabies in Deutschland: Multi-Source-Analyse von Primär- und Sekundärdaten [Epidemiology of scabies in Germany: Multisource analysis of primary and secondary data]. Hautarzt 2022, 73, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Dei-Cas, I.; Carrizo, D.; Giri, M.; Boyne, G.; Domínguez, N.; Novello, V.; Acuña, K.; Dei-Cas, P. Infectious skin disorders encountered in a pediatric emergency department of a tertiary care hospital in Argentina: A descriptive study. Int. J. Dermatol. 2019, 58, 288–295. [Google Scholar] [CrossRef]
- Thompson, M.J.; Engelman, D.; Gholam, K.; Fuller, L.C.; Steer, A.C. Systematic review of the diagnosis of scabies in therapeutic trials. Clin. Exp. Dermatol. 2017, 42, 481–487. [Google Scholar] [CrossRef]
- Karimkhani, C.; Colombara, D.V.; Drucker, A.M.; Norton, S.A.; Hay, R.; Engelman, D.; Steer, A.; Whitfeld, M.; Naghavi, M.; Dellavalle, R.P. The global burden of scabies: A cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1247–1254. [Google Scholar] [CrossRef]
- Kobangué, L.; Guéréndo, P.; Abéyé, J.; Namdito, P.; Mballa, M.D.; Gresenguet, G. Gale sarcoptique: Aspects épidémiologiques, cliniques et thérapeutiques à Bangui [Scabies: Epidemiological, clinical and therapeutic features in Bangui]. Bull. Soc. Pathol. Exot. 2014, 107, 10–14. [Google Scholar] [CrossRef]
- Azene, A.G.; Aragaw, A.M.; Wassie, G.T. Prevalence and associated factors of scabies in Ethiopia: Systematic review and Meta-analysis. BMC Infect. Dis. 2020, 20, 380. [Google Scholar] [CrossRef]
- Romani, L.; Whitfeld, M.J.; Koroivueta, J.; Kama, M.; Wand, H.; Tikoduadua, L.; Tuicakau, M.; Koroi, A.; Ritova, R.; Andrews, R.; et al. The epidemiology of scabies and impetigo in relation to demographic and residential characteristics: Baseline findings from the skin health intervention Fiji Trial. Am. J. Trop. Med. Hyg. 2017, 97, 845–850. [Google Scholar] [CrossRef]
- Boateng, L.A. Ghana Southampton Scabies Research Partnership. Healthcare-seeking behaviour in reporting of scabies and skin infections in Ghana: A review of reported cases. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 830–837. [Google Scholar] [CrossRef]
- Galván-Casas, C.; Mitjá, O.; Esteban, S.; Kafulafula, J.; Phiri, T.; Navarro-Fernández, Í.; Román-Curto, C.; Mtenje, H.; Thauzeni, G.; Harawa, E.; et al. A facility and community-based assessment of scabies in rural Malawi. PLoS Negl. Trop. Dis. 2021, 15, e0009386. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Al-Jahdali, H.; Jradi, H.; ALMuqbil, B.I.; AlBuraikan, D.A.; Albaijan, M.A.; Ali, Y.Z.; Al Shehri, A.M. Recurrence rate of scabies in patients 14 years or older in Saudi Arabia. Saudi Med. J. 2019, 40, 1267–1271. [Google Scholar] [CrossRef]
- Kim, H.J.; Cheong, H.K. Epidemiologic Trends and Seasonality of Scabies in South Korea, 2010–2017. Korean J. Parasitol. 2019, 57, 399–404. [Google Scholar] [CrossRef]
- Lake, S.J.; Engelman, D.; Sokana, O.; Nasi, T.; Boara, D.; Grobler, A.C.; Osti, M.H.; Andrews, R.; Marks, M.; Whitfeld, M.J.; et al. Defining the need for public health control of scabies in Solomon Islands. PLoS Negl. Trop. Dis. 2021, 15, e0009142. [Google Scholar] [CrossRef]
- Liu, J.M.; Wang, H.W.; Chang, F.W.; Liu, Y.P.; Chiu, F.H.; Lin, Y.C.; Cheng, K.C.; Hsu, R.J. The effects of climate factors on scabies. A 14-year population-based study in Taiwan. Parasite 2016, 23, 54. [Google Scholar] [CrossRef]
- Launay, T.; Bardoulat, I.; Lemaitre, M.; Blanchon, T.; Fardet, L. Effects of the COVID-19 pandemic on head lice and scabies infestation dynamics: A population-based study in France. Clin. Exp. Dermatol. 2022, 47, 867–872. [Google Scholar] [CrossRef]
- Schmidt-Guerre, A.R.; Aranda-Hulin, B.; Maumy-Bertrand, M.; Aubin, F. Diagnosis and treatment of scabies by general practitioners: A survey of practices in France. Ann. Dermatol. Venereol. 2018, 145, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Reichert, F.; Schulz, M.; Mertens, E.; Lachmann, R.; Aebischer, A. Reemergence of Scabies Driven by Adolescents and Young Adults, Germany, 2009–2018. Emerg. Infect. Dis. 2021, 27, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Louka, C.; Logothetis, E.; Engelman, D.; Samiotaki-Logotheti, E.; Pournaras, S.; Stienstra, Y. Scabies epidemiology in health care centers for refugees and asylum seekers in Greece. PloS Negl. Trop. Dis. 2022, 16, e0010153. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.R.; Pender, E.K.; Laing, M.E.; Markham, T. Unexpected consequences of SARS-CoV-2 pandemic: Scabies infestation. Clin. Exp. Dermatol. 2022, 47, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- De Lucia, M.; Potestio, L.; Costanzo, L.; Fabbrocini, G.; Gallo, L. Scabies outbreak during COVID-19: An Italian experience. Int. J. Dermatol. 2021, 60, 1307–1308. [Google Scholar] [CrossRef] [PubMed]
- Korycinska, J.; Dzika, E.; Kloch, M. Epidemiology of scabies in relation to socio-economic and selected climatic factors in north-east Poland. Ann. Agric. Environ. Med. 2020, 27, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pallás, I.; Aldea-Manrique, B.; Ramírez-Lluch, M.; Manuel Vinuesa-Hernando, J.; Ara-Martín, M. Scabies outbreak during home confinement due to the SARS-CoV-2 pandemic. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e781–e783. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Bravo, L.; Fernandez-Martinez, B.; Gómez-Barroso, D.; Gherasim, A.; García-Gómez, M.; Benito, A.; Herrador, Z. Scabies in Spain? A comprehensive epidemiological picture. PLoS ONE 2021, 16, e0258780. [Google Scholar] [CrossRef]
- Baykal, C.; Atci, T.; Kutlay, A.; Baykut, B.; Türkoğlu, Z. Scabies outbreak in Turkey in 2018–2019. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e384–e385. [Google Scholar] [CrossRef]
- Hewitt, K.A.; Nalabanda, A.; Cassell, J.A. Scabies outbreaks in residential care homes: Factors associated with late recognition, burden and impact. A mixed methods study in England. Epidemiol. Infect. 2015, 143, 1542–1551. [Google Scholar] [CrossRef]
- Richardson, N.A.; Cassell, J.A.; Head, M.G.; Lanza, S.; Schaefer, C.; Walker, S.L.; Middleton, J. Scabies outbreak management in refugee/migrant camps across Europe 2014–17: A retrospective qualitative interview study of healthcare staff experiences and perspectives. medRxiv 2021. [Google Scholar] [CrossRef]
- World Health Organization. August 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/scabies (accessed on 12 August 2022).
- Search—UpToDate. Available online: https://www.uptodate.com/contents/scabies-epidemiology-clinical-features-and-diagnosis (accessed on 15 August 2022).
- Chosidow, O. Clinical practices. Scabies. N. Engl. J. Med. 2006, 354, 1718–1727. [Google Scholar] [CrossRef]
- Fuller, L.C. Epidemiology of scabies. Curr. Opin. Infect. Dis. 2013, 26, 123–126. [Google Scholar] [CrossRef]
- Bowman, D.D. Georgis’ Parasitology for Veterinarians, 10th ed.; Elsevier Health Sciences: Saint Louis, MO, USA, 2014; p. 68. ISBN 9781455739882. [Google Scholar]
- Lugović-Mihić, L.; Delaš-Aždajić, M.; Bešlić, I. Scabies cases misdiagnosed and treated as allergic diseases: A case series. Acta Clin. Croat. 2022, 61, 349–353. [Google Scholar]
- Sánchez-Borges, M.; González-Aveledo, L.; Capriles-Hulett, A.; Caballero-Fonseca, F. Scabies, crusted (Norwegian) scabies and the diagnosis of mite sensitisation. Allergol. Immunopathol. 2018, 46, 276–280. [Google Scholar] [CrossRef]
- Engelman, D.; Yoshizumi, J.; Hay, R.J.; Osti, M.; Micali, G.; Norton, S.; Walton, S.; Boralevi, F.; Bernigaud, C.; Bowen, A.C.; et al. The 2020 International Alliance for the Control of Scabies Consensus Criteria for the Diagnosis of Scabies. Br. J. Dermatol. 2020, 183, 808–820. [Google Scholar] [CrossRef]
- Stamm, L.V.; Strowd, L.C. Ignoring the “Itch”: The global health problem of scabies. Am. J. Trop. Med. Hyg. 2017, 97, 1647–1649. [Google Scholar] [CrossRef]
- Pomares, C.; Marty, P.; Delaunay, P. Isolated itching of the genitals. Am. J. Trop. Med. Hyg. 2014, 90, 589–590. [Google Scholar] [CrossRef][Green Version]
- Eshagh, K.; DeKlotz, C.M.; Friedlander, S.F. Infant with a papular eruption localized to the back. JAMA Pediatr. 2014, 168, 379–380. [Google Scholar] [CrossRef]
- Swe, P.M.; Christian, L.D.; Lu, H.C.; Sriprakash, K.S.; Fischer, K. Complement inhibition by Sarcoptes scabiei protects Streptococcus pyogenes—An in vitro study to unravel the molecular mechanisms behind the poorly understood predilection of S. pyogenes to infect mite-induced skin lesions. PLoS Negl. Trop. Dis. 2017, 11, e0005437. [Google Scholar] [CrossRef]
- Bernigaud, C.; Fischer, K.; Chosidow, O. The Management of Scabies in the 21st Century: Past, Advances and Potentials. Acta Derm. Venereol. 2020, 100, 225–234. [Google Scholar] [CrossRef]
- Jonsson, P.; Lindelöf, B.; Nordlind, K.; Bornstein, S. Vid klåda, tänk alltid på skabb! [In case of pruritus, always consider scabies!]. Lakartidningen 2017, 114, 1–7. [Google Scholar]
- Hengge, U.R.; Currie, B.J.; Jager, G.; Lupi, O.; Schwartz, R.A. Scabies: A ubiquitous neglected skin disease. Lancet Infect. Dis. 2006, 6, 769–779. [Google Scholar] [CrossRef]
- Micheletti, R.G.; Dominguez, A.R.; Wanat, K.A. Bedside diagnostics in dermatology: Parasitic and noninfectious diseases. J. Am. Acad. Dermatol. 2017, 77, 221–230. [Google Scholar] [CrossRef]
- Nair, P.A.; Vora, R.V.; Jivani, N.B.; Gandhi, S.S. A study of clinical profile and quality of life in patients with scabies at a rural tertiary care centre. J. Clin. Diagn. Res. 2016, 10, WC01–WC05. [Google Scholar] [CrossRef]
- Khalil, S.; Abbas, O.; Kibbi, A.G.; Kurban, M. Scabies in the age of increasing drug resistance. PLoS Negl. Trop. Dis. 2017, 11, e0005920. [Google Scholar] [CrossRef]
- Executive Committee of Guideline for the Diagnosis and Treatment of Scabies. Guideline for the diagnosis and treatment of scabies in Japan (third edition): Executive Committee of Guideline for the Diagnosis and Treatment of Scabies. J. Dermatol. 2017, 44, 991–1014. [Google Scholar] [CrossRef]
- Salavastru, C.M.; Chosidow, O.; Boffa, M.J.; Janier, M.; Tiplica, G.S. European guideline for the management of scabies. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1248–1253. [Google Scholar] [CrossRef]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Romani, L.; Steers, A.C.; Whitfeld, M.J.; Kaldor, J.M. Prevalence of scabies and impetigo worldwide: A systematic review. Lancet Infect. Dis. 2015, 15, 960–967. [Google Scholar] [CrossRef]
- Anderson, K.L.; Strowd, L.C. Epidemiology, Diagnosis, and Treatment of Scabies in a Dermatology Office. J. Am. Board Fam. Med. 2017, 30, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Çetinkaya, Ü.; Şahin, S.; Ulutabanca, R.Ö. The Epidemiology of Scabies and Pediculosis in Kayseri. Turk. Parazitol. Derg. 2018, 42, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Downs, A.M.; Harvey, I.; Kennedy, C.T. The epidemiology of head lice and scabies in the UK. Epidemiol. Infect. 1999, 122, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Lassa, S.; Campbell, M.J.; Bennett, C.E. Epidemiology of scabies prevalence in the U.K. from general practice records. Br. J. Dermatol. 2011, 164, 1329–1334. [Google Scholar] [CrossRef]
- Mulligan, K.M.; Cullison, C.R.; Zheng, D.X.; Tripathi, R.; Beveridge, M.G.; Ray, A.J.; Scott, J.F. Sociodemographic factors associated with scabies in the inpatient setting. Am. J. Infect. Control 2021, 49, 1558–1560. [Google Scholar] [CrossRef]
- Nittari, G.; Battineni, G.; Messinetti, M.; Campanozzi, L.; Sirignano, A. Critical reflections and solutions for health problems of Italian refugees. Clin. Ter. 2021, 172, 158–162. [Google Scholar]
- Wollina, U.; Gaber, B.; Mansour, R.; Lagner, D.; Hansel, G.; Koch, A. Dermatologic challenges of health care for displaced people. Lessons from a German emergency refugee camp. Our Dermatol. Online 2016, 7, 136–138. [Google Scholar] [CrossRef]
- Kuhne, A.; Gilsdorf, A. Infectious disease outbreaks in centralized homes for asylum seekers in Germany from 2004–2014. Bundesgesundheitsblatt Gesundh. Gesundh. 2016, 59, 570–577. [Google Scholar]
- Isenring, E.; Fehr, J.; Gültekin, N.; Schlagenhauf, P. Infectious disease profiles of Syrian and Eritrean migrants presenting in Europe: A systematic review. Travel Med. Infect. Dis. 2018, 25, 65–76. [Google Scholar] [CrossRef]
- Debe Worku, E.; Asemahagn, M.A.; Endalifer, M.L. Determinants of scabies outbreak in Takusa district of Amhara Region, Northwest Ethiopia. J. Public Health Afr. 2020, 11, 1325. [Google Scholar] [CrossRef]
- Ansart, S.; Perez, L.; Jaureguiberry, S.; Danis, M.; Bricaire, F.; Caumes, E. Spectrum of dermatoses in 165 travelers returning from the tropics with skin diseases. Am. J. Trop. Med. Hyg. 2007, 76, 184–186. [Google Scholar] [CrossRef]
- Search—UpToDate. Available online: https://www.uptodate.com/contents/scabies-beyond-the-basics (accessed on 15 August 2022).
- El-Moamly, A.A. Scabies as a part of the World Health Organization roadmap for neglected tropical diseases 2021–2030: What we know and what we need to do for global control. Trop. Med. Health 2021, 49, 64. [Google Scholar] [CrossRef]
- Lapeere, H.; Naeyaert, J.M.; De Weert, J.; De Maeseneer, J.; Brochez, L. Incidence of scabies in Belgium. Epidemiol. Infect. 2008, 136, 395–398. [Google Scholar] [CrossRef]
- Falagas, M.E.; Theocharis, G.; Spanos, A.; Vlara, L.A.; Issaris, E.A.; Panos, G.; Peppas, G. Effect of meteorological variables on the incidence of respiratory tract infections. Respir. Med. 2008, 102, 733–737. [Google Scholar] [CrossRef]
- Kartal, S.P.; Çelik, G.; Sendur, N.; Aytekin, S.; Serdaroğlu, S.; Doğan, B.; Yazıcı, A.C.; Çiçek, D.; Borlu, M.; Kaçar, N.G.; et al. Multicenter study evaluating the impact of COVID-19 outbreak on dermatology outpatients in Turkey. Dermatol. Ther. 2020, 33, e14485. [Google Scholar] [CrossRef]
- Isoletta, E.; Vassallo, C.; Brazzelli, V.; Giorgini, C.; Tomasini, C.F.; Sabena, A.; Perlini, S.; De Silvestri, A.; Barruscotti, S. Emergency accesses in Dermatology Department during the COVID-19 pandemic in a referral third level center in the north of Italy. Dermatol. Ther. 2020, 33, e14027. [Google Scholar] [CrossRef]
- Kutlu, Ö.; Aktaş, H. The explosion in scabies cases during COVID-19 pandemic. Dermatol. Ther. 2020, 33, e13662. [Google Scholar] [CrossRef]
- May, P.J.; Tong, S.Y.C.; Steer, A.C.; Currie, B.J.; Andrews, R.M.; Carapetis, J.R.; Bowen, A.C. Treatment, prevention and public health management of impetigo, scabies, crusted scabies and fungal skin infections in endemic populations: A systematic review. Trop. Med. Int. Health 2019, 24, 280–293. [Google Scholar] [CrossRef]
- Bhat, S.A.; Mounsey, K.E.; Liu, X.; Walton, S.F. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasites Vectors 2017, 10, 385. [Google Scholar] [CrossRef]
- White, L.C.; Lanza, S.; Middleton, J.; Hewitt, K.; Freire-Moran, L.; Edge, C.; Nicholls, M.; Rajan-Iyer, J.; Cassell, J.A. The management of scabies outbreaks in residential care facilities for the elderly in England: A review of current health protection guidelines. Epidemiol. Infect. 2016, 144, 3121–3130. [Google Scholar] [CrossRef]
- Sunderkötter, C.; Feldmeier, H.; Fölster-Holst, R.; Geisel, B.; Klinke-Rehbein, S.; Nast, A.; Philipp, S.; Sachs, B.; Stingl, J.; Stoevesandt, J.; et al. S1 guidelines on the diagnosis and treatment of scabies—Short version. J. Dtsch. Dermatol. Ges. 2016, 14, 1155–1167. [Google Scholar] [CrossRef]
| COUNTRY | AUTHORS | ANALYSED DATA/SOURCE | RESULTS |
|---|---|---|---|
| ARGENTINA | Dei-Cas et al. [8] | A prospective study that included all children aged 15 years or younger who attended the Pediatric Emergency Department of Presidente Peron Hospital (Avellaneda, Buenos Aires, Argentina) between 1 January and 31 December 2016 | Scabies was the most frequent parasitic skin infection and fourth most common infectious skin disorder. |
| CENTRAL AFRICA | Kobangué et al. [11] | Scabies frequency in Bangui assessed using records and clinical confirmation at the dermatology department of Bangui (January 2006–December 2010) (a cross-sectional study) | Average hospital scabies prevalence was 5.88%, highest for those 0–9 years of age (33%). By school age group: preschool age and pupils/students, respectively, 25.5% and 26.3%). |
| ETHIOPIA | Azene et al. [12] | Systematic review and meta-analysis of overall scabies prevalence and associated factors in Ethiopia; due to high heterogeneity across the studies, the random effects meta-analysis model was used to fix the overall prevalence and associated factors of scabies | Overall scabies prevalence was 14.5% and was significantly associated with (large) family size and bed sharing. The highest prevalence, 19.6%, was seen in the Amhara region. |
| FIJI | Romani et al. [13] | This mass anti-scabies treatment trial began by looking at the occurrence and predictors of scabies in 2051 participants at baseline from six island communities | High scabies burden and prevalence in Fiji (36.4%), highest in children 5–9 years (55.7%). Important scabies-related factors/findings were overcrowding, young age, and typical clinical distribution. |
| GHANA | Boateng et al. [14] | Diagnosed skin infections in urban (Greater Accra) and rural (Oti region) areas, reported by study health centres in Ghana (from a 6-month period in 2019) | Among skin infections, 11.6% were scabies cases, the third most common diagnosis behind bacterial dermatitis (26.5%) and tinea (19.5%). Males predominated scabies cases (64.4%). |
| GLOBAL | Karimkhani et al. [10] | Sources of scabies epidemiological data came from an extensive literature search and hospital insurance records. Data was analysed with a Bayesian meta-regression modelling tool. Estimated DALYs for 195 countries were divided into 21 world regions, 20 age groups, and by both sexes between 1990 and 2015 | Scabies was responsible for 0.21% of DALYs from all conditions studied by GBD 2015 worldwide. |
| MALAWI | Galván-Casas et al. [15] | Scabies frequency in rural Malawi; data from a community-based outreach programme (integrated dermatology clinics and tele-dermatology care, including patient visits, screenings, and treatments) | Total/overall scabies cases increased from 2.9% to 39.2% during 2015–2018; the high prevalence confirmed scabies is a major public health issue in parts of Malawi. |
| SAUDI ARABIA | Ahmed et al. [16] | Scabies rate in Saudi Arabia and associated factors (a multi-centre retrospective study on adults diagnosed with ≥1 episode of scabies during January 2016–September 2018); 468 adult patients participated | High scabies recurrence rate among adults, with these risk factors: male gender, first tertile (January to April), and living in areas of high humidity. Of patients, 46.8% had recurrences. |
| SOUTH KOREA | Kim et al. [17] | The national annual and seasonal trend of scabies prevalence based on the National Health Insurance Service database | Annual prevalence (per 1000 persons): those <40 years (0.56–0.69); peak at 3.0–4.1 for persons >80 years; women consistently predominated. High prevalence (>6000 cases) in autumn (when temperatures >25 °C at 2 months prior. |
| SOLOMON ISLANDS | Lake et al. [18] | Prevalence of scabies was analysed in 20 villages in the Western Province (5239 participants); diagnosis was based on the 2020 International Alliance for the Control of Scabies diagnostic criteria | Overall prevalence was 15.0%; considerable variation by village (3.3–42.6%); highest prevalence in children aged <2 years (27%), and significantly higher prevalence in males (16.7%) than females (13.5%). |
| TAIWAN | Liu et al. [19] | A nationwide population-based study using data from Taiwan’s National Health Insurance Research Database for the period from January 2000 to December 2013 for a randomly selected sample of one million people from the 23 million people in the database in 2000 | The total number of patients infested with scabies was 14,883; the mean age was 52.4 ± 21.0 years. A diagnosis was made according to the patient’s history and a physical examination by a licensed physician. |
| COUNTRY | AUTHORS | ANALYSED DATA/SOURCE | RESULTS |
|---|---|---|---|
| CROATIA | Lugović-Mihić et al. [6] | Retrospective medical records and national data from communicable disease reports during 2007–2017 | Six-fold increase in scabies diagnoses in Croatia during 2007–2017, especially in children and young adults; the highest incidence was during 2014–2017 in border counties, probably due to migration flows. The capital, Zagreb, saw a three-fold increase during 2014–2017. |
| FRANCE | Launay et al. [20] | Data from a healthcare sciences company during lockdown (March–December 2020), including dispensing data of prescription and over-the-counter anti-scabies drugs (from 60% of all French retail pharmacies) | The mean reduction in observed vs. expected sales for topical and oral anti-scabies treatments was 14% and 4%, respectively. |
| Schmidt-Guerre et al. [21] | Scabies cases diagnosed during January-June 2015 based on a questionnaire given to general practitioners of the Doubs department in France | GPs frequently diagnosed scabies; 89% had diagnosed ≥1 case during the previous 6 months. | |
| GERMANY | Augustin et al. [7] | Multisource analyses of treatment data from a nationwide statutory health insurance company, the Federal Statistical Office, and data from skin screenings | Scabies case numbers have been rising since 2009, especially since 2014. During 2010–2015 there was a 52.8% increase in outpatients; during 2010–2016 the inpatient increase was about 306%. |
| Reichert et al. [22] | Analysis of inpatient and outpatient claims data from 2009 to 2018 in Germany | Scabies diagnoses increased 9-fold during 2009-2018; the highest proportion of scabies was seen in patients 15–24 years old. | |
| Sunderkötter et al. [1] | Review article on scabies frequency in Germany | Increased scabies diagnoses (no exact quantified data on age-specific and regional incidences); possible regional increases due to migration and an increase in STDs among young adults. | |
| GREECE | Louka et al. [23] | Data from the Greek National Public Health Organization surveillance system for June 2016–July 2020 (retrospective study), submitted by staff at health centres for refugees/asylum seekers | Scabies infestation increased over time, followed by several outbreaks; in the refugee/asylum seeker populations, scabies ranked third among most frequent infectious diseases. |
| IRELAND | Griffin et al. [24] | Data from a dermatological clinic in Galway for the period March 2020–July 2021 compared to previous 4 years | A significant increase in scabies cases during March 2020–July 2021 compared with averages for the same period of the previous 4 years. |
| ITALY | De Lucia et al. [25] | Scabies admissions to a dermatological clinic in Naples during lockdown (March 2020–March 2021) | A significant increase in scabies cases, especially among patients <18 and >65 years. |
| NETHERLANDS | van Deursen et al. [5] | Two national data sources on scabies analysed during 2011–2020 | Increased scabies incidence by more than 3-fold during 2011–2020, mainly affecting adolescents and (young) adults. |
| NORWAY | Amato et al. [2] | Data from Norwegian Syndromic Surveillance System about mite infestations compared with anti-scabies treatment sales during 2006–2018 | Increased scabies management and incidence rates since 2013, based on increased consultations/sales of anti-scabies treatments. |
| POLAND | Korycinska et al. [26] | Data reports on scabies from the Polish National Health Fund for the period 2007–2014 | Highest scabies rates for the 10–19 age group; seasonality (predominantly during the autumn and winter months). |
| SPAIN | Martínez-Pallás et al. [27] | Scabies frequency data during March–May 2020 compared to the averages for the same period for the previous five years in Zaragoza | A significant increase in scabies cases during the confinement period. |
| Redondo-Bravo et al. [28] | Nationwide retrospective study of four databases (hospital admissions, patients attended at primary healthcare, outbreaks, and occupational diseases) during 1997–2019 | An increasing trend in scabies admissions during 2014–2017; among hospital admissions, the elderly predominated, while children and young adults were treated at primary health centres. | |
| TURKEY | Baykal et al. [29] | Retrospective database search on scabies prevalence from two Istanbul tertiary-level dermatology centres (January 2016–December 2019) | 2016 and 2017 saw a stable frequency of scabies diagnoses; 2018 and 2019 saw a scabies outbreak that peaked in the fourth quarter of 2019 (in both centres). |
| UNITED KINGDOM | Hewitt et al. [30] | Data from seven care homes reporting suspected scabies outbreaks in southern England over a 6-month period (November 2012–April 2013) | Scabies attack rates varied (2−50%). Cases were diagnosed clinically by GPs or care staff (none by dermatologists). Most outbreaks were attributable to delayed diagnoses. |
| VARIOUS COUNTRIES (Greece, France, The Netherlands, Serbia, Belgium, Turkey, Macedonia) | Richardson et al. [31] | A qualitative study, retrospective semi-structured telephone interviews for scabies data during November 2017–February 2018 | Clinicians confirmed scabies by clinical presentation; treatment and outbreak management varied highly. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delaš Aždajić, M.; Bešlić, I.; Gašić, A.; Ferara, N.; Pedić, L.; Lugović-Mihić, L. Increased Scabies Incidence at the Beginning of the 21st Century: What Do Reports from Europe and the World Show? Life 2022, 12, 1598. https://doi.org/10.3390/life12101598
Delaš Aždajić M, Bešlić I, Gašić A, Ferara N, Pedić L, Lugović-Mihić L. Increased Scabies Incidence at the Beginning of the 21st Century: What Do Reports from Europe and the World Show? Life. 2022; 12(10):1598. https://doi.org/10.3390/life12101598
Chicago/Turabian StyleDelaš Aždajić, Marija, Iva Bešlić, Ana Gašić, Nikola Ferara, Lovre Pedić, and Liborija Lugović-Mihić. 2022. "Increased Scabies Incidence at the Beginning of the 21st Century: What Do Reports from Europe and the World Show?" Life 12, no. 10: 1598. https://doi.org/10.3390/life12101598
APA StyleDelaš Aždajić, M., Bešlić, I., Gašić, A., Ferara, N., Pedić, L., & Lugović-Mihić, L. (2022). Increased Scabies Incidence at the Beginning of the 21st Century: What Do Reports from Europe and the World Show? Life, 12(10), 1598. https://doi.org/10.3390/life12101598







