The Effectiveness of Dry Needling in Patients with Hip or Knee Osteoarthritis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Eligibility Criteria and Study Selection
2.4. Data Extraction
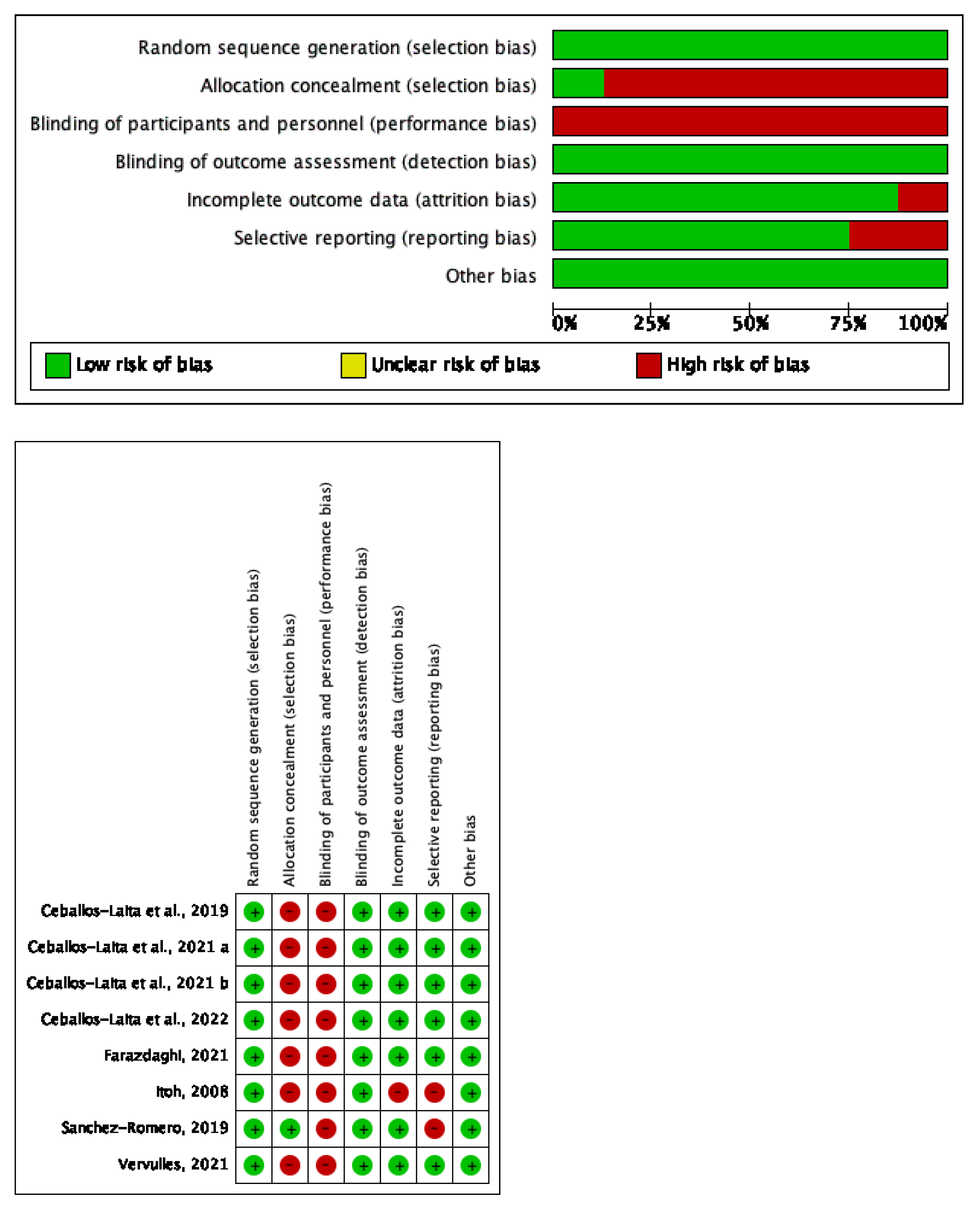
2.5. Risk of Bias and Quality of Evidence
2.6. Data Synthesis and Analysis
3. Results
3.1. Literature Search and Screening
3.2. Characteristics of the Eligible Studies
3.3. Outcome Measures
3.4. Study Quality and Risk of Bias
3.5. Synthesis of Results
3.5.1. Pain Intensity
3.5.2. Physical Function
3.5.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy
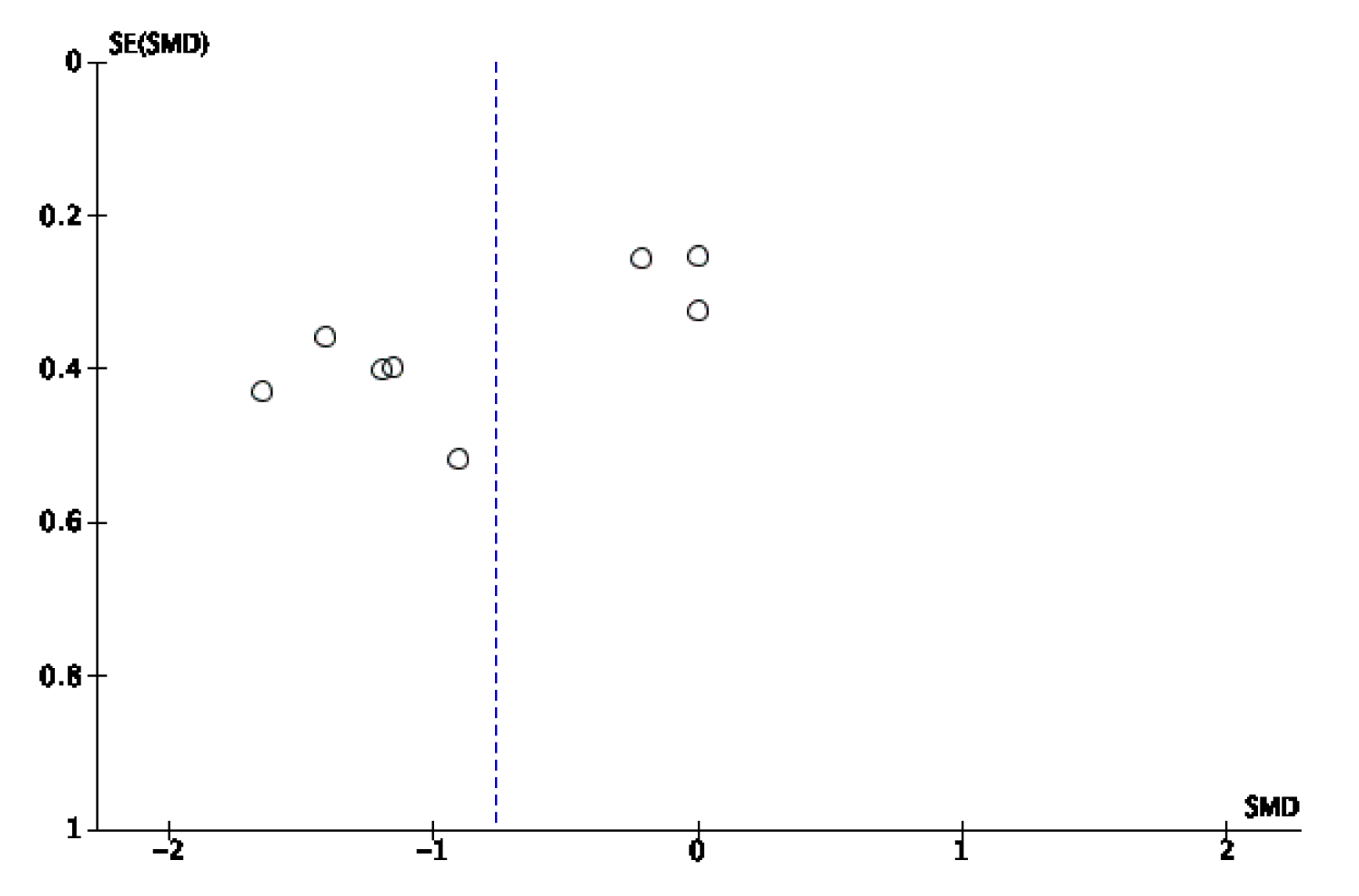
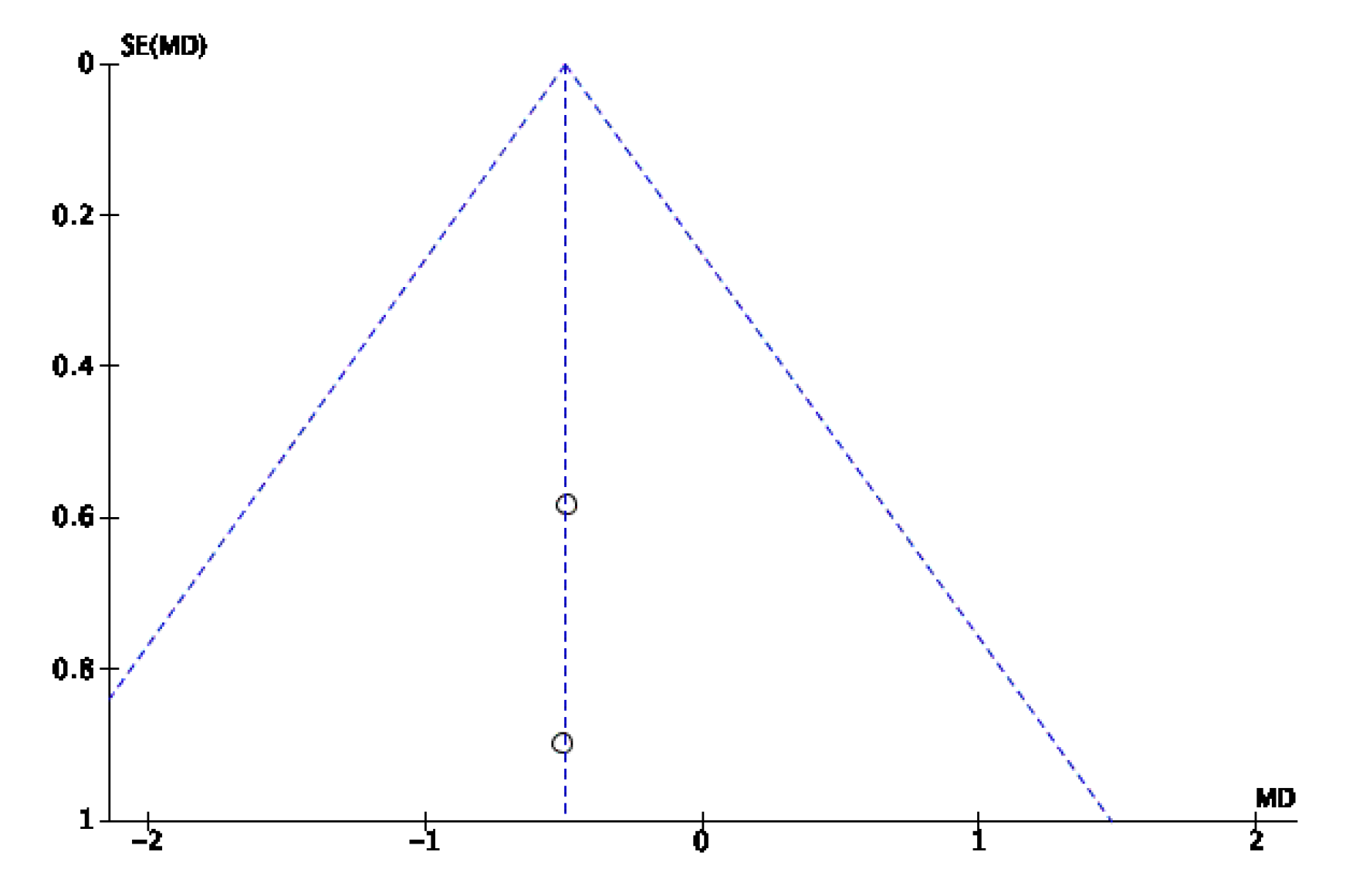
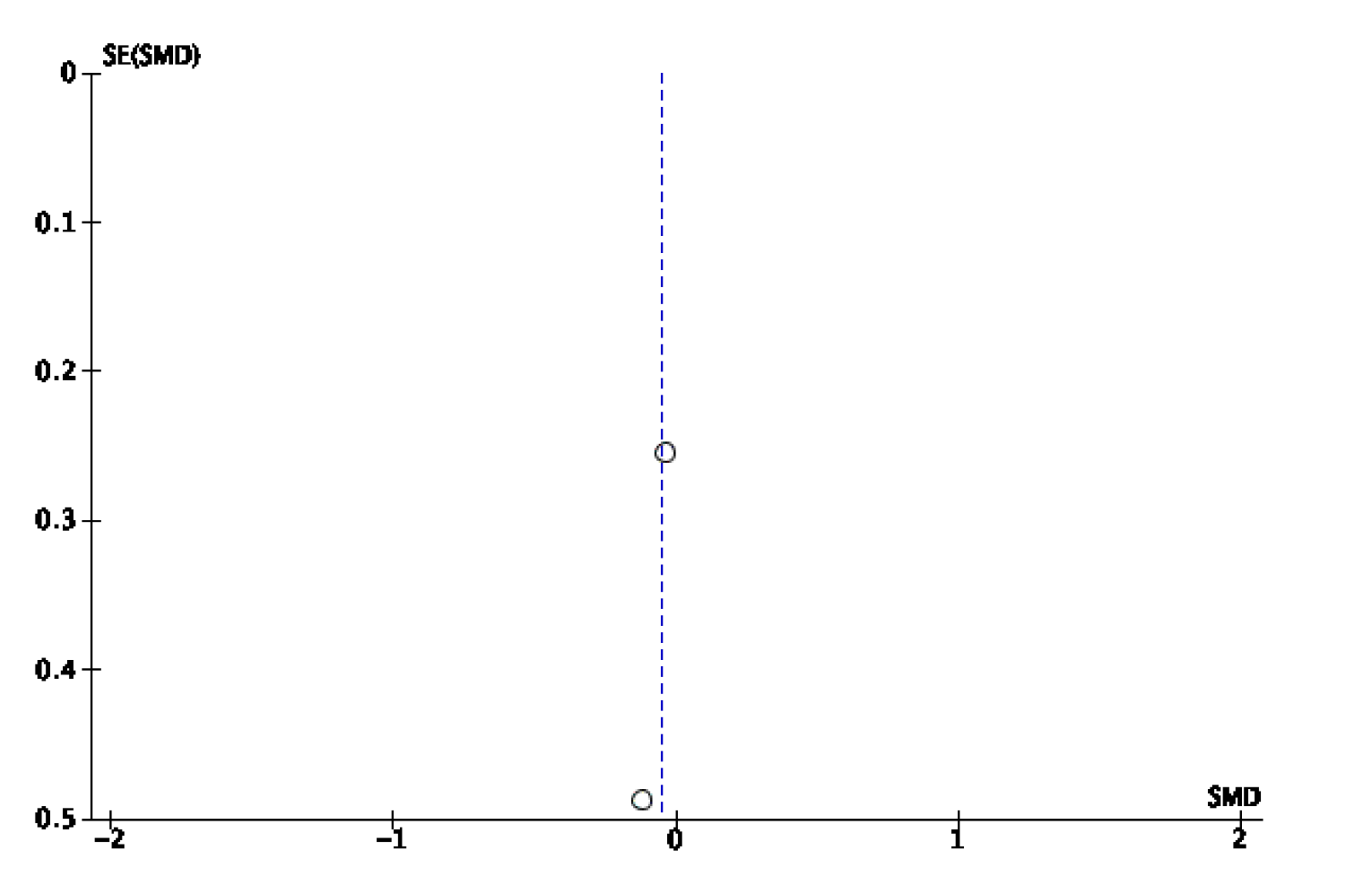
Appendix B. Funnel Plots



Appendix C. GRADE Summary
| Assessment Cernainly | No. de Pacientes | Effect | Certainly | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectly Evidence | Imprecision | Otrhers | [Intervention Group ] | [Comparation Group] | Relative(95% CI) | Absolute(95% CI) | ||
| Pain intensity short-term | ||||||||||||
| 7 | RCTs | Serious a | Serious b | Not serious | Serious d | None | 131 | 115 | - | SMD 0.75 less (1.27 less to 0.23 less) | ⨁◯◯◯Very Low | Critical |
| Function short term | ||||||||||||
| 6 | RCTs | Serious a | Serious b | Not serious | Serious d | None | 100 | 30 | - | SMD 0.96 less (1.58 less to 0.34 less) | ⨁◯◯◯Very Low | Critical |
| Pain intensity medium term | ||||||||||||
| 2 | RCTs | Serious a | Serious c | Not serious | Serious e | None | 39 | 38 | - | SMD 0.49 less (1.45 less to 0.46 higher) | ⨁◯◯◯Very Low | Critical |
| Pain intensity long term | ||||||||||||
| 2 | RCTs | Serious a | Serious c | Not serious | Serious e | None | 39 | 38 | - | SMD 0.24 less (0.68 less to 0.2 higher) | ⨁◯◯◯Very Low | Critical |
| Function medium term | ||||||||||||
| 2 | RCTs | Serious a | Serious c | Not serious | Serious e | None | 39 | 38 | - | SMD 0.14 less (0.58 less to 0.3 higher) | ⨁◯◯◯Very Low | Critical |
| Function long term | ||||||||||||
| 2 | RCTs | Serious a | Serious c | Not serious | Serious e | None | 39 | 38 | - | SMD 0.05 less (0.5 less to 0.39 higher) | ⨁◯◯◯Very Low | Critical |
References
- Bennell, K. Physiotherapy management of hip osteoarthritis. J. Physiother. 2013, 59, 145–157. [Google Scholar] [CrossRef]
- Guillemin, F.; Rat, A.; Mazieres, B.; Pouchot, J.; Fautrel, B.; Euller-Ziegler, L.; Fardellone, P.; Morvan, J.; Roux, C.; Verrouil, E.; et al. Prevalence of symptomatic hip and knee osteoarthritis: A two-phase population-based survey. Osteoarthr. Cartil. 2011, 19, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Severo, M.; Santos, R.A.; Barros, H.; Branco, J.; Lucas, R.; Costa, L.; Ramos, E. Knee and hip radiographic osteoarthritis features: Differences on pain, function and quality of life. Clin. Rheumatol. 2015, 35, 1555–1564. [Google Scholar] [CrossRef]
- Slemenda, C.; Brandt, K.D.; Heilman, D.K.; Mazzuca, S.; Braunstein, E.M.; Katz, B.P.; Wolinsky, F. Quadriceps Weakness and Osteoarthritis of the Knee. Ann. Intern. Med. 1997, 127, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.M. Myofascial trigger point, falls in the elderly, idiopathic knee pain and osteoarthritis: An alternative concept. Med. Hypotheses 2013, 80, 806–809. [Google Scholar] [CrossRef]
- Becker, R.; Berth, A.; Nehring, M.; Awiszus, F. Neuromuscular quadriceps dysfunction prior to osteoarthritis of the knee. J. Orthop. Res. 2004, 22, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Herzog, W.; Block, J.; Bennell, K. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat. Rev. Rheumatol. 2010, 7, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.; Glass, N. Is Quadriceps Muscle Weakness a Risk Factor for Incident or Progressive Knee Osteoarthritis? Phys. Sportsmed. 2011, 39, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Herzog, W.; Longino, D.; Clark, A. The role of muscles in joint adaptation and degeneration. Langenbeck’s Arch. Surg. 2003, 388, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Dor, A.; Kalichman, L. A myofascial component of pain in knee osteoarthritis. J. Bodyw. Mov. Ther. 2017, 21, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Romero, E.A.; Fernández Carnero, J.; Villafañe, J.H.; Calvo-Lobo, C.; Ochoa Sáez, V.; Burgos Caballero, V.; Pecos Martín, D. Prevalence of myofascial trigger points in patients with mild to moderate painful knee osteoarthritis: A secondary analysis. J. Clin. Med. 2020, 9, 2561. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.; Travell, J.G.; Simons, L. Myofascial Pain and Dysfunction: The Trigger Point Manual, 2nd ed.; Editorial Panamericana: Madrid, Spain, 2007; p. 44. [Google Scholar]
- Dommerholt, J.; Mayoral del Moral, O.; Gröbli, C. Trigger Point Dry Needling. J. Man. Manip. Ther. 2006, 14, 70E–87E. [Google Scholar] [CrossRef]
- Hong, C.-Z.; Torigoe, Y. Electrophysiological Characteristics of Localized Twitch Responses in Responsive Taut Bands of Rabbit Skeletal Muscle Fibers. J. Musculoskelet. Pain 1994, 2, 17–43. [Google Scholar] [CrossRef]
- Hong, C.-Z.; Torigoe, Y.; Yu, J. The Localized Twitch Responses in Responsive Taut Bands of Rabbit Skeletal Muscle Fibers Are Related to the Reflexes at Spinal Cord Level. J. Musculoskelet. Pain 1995, 3, 15–33. [Google Scholar] [CrossRef]
- Llurda-Almuzara, L.; Labata-Lezaun, N.; Meca-Rivera, T.; Navarro-Santana, M.J.; Cleland, J.A.; Fernández-De-Las-Peñas, C.; Pérez-Bellmunt, A. Is Dry Needling Effective for the Management of Plantar Heel Pain or Plantar Fasciitis? An Updated Systematic Review and Meta-Analysis. Pain Med. 2021, 22, 1630–1641. [Google Scholar] [CrossRef]
- Pourahmadi, M.; Dommerholt, J.; Fernández-De-Las-Peñas, C.; Koes, B.W.; Mohseni-Bandpei, M.A.; Mansournia, M.A.; Delavari, S.; Keshtkar, A.; Bahramian, M. Dry Needling for the Treatment of Tension-Type, Cervicogenic, or Migraine Headaches: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzab068. [Google Scholar] [CrossRef]
- Sánchez-Infante, J.; Navarro-Santana, M.J.; Bravo-Sánchez, A.; Jiménez-Diaz, F.; Abián-Vicén, J. Is dry needling applied by physical therapists effective for pain in musculoskeletal conditions? a systematic review and meta-analysis. Phys Ther. 2021, 101, pzab070. [Google Scholar] [CrossRef]
- Rahou-el-bachiri, Y.; Navarro-santana, M.J.; Guido, F.G.; Cleland, J.A.; Ibai, L. Effects of Trigger Point Dry Needling for the Management of Knee Pain Syndromes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2044. [Google Scholar] [CrossRef]
- Lin, X.; Li, F.; Lu, H.; Zhu, M.; Peng, T.Z.; Albadrany, Y. Acupuncturing of myofascial pain trigger points for the treatment of knee osteoarthritis: A systematic review and meta-analysis. Medicine 2022, 101, E28838. [Google Scholar] [CrossRef]
- Ughreja, R.A.; Prem, V. Effectiveness of dry needling techniques in patients with knee osteoarthritis: A systematic review and meta-analysis. J. Bodyw. Mov. Ther. 2021, 27, 328–338. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Alarcon, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Wolfe, F. The American College of Rheumatology criteria for the classifi-cation and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991, 34, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, A.P.; De Vet, H.C.; De Bie, R.A.; Kessels, A.G.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef]
- Young, J.L.; Rhon, D.; de Zoete, R.M.; Cleland, J.A.; Snodgrass, S.J. The influence of dosing on effect size of exercise therapy for musculoskeletal foot and ankle disorders: A systematic review. Braz. J. Phys. Ther. 2017, 22, 20–32. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Xie, C.X.; Machado, G.C. Clinimetrics: Grading of Recommendations, Assessment, Development and Evaluation (GRADE). J. Physiother. 2020, 67, 66. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Cciences; Lawrence Erlbaum Associates Publishers: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2019; pp. 143–176. [Google Scholar]
- Sánchez-Romero, E.A.; Pecos-Martín, D.; Calvo-Lobo, C.; Ochoa-Sáez, V.; Burgos-Caballero, V.; Fernández-Carnero, J. Effects of dry needling in an exercise program for older adults with knee osteoarthritis. Medicine 2018, 97. [Google Scholar] [CrossRef]
- Pang, J.C.Y.; Fu, A.S.N.; Lam, S.K.H.; Fu, A.C.L. Effectiveness of ultrasound-guided dry needling in physiotherapy management of knee osteoarthri-tis: A randomized, double-blinded and controlled study. Physiotherapy 2022, 114, e176–e177. [Google Scholar] [CrossRef]
- Ceballos-Laita, L.; Jiménez-Del-Barrio, S.; Marín-Zurdo, J.; Moreno-Calvo, A.; Bone, J.M.; Albarova-Corral, M.I.; Estébanez-De-Miguel, E. Effects of dry needling on pain, pressure pain threshold and psychological distress in patients with mild to moderate hip osteoarthritis: Secondary analysis of a randomized controlled trial. Complement. Ther. Med. 2020, 51, 102443. [Google Scholar] [CrossRef]
- Itoh, K.; Hirota, S.; Katsumi, Y.; Ochi, H.; Kitakoji, H. Trigger point acupuncture for treatmente of knee osteoarthritis. A preliminary RCT for a pragmatic trial. Acupunct Med. 2008, 26, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Romero, E.A.; Fernández-Carnero, J.; Calvo-Lobo, C.; Ochoa Sáez, V.; Burgos Caballero, V.; Pecos-Martín, D. Is a Combination of Exercise and Dry Needling Effective for Knee OA? Pain Med. 2020, 21, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Vervullens, S.; Meert, L.; Baert, I.; Delrue, N.; Heusdens, C.H.W.; Hallemans, A.; Van Criekinge, T.; Smeets, R.J.E.M.; De Meulemeester, K. The effect of one dry needling session on pain, central pain processing, muscle co-contraction and gait characteristics in patients with knee osteoarthritis: A randomized controlled trial. Scand. J. Pain 2021, 22, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Laita, L.; Jiménez-Del-Barrio, S.; Marín-Zurdo, J.; Moreno-Calvo, A.; Bone, J.M.; Albarova-Corral, M.I.; Estébanez-De-Miguel, E. Effects of dry needling in HIP muscles in patients with HIP osteoarthritis: A randomized controlled trial. Musculoskelet. Sci. Pract. 2019, 43, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Laita, L.; Jiménez-del-Barrio, S.; Marín-Zurdo, J.; Moreno-Calvo, A.; Marín-Boné, J.; Albarova-Corral, M.I.; Estébanez-de-Miguel, E. Effectiveness of Dry Needling Therapy on Pain, Hip Muscle Strength and Physical Function in Patients with Hip Osteoarthritis: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2021, 102, 959–966. [Google Scholar] [CrossRef]
- Ceballos-Laita, L.; Jiménez-Del-Barrio, S.; Marín-Zurdo, J.; Moreno-Calvo, A.; Marín-Boné, J.; Albarova-Corral, M.I.; Estébanez-De-Miguel, E. Comparison of dry needling and self-stretching in muscle extensibility, pain, stiffness, and physical function in hip osteoarthritis: A randomized controlled trial. Complement. Ther. Clin. Pract. 2022, 49, 101667. [Google Scholar] [CrossRef]
- Farazdaghi, M.R.; Yoosefinejad, A.K.; Abdollahian, N.; Rahimi, M.; Motealleh, A. Dry needling trigger points around knee and hip joints improves function in patients with mild to moderate knee osteoarthritis. J. Bodyw. Mov. Ther. 2021, 27, 597–604. [Google Scholar] [CrossRef]
- Kamper, S.J. Blinding: Linking Evidence to Practice. J. Orthop. Sports Phys. Ther. 2018, 48, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Halle, J.S.; Halle, R.J. Pertinent dry needling considerations for minimizing adverse effects—Part one. Int. J. Sports Phys. Ther. 2016, 11, 651–662. [Google Scholar]



| Population | Intervention | Control | Outcomes |
|---|---|---|---|
| Osteoarthritis | Dry needling | - | symptom |
| Hip osteoarthritis | Trigger point acupuncture | pain | |
| Knee osteoarthritis | Intramuscular stimulation | function | |
| Thumb osteoarthritis | physical function | ||
| Spinal osteoarthritis | functional capacity |
| Participants | Intervention | Outcome (Tool) | Main Results | Follow-Up | |||||
|---|---|---|---|---|---|---|---|---|---|
| Author | N (Sex Ratio) | Mean Age (SD) | Diagnosis | DN Group | Control Group | ||||
| Itoh et al., 2008 [34] | 15 | DN: 74.20 (8.10) Control: 73.30 (6.50) | Knee OA | 5 sessions (1 per week) (n = 8) | 5 sessions of sham DN (1 per week) (n = 7) | -Pain (VAS) -Physical function (WOMAC) | ↑ VAS and WOMAC in DN group vs. control group | ↑ VAS and WOMAC in DN group vs. control group at 10 weeks of follow-up but not at 20 weeks of follow-up | |
| Ceballos-Laita et al., 2019 [37] | 30 (17M/13F) | DN: 55.5 (4.70)Control: 58.6 (6.60) | Hip OA | 3 sessions (1 per week) (n = 15) | 3 sessions of sham DN (1 per week) (n = 15) | -Pain (VAS) -Physical function (SPWT) | ↑ VAS and SPWT in DN group vs. control group | No data | |
| Sanchez-Romero et al., 2020 [35] | 62 (18M/44F) | DN: 72.97 (6.29) Control: 71.65 (5.00) | Knee OA | 6 sessions (1 per week) + Exercise therapy. (n =31) | Exercise therapy (n =31) | -Pain (VAS) -Physical function (WOMAC) | No between-groups differences | No between-groups differences at 6, 9 or 12 months of follow-up | |
| Ceballos-Laita et al., 2021 [38] | 45 (20M/25F) | DN: 57.53 (3.88) Control: 54.67 (4.48) Other: 58.20 (5.08) | Hip OA | 3 sessions (1 per week) (n = 15) | 3 sessions of sham DN (1 per week) (n = 15) | No additional intervention (n = 15) | -Pain (VAS) -Physical function (WOMAC PF) | ↑ VAS, and WOMAC PF in DN group vs. control and sham groups | No data |
| Farazdaghi et al., 2021 [40] | 40 (40F) | NR | Knee OA | 3 sessions (3 per week) (n = 20) | 3 sessions of sham DN in one week (n = 20) | -Pain (VAS) -Physical function (SPWT) | ↑ VAS and SPWT in DN group vs. control group | No data | |
| Vervullens et al., 2021 [36] | 61 (27M/34F) | DN: 63.00 (10.00) Control: 66.00 (10.00) | Knee OA | 1 session (n = 31) | 1 session of sham DN (n = 30) | -Pain (VAS) | No between-groups differences | No data | |
| Ceballos-Laita et al., 2022 [39] | 38 (18M/20F) | DN: 53.60 (4.30) Control: 55.0 (4.10) | Hip OA | 3 sessions (1 per week) (n = 19) | Self-stretching protocol for 3 weeks (n = 19) | -Pain (WOMAC P) -Physical function (WOMAC PF) | No between-groups differences | No data |
| Study | MtrP Criteria | Muscles Treated | Gauge (mm) | LTR |
|---|---|---|---|---|
| Itoh et al., 2008 [34] | YES | quadriceps, iliopsoas, sartorius, adductors, popliteus, gluteus minimus and hamstrings | 0.2 × 50 | YES |
| Ceballos-Laita et al., 2019 [37] | YES | iliopsoas, rectus femoris, tensor fasciae latae, gluteus medius and minimus | 0.25 × 50 | YES |
| Sanchez-Romero et al., 2020 [35] | YES | NR | 0.3 × 40 0.3 × 60 0.3 × 75 | YES |
| Ceballos-Laita et al., 2021 [38] | YES | iliopsoas, rectus femoris, tensor fasciae latae, gluteus medius and minimus | 0.25 × 50 | YES |
| Farazdaghi et al., 2021 [40] | YES | hip adductors, abductors, flexors and extensors, and knee flexors and extensors | 0.24 × 40 | YES |
| Vervullens et al., 2021 [36] | YES | gastrocnemius, vastus medialis, vastus lateralis, rectus femoris, biceps femoris, semitendinosus, semimembranosus, adductor longus, adductor brevis | 0.3 × 40 0.3 × 70 | YES |
| Ceballos-Laita et al., 2022 [39] | YES | iliopsoas, rectus femoris, tensor fasciae latae, gluteus medius and minimus | 0.25 × 50 | YES |
| Study | Items | Total Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Itoh et al., 2008 [34] | Y | Y | N | Y | Y | N | Y | N | N | Y | Y | 6/10 |
| Ceballos-Laita et al., 2019 [37] | Y | Y | N | Y | Y | N | Y | Y | N | Y | Y | 7/10 |
| Sanchez-Romero et al., 2020 [35] | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 8/10 |
| Ceballos-Laita et al., 2021 [38] | Y | Y | N | Y | Y | Y | N | Y | N | Y | Y | 7/10 |
| Farazdaghi et al., 2021 [40] | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | 8/10 |
| Vervullens et al., 2021 [36] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7/10 |
| Ceballos-Laita et al., 2022 [39] | Y | Y | N | Y | Y | N | Y | Y | N | Y | Y | 7/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-del-Barrio, S.; Medrano-de-la-Fuente, R.; Hernando-Garijo, I.; Mingo-Gómez, M.T.; Estébanez-de-Miguel, E.; Ceballos-Laita, L. The Effectiveness of Dry Needling in Patients with Hip or Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Life 2022, 12, 1575. https://doi.org/10.3390/life12101575
Jiménez-del-Barrio S, Medrano-de-la-Fuente R, Hernando-Garijo I, Mingo-Gómez MT, Estébanez-de-Miguel E, Ceballos-Laita L. The Effectiveness of Dry Needling in Patients with Hip or Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Life. 2022; 12(10):1575. https://doi.org/10.3390/life12101575
Chicago/Turabian StyleJiménez-del-Barrio, Sandra, Ricardo Medrano-de-la-Fuente, Ignacio Hernando-Garijo, María Teresa Mingo-Gómez, Elena Estébanez-de-Miguel, and Luis Ceballos-Laita. 2022. "The Effectiveness of Dry Needling in Patients with Hip or Knee Osteoarthritis: A Systematic Review and Meta-Analysis" Life 12, no. 10: 1575. https://doi.org/10.3390/life12101575
APA StyleJiménez-del-Barrio, S., Medrano-de-la-Fuente, R., Hernando-Garijo, I., Mingo-Gómez, M. T., Estébanez-de-Miguel, E., & Ceballos-Laita, L. (2022). The Effectiveness of Dry Needling in Patients with Hip or Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Life, 12(10), 1575. https://doi.org/10.3390/life12101575