GC–MS Profiling of Naturally Extracted Essential Oils: Antimicrobial and Beverage Preservative Actions
Abstract
1. Introduction
2. Materials and Methods
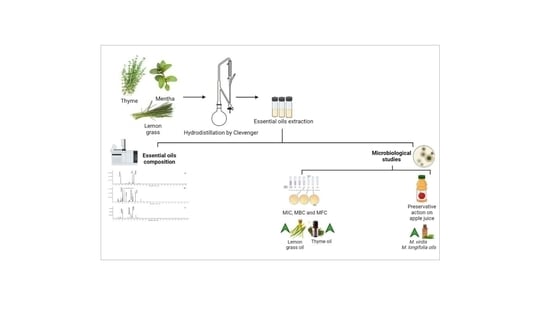
2.1. Plant Materials, Microorganisms, and a Prepared Juice Sample
2.1.1. Plant and Standard Volatiles
2.1.2. Preparation of Volatile Oils
2.1.3. Microorganisms
2.1.4. Juice Sample
2.2. Methods of Analysis
2.2.1. The Disk Diffusion Susceptibility Test
2.2.2. Minimum Inhibitory Concentration (MIC) Determination
2.2.3. Minimum Bactericidal Concentration (MBC) and Minimum Fungicidal Concentration (MFC) Determination
2.2.4. Induction of Microorganisms in Juice
2.2.5. Bacterial Count/Viable Count
2.2.6. GC–MS analysis of Essential Oils
3. Results
3.1. The Disk Diffusion Susceptibility Test
3.2. Minimum Inhibitory Concentration (MIC) Determination
3.3. Bacterial Count/Viable Count
3.4. Physical and Chemical (GC-MS) Analyses of Essential Oils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, S.C.; Lin, C.H.; Aljuffali, I.A.; Fang, J.Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Park, K.H.; Bahk, G.J. Interrelationships between Multiple Climatic Factors and Incidence of Foodborne Diseases. Int. J. Environ. Res. Public Health 2018, 15, 2482. [Google Scholar] [CrossRef] [PubMed]
- Valero, A.; Rodríguez, M.-Y.; Posada-Izquierdo, G.D.; Pérez-Rodríguez, F.; Carrasco, E.; García-Gimeno, R.M. Risk factors influencing microbial contamination in food service centers. In Significance, Prevention and Control of Food Related Diseases; IntechOpen: London, UK, 2016; pp. 27–58. [Google Scholar]
- World Health Organization. The Burden of Foodborne Diseases in the WHO European Region; WHO Regional Office for Europe: Copenhagen, Denmark, 2017. [Google Scholar]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.-N.; Tang, G.-Y.; Li, H.-B. Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. Fems. Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.N.; Kurup, A.; Tan, M.Y.; Kwa, A.L.H.; Liau, K.H.; Wilcox, M.H. Management of complicated skin and soft tissue infections with a special focus on the role of newer antibiotics. Infect. Drug Resist. 2018, 11, 1959–1973. [Google Scholar] [CrossRef] [PubMed]
- de Barros, J.C.; da Conceicao, M.L.; Neto, N.J.G.; da Costa, A.C.V.; Siqueira, J.P.; Basilio, I.D.; de Souza, E.L. Interference of Origanum vulgare L. essential oil on the growth and some physiological characteristics of Staphylococcus aureus strains isolated from foods. Lwt-Food Sci. Technol. 2009, 42, 1139–1143. [Google Scholar] [CrossRef]
- Jubelin, G.; Desvaux, M.; Schuller, S.; Etienne-Mesmin, L.; Muniesa, M.; Blanquet-Diot, S. Modulation of Enterohaemorrhagic Escherichia coli Survival and Virulence in the Human Gastrointestinal Tract. Microorganisms 2018, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Kolenda, R.; Burdukiewicz, M.; Schierack, P. A systematic review and meta-analysis of the epidemiology of pathogenic Escherichia coli of calves and the role of calves as reservoirs for human pathogenic E. coli. Front. Cell Infect. Mi 2015, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Wong, W.F.; Madhavan, P.; Yong, V.C.; Looi, C.Y. Transcriptomic and Genomic Approaches for Unravelling Candida albicans Biofilm Formation and Drug Resistance-An Update. Genes 2018, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef]
- Bondi, M.; Lauková, A.; de Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural preservatives to improve food quality and safety. Hindawi J. Food Qual. 2017, 2017, 1–3. [Google Scholar] [CrossRef]
- El-Kased, R.F. Natural antibacterial remedy for respiratory tract infections. Asian Pac. J. Trop Bio. 2016, 6, 270–274. [Google Scholar] [CrossRef]
- Yuste, J.; Fung, D.Y.C. Evaluation of Salmonella typhimurium, Yersinia enterocolitica and Staphylococcus aureus counts in apple juice with cinnamon, by conventional media and thin agar layer method. Food Microbiol. 2003, 20, 365–370. [Google Scholar] [CrossRef]
- Trinetta, V.; Morgan, M.T.; Coupland, J.N.; Yucel, U. Essential Oils Against Pathogen and Spoilage Microorganisms of Fruit Juices: Use of Versatile Antimicrobial Delivery Systems. J. Food Sci. 2017, 82, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Mota, V.D.; Turrini, R.N.T.; Poveda, V.D. Antimicrobial activity of Eucalyptus globulus oil, xylitol and papain: A pilot study. Rev. Esc. Enferm. Usp. 2015, 49, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Adelakun, O.E.; Oyelade, O.J.; Olanipekun, B.F. Use of essential oils in food preservation. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amesterdam, The Netherlands, 2016; pp. 71–84. [Google Scholar]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Plant-based phenolic molecules as natural preservatives in comminuted meats: A review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Mintah, S.O.; Asafo-Agyei, T.; Archer, M.-A.; Junior, P.A.-A.; Boamah, D.; Kumadoh, D.; Appiah, A.; Ocloo, A.; Boakye, Y.D.; Agyare, C. Medicinal plants for treatment of prevalent diseases. In Pharmacognosy-Medicinal Plants; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Ramos da Silva, L.R.; Ferreira, O.O.; Cruz, J.N.; de Jesus Pereira Franco, C.; Oliveira dos Anjos, T.; Cascaes, M.M.; Almeida da Costa, W.; Helena de Aguiar Andrade, E.; Santana de Oliveira, M. Lamiaceae Essential Oils, Phytochemical Profile, Antioxidant, and Biological Activities. Evid.-Based Complementary Altern. Med. 2021, 2021, 6748052. [Google Scholar] [CrossRef] [PubMed]
- Moumni, S.; Elaissi, A.; Trabelsi, A.; Merghni, A.; Chraief, I.; Jelassi, B.; Chemli, R.; Ferchichi, S. Correlation between chemical composition and antibacterial activity of some Lamiaceae species essential oils from Tunisia. BMC Complementary Med. Ther. 2020, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; Gago, C.; Antunes, M.D.; Lagoas, S.; Faleiro, M.L.; Megias, C.; Cortes-Giraldo, I.; Vioque, J.; Figueiredo, A.C. Antibacterial, Antioxidant, and Antiproliferative Activities of Corymbia citriodora and the Essential Oils of Eight Eucalyptus Species. Medicines 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.S.; Ferreira, M.d.F.; Moreira, J.L.; Santos, L. Simultaneous Distillation–Extraction of Essential Oils from Rosmarinus officinalis L. Cosmetics 2021, 8, 117. [Google Scholar] [CrossRef]
- Pharmacopoeia, E. Egyptian Pharmacopoeia, General Organization for Governmental; Printing Office, Ministry of Health: Cairo, Egypt, 1984; pp. 31–33.
- Padla, E.P.; Solis, L.T.; Ragasa, C.Y. Antibacterial and antifungal properties of ent-kaurenoic acid from Smallanthus sonchifolius. Chin. J. Nat. Med. 2012, 10, 408–414. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Gad, H.A.; El-Kersh, D.M. Characterization of Four Piper Essential Oils (GC/MS and ATR-IR) Coupled to Chemometrics and Their anti-Helicobacter pylori Activity. Acs Omega 2021, 6, 25652–25663. [Google Scholar] [CrossRef] [PubMed]
- Fokom, R.; Adamou, S.; Essono, D.; Ngwasiri, D.P.; Eke, P.; Mofor, C.T.; Tchoumbougnang, F.; Fekam, B.F.; Zollo, P.H.A.; Nwaga, D.; et al. Growth, essential oil content, chemical composition and antioxidant properties of lemongrass as affected by harvest period and arbuscular mycorrhizal fungi in field conditions. Ind. Crop. Prod. 2019, 138, 111477. [Google Scholar] [CrossRef]
- Gao, S.; Liu, G.; Li, J.; Chen, J.; Li, L.; Li, Z.; Zhang, X.; Zhang, S.; Thorne, R.F.; Zhang, S. Antimicrobial activity of lemongrass essential oil (Cymbopogon flexuosus) and its active component citral against dual-species biofilms of Staphylococcus aureus and Candida species. Front. Cell Infect. Mi 2020, 10, 603858. [Google Scholar] [CrossRef] [PubMed]
- Hudaib, M.; Speroni, E.; Di Pietra, A.M.; Cavrini, V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J. Pharm. Biomed. 2002, 29, 691–700. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Ahd, A.A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharm. Mag. 2010, 6, 172–175. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Łysakowska, M.; Denys, P.; Kowalczyk, E. The antimicrobial activity of thyme essential oil against multidrug resistant clinical bacterial strains. Microb. Drug Resist. 2012, 18, 137–148. [Google Scholar] [CrossRef]
- Shala, A.Y.; Gururani, M.A. Phytochemical Properties and Diverse Beneficial Roles of Eucalyptus globulus Labill.: A Review. Horticulturae 2021, 7, 450. [Google Scholar] [CrossRef]
- Mkaddem, M.; Bouajila, J.; Ennajar, M.; Lebrihi, A.; Mathieu, F.; Romdhane, M. Chemical Composition and Antimicrobial and Antioxidant Activities of Mentha (longifolia L. and viridis) Essential Oils. J. Food Sci. 2009, 74, M358–M363. [Google Scholar] [CrossRef] [PubMed]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Emad, A.M.; Rasheed, D.M.; El-Kased, R.F.; El-Kersh, D.M. Antioxidant, Antimicrobial Activities and Characterization of Polyphenol-Enriched Extract of Egyptian Celery (Apium graveolens L., Apiaceae) Aerial Parts via UPLC/ESI/TOF-MS. Molecules 2022, 27, 698. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.C.; Meireles, L.M.; Lemos, M.F.; Guimaraes, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Allenspach, M.D.; Valder, C.; Steuer, C. Absolute quantification of terpenes in conifer-derived essential oils and their antibacterial activity. J. Anal. Sci. Technol. 2020, 11, 12. [Google Scholar] [CrossRef]
- Oz, M.; Lozon, Y.; Sultan, A.; Yang, K.H.S.; Galadari, S. Effects of monoterpenes on ion channels of excitable cells. Pharm. Ther. 2015, 152, 83–97. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Somolinos, M.; Garcia, D.; Manas, P.; Condon, S.; Pagan, R. Effect of environmental factors and cell physiological state on Pulsed Electric Fields resistance and repair capacity of various strains of Escherichia coli. Int. J. Food Microbiol. 2008, 124, 260–267. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Górny, A.; Grabarczyk, M.; Wińska, K. Can eucalyptol replace antibiotics? Molecules 2021, 26, 4933. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y. Chemical Composition and In Vitro Antibacterial Activity of Mentha spicata Essential Oil against Common Food-Borne Pathogenic Bacteria. J. Pathog. 2015, 2015, 916305. [Google Scholar] [CrossRef] [PubMed]
- Mariod, A.A. Effect of essential oils on organoleptic (smell, taste, and texture) properties of food. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 131–137. [Google Scholar]


| Oil/ Microorganism | E. coli | S. aureus | C. albicans | |
|---|---|---|---|---|
| Mentha virdis | Oil Concentration (%) | Zones of Inhibition (mm) | ||
| 100 | 7 ± 0.42 | 10 ± 0.60 | 29.5 ± 1.50 | |
| 50 | 6 ± 0.21 | 12 ± 0.80 | 7 ± 0.58 | |
| 25 | 6 ± 0.23 | 8 ± 0.70 | 11.5 ± 0.95 | |
| Lavender | 100 | 5 ± 0.12 | - | - |
| 50 | 6.5 ± 0.22 | - | - | |
| 25 | 5.5 ± 0.13 | - | - | |
| Rosemary | 100 | 6 ± 0.22 | 13 ± 1.20 | - |
| 50 | 6 ± 0.40 | 8 ± 0.90 | - | |
| 25 | 2 ± 0.0 | 9 ± 0.80 | - | |
| Eucalyptus | 100 | 6 ± 0.20 | 12 ± 0.99 | 12.5 ± 1.00 |
| 50 | 10 ± 0.3 | - | 9 ± 0.95 | |
| 25 | 13 ± 0.35 | - | 8 ± 0.55 | |
| Thyme | 100 | 27 ± 1.5 | 22 ± 1.20 | 60 ± 1.50 |
| 50 | 17 ± 1.0 | 11 ± 0.99 | 45 ± 1.00 | |
| 25 | 3 ± 0.003 | - | - | |
| Lemon grass | 100 | 12 ± 0.90 | 40 ± 1.20 | 25 ± 0.80 |
| 50 | 13 ± 0.90 | 20 ± 1.20 | 16 ± 0.95 | |
| 25 | 9 ± 0.60 | 18 ± 1.10 | 10 ± 0.55 | |
| M. longifolia | 100 | 6 ± 0.30 | 4 ± 0.00 | 9 ± 0.00 |
| 50 | 6.5 ± 0.29 | - | 8 ± 0.85 | |
| 25 | 7.5 ± 0.30 | 10 ± 0.70 | 8 ± 0.95 | |
| Oregano | 100 | 7 ± 0.30 | - | - |
| 50 | 6 ± 0.28 | 8 ± 0.31 | - | |
| 25 | 8 ± 0.31 | - | - | |
| Limonene | 100 | 36 ± 2.82 | - | 36 ± 1.20 |
| 50 | 34 ± 1.22 | 10 ± 0.68 | 34 ± 1.30 | |
| 25 | 16 ± 1.10 | - | 16 ± 0.95 | |
| Oils | E. coli | S. aureus | C. albicans | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC (µL/mL) | MBC (µL/mL) | MBC: MIC | MIC (µL/mL) | MBC (µL/mL) | MBC: MIC | MIC (µL/mL) | MBC (µL/mL) | MBC: MIC | |
| Mentha virdis | 1.6 ± 0.02 | ND | NC | 12.5 ± 0.85 | ND | NC | 3.2 ± 0.85 | ND | NC |
| Lavender | 25 ± 1.20 | ND | NC | NC | NC | NC | NC | NC | NC |
| Rosemary | 4.0 ± 0.03 | ND | NC | NC | NC | NC | NC | NC | NC |
| Oregano | 25 ± 0.95 | ND | NC | 6.3 ± 0.55 | ND | NC | NC | NC | NC |
| Eucalyptus | 6.3 ± 0.80 | ND | NC | 25 ± 1.10 | ND | NC | 6.3 ± 0.95 | ND | NC |
| Thyme | 1.6 ± 0.40 | ND | NC | 1.6 ± 0.25 | 3.2 | 2:1 | 0.8 ± 0.05 | 1.6 | 2:1 |
| Lemon grass | 0.1 ± 0.00 | 0.2 | 2:1 | 0.2 ± 0.00 | 0.4 | 2:1 | 0.8 ± 0.06 | 1.6 | 2:1 |
| M. longifolia | 0.2 ± 0.00 | 0.4 | 2:1 | 50 ± 1.95 | ND | NC | 12.5 ± 1.25 | ND | NC |
| Limonene | 1.6 ± 0.45 | ND | NC | 100 ± 2.55 | NC | NC | 1.6 ± 0.85 | ND | NC |
| Physical Property | EOs Yield (mL/500 g Plant) | Specific Gravity (25 °C) | Relative Density (g/cm3) | Refractive Index (20 °C) | Appearance | Color | Odor |
|---|---|---|---|---|---|---|---|
| Lemon grass oil | 6.4 ± 1.2 | 0.9012 ± 0.03 | 0.893 ± 0.01 | 1.4862 ± 0.16 | Clear oil | Pale yellow | citrus |
| Thyme oil | 6.0 ± 0.10 | 0.9180 ± 0.04 | 0.865 ± 0.06 | 1.4823 ± 0.15 | Clear oil | Pale yellow | thyme |
| Mentha oil | 5.5 ± 0.21 | 0.9160 ± 0.01 | 0.910 ± 0.08 | 1.4638 ± 0.11 | Clear oil | Pale yellow | mentha |
| Peak No. | Rt. (Min.) | Name of Compound | KI (Obs.) | KI (Lit.) | Area (%) | Chemical Class | Identification | ||
|---|---|---|---|---|---|---|---|---|---|
| Lemon Grass Oil | Thyme Oil | Mentha Oil | |||||||
| 1 | 7.17 | β-Thujene | 910 | 902 | _ | 2.09 ± 0.720 | _ | Monoterpene | MS, KI |
| 2 | 7.19 | α-thujene | 910 | 905 | _ | _ | 0.04 ± 0.001 | Monoterpenes | MS, KI |
| 3 | 7.37 | α-pinene | 917 | 917 | _ | 1.68 ± 0.0320 | 1.2 ± 0.030 | Monoterpenes | MS, KI |
| 4 | 7.72 | 2,4(10)-Thujadiene | 930 | 946 | _ | 0.04 ± 0.0050 | _ | Monoterpene | MS, KI |
| 5 | 7.81 | Camphene | 933 | 929 | _ | 0.86 ± 0.020 | 0.21 ± 0.004 | Monoterpenoids | MS, KI |
| 6 | 8.57 | Sabinene | 961 | 964 | _ | _ | 1.07 ± 0.560 | Monoterpenes | MS, KI |
| 7 | 8.58 | 4(10)-Thujene | 961 | 969 | _ | 0.19 ± 0.001 | _ | Monoterpene | MS, KI |
| 8 | 8.65 | β-pinene | 964 | 965 | _ | 0.62 ± 0.050 | 1.54 ± 0.580 | Monoterpenes | MS, KI |
| 9 | 8.82 | Vinyl amyl carbinol | 970 | 969 | _ | 1.23 ± 0.063 | _ | Alkenyl alcohol | MS, KI |
| 10 | 9.16 | β-Myrcene | 982 | 985 | 11.69 ± 1.300 | 3.36 ± 0.730 | 0.94 ± 0.020 | Monoterpenes | MS, KI |
| 11 | 9.33 | 3-octanol | 988 | 988 | _ | 0.15 ± 0.003 | _ | Aliphatic alcohol | MS, KI |
| 12 | 9.49 | pseudolimonene | 994 | 996 | _ | _ | 0.3 ± 0.002 | Monoterpenes | MS, KI |
| 13 | 9.51 | a-Phellandrene | 995 | 994 | _ | 0.22 ± 0.002 | _ | Monoterpene | MS, KI |
| 14 | 9.69 | 3-Carene | 1001 | 1004 | _ | 0.14 ± 0.003 | _ | Monoterpene | MS, KI |
| 15 | 9.9 | 4-Carene | 1008 | 1011 | _ | 2.53 ± 0.430 | _ | Monoterpene | MS, KI |
| 16 | 9.9 | 2-carene | 1008 | 1010 | _ | _ | 0.07 ± 0.001 | Monoterpenes | MS, KI |
| 17 | 10.17 | O-cymene | 1017 | 1021 | _ | _ | 0.12 ± 0.001 | Monoterpenes | MS, KI |
| 18 | 10.39 | Eucalyptol | 1024 | 1020 | 0.05 ± 0.010 | 24.30 ± 3.530 | 17.40 ± 3.220 | Monoterpene oxide | MS, KI |
| 19 | 10.59 | trans-β-ocimene | 1030 | 1042 | _ | _ | 0.19 ± 0.030 | Monoterpenes | MS, KI |
| 20 | 10.93 | β-ocimene | 1041 | 1046 | 0.26 ± 0.020 | 0.04 | 0.07 ± 0.005 | Monoterpenes | MS, KI |
| 21 | 11.23 | γ-terpinene | 1051 | 1055 | _ | 15.2 ± 1.720 | 0.17 ± 0.020 | Monoterpenes | MS, KI |
| 22 | 11.49 | trans-sabinene hydrate | 1059 | 1051 | _ | 1.2 ± 0.040 | 0.33 ± 0.060 | Monoterpenes | MS, KI |
| 23 | 11.67 | Linalool oxide | 1065 | 1071 | _ | 0.28 ± 0.002 | _ | Monoterpene | MS, KI |
| 24 | 12.52 | β-linalool | 1092 | 1098 | 1.58 ± 0.340 | 4.79 ± 0.050 | 0.64 ± 0.030 | Monoterpenes | MS, KI |
| 25 | 12.63 | Cis-verbenol | 1096 | 1095 | 0.90 ± 0.030 | _ | _ | Monoterpenes | MS, KI |
| 26 | 13.74 | Pinen-3-ol | 1131 | 1131 | _ | _ | 0.12 ± 0.006 | Monoterpene ketone | MS, KI |
| 27 | 13.89 | Camphor isomer | 1136 | 1141 | _ | _ | 1.50 ± 0.400 | Monoterpenoid ketone | MS, KI |
| 28 | 13.9 | Camphor | 1137 | 1131 | 0.40 ± 0.010 | 0.97 ± 0.050 | _ | Monoterpene ketone | MS, KI |
| 29 | 14.19 | Isomenthone | 1146 | 1148 | _ | 0.60 ± 0.030 | _ | Monoterpenoid | MS, KI |
| 30 | 14.52 | t-verbenol | 1156 | 1148 | 2.27 ± 0.010 | _ | _ | Monoterpenes | MS, KI |
| 31 | 14.59 | Isoborneol | 1056 | 1159 | _ | 2.5 ± 0.060 | 0.86 ± 0.300 | Monoterpene alcohol | MS, KI |
| 32 | 14.79 | Menthol | 1165 | 1164 | __ | 0.88 ± 0.040 | _ | Monoterpenoid | MS, KI |
| 33 | 14.92 | Terpinen-4-ol | 1170 | 1177 | _ | 2.19 ± 0.060 | 0.77 ± 0.030 | Monoterpene alcohol | MS, KI |
| 34 | 15.1 | Levomenthol | 1175 | 1172 | _ | 0.03 ± 0.005 | _ | Monoterpenoid | MS, KI |
| 35 | 15.13 | Isoneral | 1174 | 1174 | 2.91 ± 0.02 | _ | _ | MS, KI | |
| 36 | 15.39 | α-Terpineol | 1185 | 1189 | _ | 0.56 ± 0.005 | _ | Monoterpene alcohol | MS, KI |
| 37 | 15.58 | Dihydrocarveol | 1191 | 1202 | _ | _ | 12.9 ± 2.600 | Monoterpene alcohol | MS, KI |
| 38 | 15.61 | Trans-Dihydrocarvone | 1192 | 1195 | _ | 0.14 ± 0.003 | 0.35 ± 0.030 | Monoterpenoid | MS, KI |
| 39 | 15.764 | Decanal | 1197 | 1207 | 0.42 ± 0.020 | _ | _ | Monoterpenes | MS, KI |
| 40 | 16.45 | trans-carveol | 1220 | 1219 | _ | _ | 5.99 ± 1.600 | Monoterpene alcohol | MS, KI |
| 41 | 16.518 | Citronellol | 1223 | 1228 | 1.23 ± 0.040 | 1.62 ± 0.040 | _ | Monoterpenoid | MS, KI |
| 42 | 16.84 | carveol | 1234 | 1246 | _ | _ | 2.65 ± 0.860 | Monoterpene alcohol | MS, KI |
| 43 | 16.921 | Isothymol methyl ether | 1237 | 1244 | _ | 6.14 ± 0.720 | _ | Aromatic monoterpenoid | MS, KI |
| 44 | 17.02 | Neral (β-citral) | 1240 | 1240 | 34.99 ± 3.530 | _ | _ | Monoterpene aldehyde | MS, KI |
| 45 | 17.22 | carvone | 1247 | 1248 | _ | _ | 42.5 ± 6.800 | Monoterpene ketone | MS, KI |
| 46 | 17.35 | cis-Geraniol | 1252 | 1254 | 4.24 ± 0.520 | 0.39 ± 0.002 | _ | Monoterpene alcohol | MS, KI |
| 47 | 17.52 | Piperitone | 1258 | 1254 | _ | 0.08 ± 0.003 | _ | Monoterpene ketone | MS, KI |
| 48 | 17.7 | Carvenone | 1263 | 1258 | _ | 0.01 ± 0.002 | _ | Methane monoterpenoid | MS, KI |
| 49 | 17.81 | Citronellyl formate | 1268 | 1273 | _ | 0.4 ± 0.030 | _ | fatty alcohol ester | MS, KI |
| 50 | 17.94 | Geranial | 1272 | 1277 | 36.35 ± 4.230 | _ | _ | Monoterpene aldehyde | MS, KI |
| 51 | 18.16 | Bornyl acetate | 1280 | 1284 | _ | _ | 0.1 ± 0.003 | Monoterpenes | MS, KI |
| 52 | 18.38 | 2-Undecanone | 1288 | 1292 | 0.08 ± 0.010 | _ | _ | Organic ketone | MS, KI |
| 53 | 18.47 | Carvacrol | 1299 | 1300 | _ | 1.54 ± 0.021 | _ | Monoterpenoid phenol | MS, KI |
| 54 | 18.5 | Thymol | 1292 | 1292 | _ | 17.4 ± 3.410 | _ | Monoterpenoid phenol | MS, KI |
| 55 | 18.76 | Dihydrocarvenyl acetate | 1301 | 1304 | _ | _ | 0.03 ± 0.001 | Monoterpenes | MS, KI |
| 56 | 18.77 | Undecanal | 1301 | 1303 | 0.02 ± 0.001 | _ | _ | Organic aldehyde | MS, KI |
| 57 | 18.92 | Isopulegyl acetate | 1307 | 1335 | _ | _ | 0.03 ± 0.004 | Monoterpenes | MS, KI |
| 58 | 19.36 | Dihydrocarvyl acetate | 1322 | 1344 | _ | _ | 3.97 ± 0.053 | Monoterpenes | MS, KI |
| 59 | 19.43 | Geranic acid | 1355 | 1347 | 0.06 ± 0.002 | _ | _ | Poly unsaturated fatty acid | MS, KI |
| 60 | 20.02 | Citronellol acetate | 1345 | 1355 | 0.02 ± 0.002 | _ | _ | Sesquiterpenes | MS, KI |
| 61 | 20.071 | Thymyl acetate | 1347 | 1349 | _ | 0.45 ± 0.001 | _ | Monoterpene | MS, KI |
| 62 | 20.31 | Geranic acid isomer | 1355 | 1355 | 0.25 ± 0.030 | _ | _ | Poly unsaturated fatty acid | MS, KI |
| 63 | 20.31 | Carvyl acetate | 1355 | 1346 | _ | _ | 1.51 ± 0.090 | Monoterpenes | MS, KI |
| 64 | 20.67 | Isobornyl propionate | 1368 | 1388 | _ | 0.06 ± 0.003 | _ | Sesquiterpene | MS, KI |
| 65 | 20.67 | α-copaene | 1368 | 1372 | _ | _ | 0.02 ± 0.004 | Sesquiterpene | MS, KI |
| 66 | 20.83 | Geranyl acetate | 1374 | 1368 | 0.77 ± 0.021 | _ | _ | Sesquiterpenes | MS, KI |
| 67 | 20.93 | β-Bourbonene | 1377 | 1385 | _ | 0.15 ± 0.002 | 0.76 ± 0.050 | Sesquiterpene | MS, KI |
| 68 | 21.1 | β-Elemene | 1383 | 1384 | _ | _ | 0.29 ± 0.040 | Sesquiterpene | MS, KI |
| 69 | 21.48 | Methyl eugenol | 1396 | 1395 | 1.29 ± 0.310 | _ | _ | Phenyl propanoid | MS, KI |
| 70 | 21.89 | Caryophyllene | 1411 | 1418 | 0.02 ± 0.004 | 2.46 ± 0.540 | 0.46 ± 0.030 | Sesquiterpenes | MS, KI |
| 71 | 22.14 | α-copaene | 1421 | 1380 | _ | 0.04 ± 0.002 | _ | Hydrocarbon | MS, KI |
| 72 | 22.26 | t-α-Bergamotene | 1426 | 1436 | 0.03 ± 0.001 | _ | _ | Bicyclic monoterpenes | MS, KI |
| 73 | 22.425 | Citronellyl propionate | 1433 | 1444 | _ | 0.01 ± 0.001 | _ | fatty alcohol ester | MS, KI |
| 74 | 22.54 | Germacrene D | 1437 | 1477 | _ | 0.01 ± 0.002 | 0.13 ± 0.007 | Sesquiterpene | MS, KI |
| 75 | 22.79 | cis-a-Bisabolene | 1488 | 1518 | 0.02 ± 0.004 | _ | _ | Sesquiterpenes | MS, KI |
| 76 | 22.79 | Humulene | 1447 | 1442 | _ | 0.12 ± 0.006 | 0.05 ± 0.000 | Sesquiterpene | MS, KI |
| 77 | 23.04 | ( + )-epi-Bicyclosesquiphellandrene | 1457 | 1452 | _ | _ | 0.12 ± 0.020 | Sesquiterpene | MS, KI |
| 78 | 23.241 | Geranyl isovalerate | 1464 | 1582 | _ | 0.14 ± 0.003 | _ | fatty alcohol ester | MS, KI |
| 79 | 23.34 | (Z,Z)-α-Farnesene | 1468 | 1506 | _ | _ | 0.01 ± 0.001 | Sesquiterpene | MS, KI |
| 80 | 23.38 | γ-Muurolene | 1470 | 1477 | _ | 0.66 ± 0.007 | _ | Sesquiterpene | MS, KI |
| 81 | 23.42 | Ylangene | 1471 | 1470 | _ | _ | 0.02 ± 0.003 | Sesquiterpene | MS, KI |
| 82 | 23.52 | Germacrene D isomer | 1482 | 1475 | _ | _ | 0.16 ± 0.001 | Sesquiterpene | MS, KI |
| 83 | 23.73 | α-Guaiene | 1483 | 1490 | _ | _ | 0.03 ± 0.000 | Sesquiterpene | MS, KI |
| 84 | 24.35 | γ-Muurolene | 1508 | 1491 | _ | _ | 0.1 ± 0.000 | Sesquiterpene | MS, KI |
| 85 | 24.36 | γ- Cadinene | 1508 | 1513 | _ | 0.08 ± 0.001 | _ | Sesquiterpene | MS, KI |
| 86 | 24.58 | Delta- Cadinene | 1517 | 1523 | _ | 0.11 ± 0.000 | _ | Sesquiterpene | MS, KI |
| 87 | 24.59 | Cis-Calamenene | 1517 | 1531 | _ | _ | 0.16 ± 0.000 | Sesquiterpene | MS, KI |
| 88 | 25.93 | α-Bourbonene | 1569 | 1531 | _ | 0.02 ± 0.005 | _ | Sesquiterpene | MS, KI |
| 89 | 26.01 | Spathulenol | 1573 | 1576 | _ | 0.02 ± 0.001 | _ | Sesquiterpene | MS, KI |
| 90 | 26.15 | Caryophyllene oxide | 1578 | 1577 | 0.06 ± 0.002 | 0.97 ± 0.130 | 0.08 ± 0.001 | Sesquiterpene | MS, KI |
| 91 | 26.9 | Eudesmol | 1608 | 1602 | _ | 0.02 ± 0.001 | _ | Sesquiterpene | MS, KI |
| 92 | 27.04 | Cadinol | 1614 | 1635 | _ | 0.24 ± 0.060 | _ | Sesquiterpene | MS, KI |
| Total monoterpenes (%) | 97.10 | 93.04 | 97.61 | ||||||
| Total sesquiterpenes (%) | 0.89 | 4.92 | 2.39 | ||||||
| Miscellaneous (%) | 1.70 | 1.97 | _ | ||||||
| Total percentage (Approximated %) | 100 | 100 | 100 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Kased, R.F.; El-Kersh, D.M. GC–MS Profiling of Naturally Extracted Essential Oils: Antimicrobial and Beverage Preservative Actions. Life 2022, 12, 1587. https://doi.org/10.3390/life12101587
El-Kased RF, El-Kersh DM. GC–MS Profiling of Naturally Extracted Essential Oils: Antimicrobial and Beverage Preservative Actions. Life. 2022; 12(10):1587. https://doi.org/10.3390/life12101587
Chicago/Turabian StyleEl-Kased, Reham F., and Dina M. El-Kersh. 2022. "GC–MS Profiling of Naturally Extracted Essential Oils: Antimicrobial and Beverage Preservative Actions" Life 12, no. 10: 1587. https://doi.org/10.3390/life12101587
APA StyleEl-Kased, R. F., & El-Kersh, D. M. (2022). GC–MS Profiling of Naturally Extracted Essential Oils: Antimicrobial and Beverage Preservative Actions. Life, 12(10), 1587. https://doi.org/10.3390/life12101587