Confocal Raman Micro-Spectroscopy for Discrimination of Glycerol Diffusivity in Ex Vivo Porcine Dura Mater
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Properties of Dura Mater (DM)
2.2. OCA Reagent and DM Sample Preparation
2.3. Confocal Raman Micro-Spectroscopy
2.4. Calculation of the Glycerol Concentration
2.5. Data Analysis
3. Results and Discussion
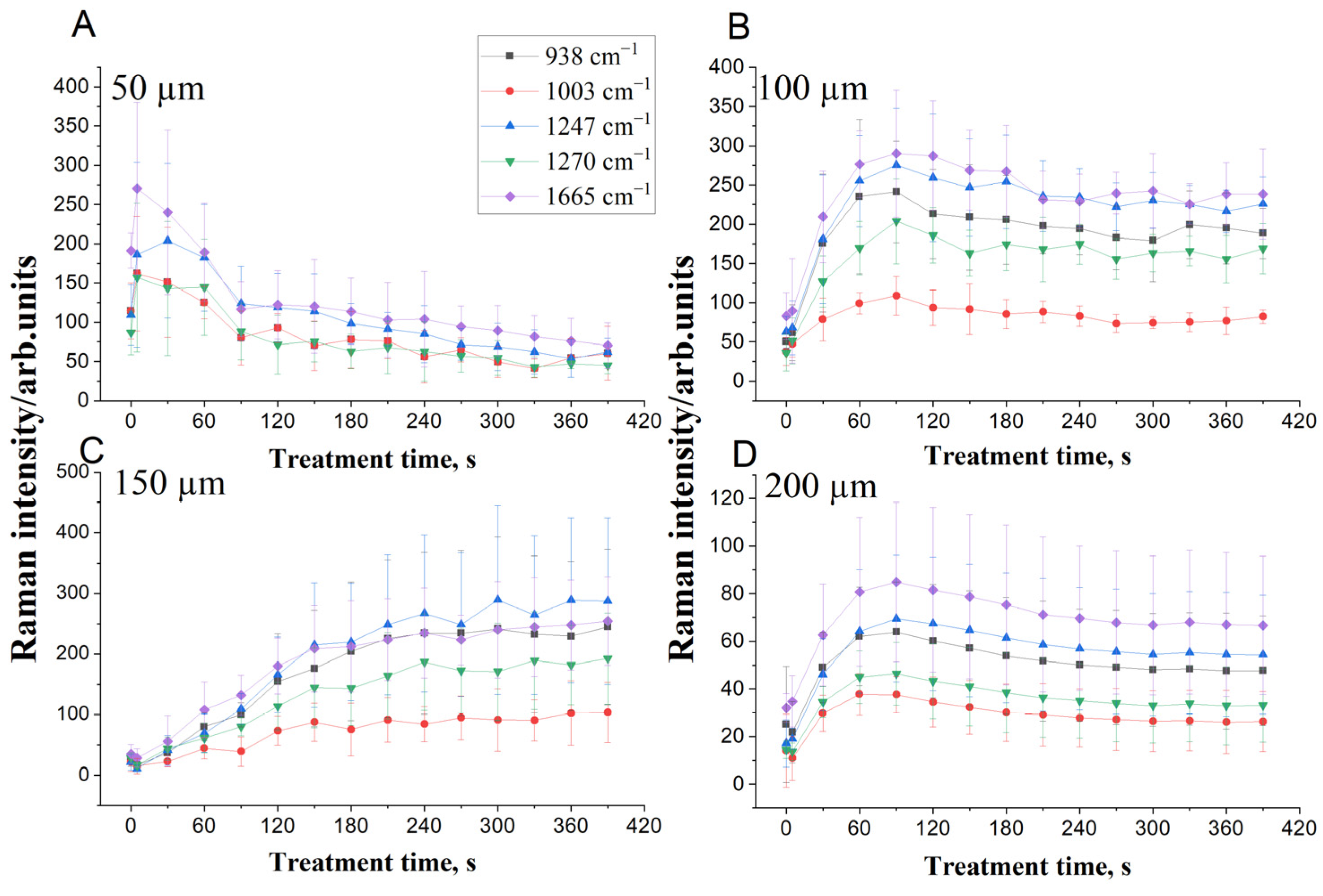
3.1. Calculation of the Glycerol Diffusion Coefficient and Its Effect on DM Collagen
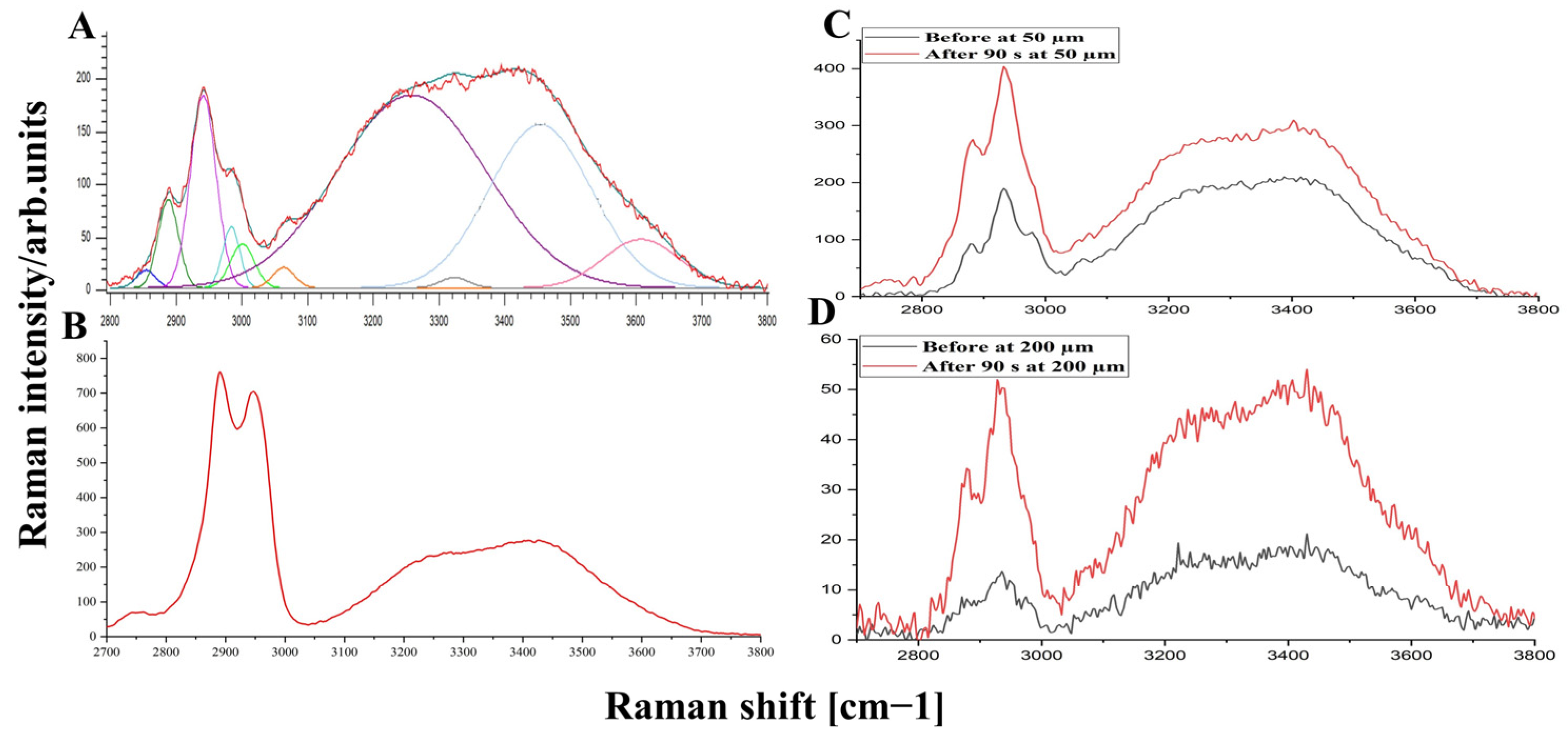
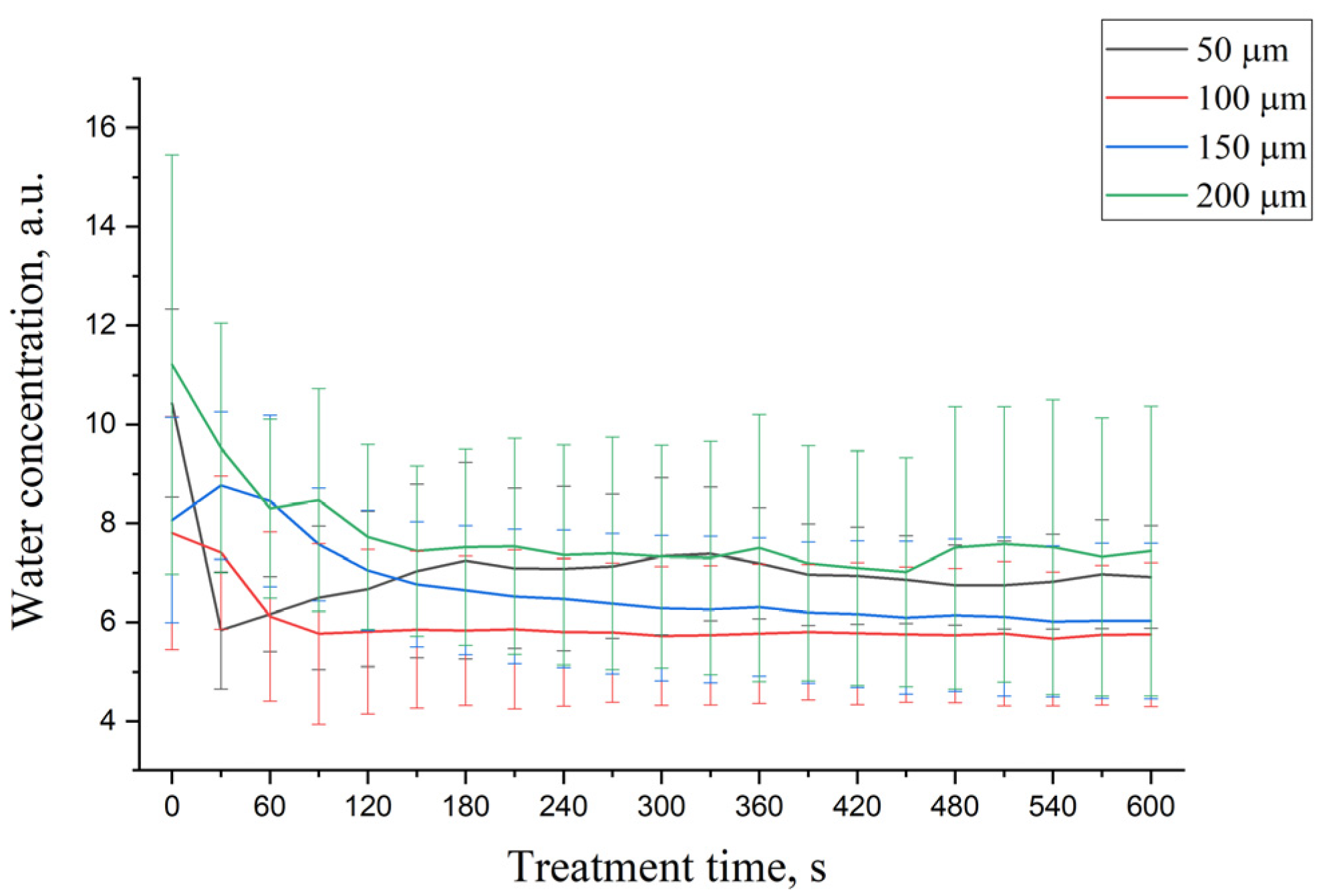
3.2. OCA Effect on Water Mobility States in DM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Latka, I.; Dochow, S.; Krafft, C.; Dietzek, B.; Popp, J. Fiber optic probes for linear and nonlinear Raman applications-Current trends and future development. Laser Photonics Rev. 2013, 7, 698–731. [Google Scholar] [CrossRef]
- Darvin, M.E.; Schleusener, J.; Lademann, J.; Choe, C.-S. Current views on non-invasive in vivo determination of physiological parameters of the stratum corneum using confocal Raman microspectroscopy. Skin Pharmacol. Physiol. 2022, 35, 125–136. [Google Scholar] [CrossRef]
- DePaoli, D.; Lemoine, É.; Ember, K.; Parent, M.; Prud’homme, M.; Cantin, L.; Petrecca, K.; Leblond, F.; Côté, D.C. Rise of Raman spectroscopy in neurosurgery: A review. J. Biomed. Opt. 2020, 25, 1. [Google Scholar] [CrossRef]
- Stevens, A.R.; Stickland, C.A.; Harris, G.; Ahmed, Z.; Oppenheimer, P.G.; Belli, A.; Davies, D.J. Raman Spectroscopy as a Neuromonitoring Tool in Traumatic Brain Injury: A Systematic Review and Clinical Perspectives. Cells 2022, 11, 1227. [Google Scholar] [CrossRef]
- Lee, K.S.; Landry, Z.; Pereira, F.C.; Wagner, M.; Berry, D.; Huang, W.E.; Taylor, G.T.; Kneipp, J.; Popp, J.; Zhang, M.; et al. Raman microspectroscopy for microbiology. Nat. Rev. Methods Prim. 2021, 1, 1–25. [Google Scholar] [CrossRef]
- Azemtsop Matanfack, G.; Pistiki, A.; Rösch, P.; Popp, J. Raman stable isotope probing of bacteria in visible and deep uv-ranges. Life 2021, 11, 1003. [Google Scholar] [CrossRef]
- Mandrell, C.T.; Holland, T.E.; Wheeler, J.F.; Esmaeili, S.M.A.; Amar, K.; Chowdhury, F.; Sivakumar, P. Machine learning approach to raman spectrum analysis of mia paca-2 pancreatic cancer tumor repopulating cells for classification and feature analysis. Life 2020, 10, 181. [Google Scholar] [CrossRef]
- Tuchin, V.V. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnostics, 3rd ed.; PM 254; SPIE Press: Bellingham, WA, USA, 2015; p. 988. [Google Scholar]
- Tuchin, V.V. Handbook of Optical Biomedical Diagnostics. Light-Tissue Interaction, 2nd ed.; PM 262; SPIE Press: Bellingham, WA, USA, 2016; Volume 1, p. 864. Available online: https://spie.org/Publications/Book/2219613?SSO=1 (accessed on 10 August 2021).
- Neprokin, A.; Broadway, C.; Myllylä, T.; Bykov, A.; Meglinski, I. Photoacoustic Imaging in Biomedicine and Life Sciences. Life 2022, 12, 588. [Google Scholar] [CrossRef]
- Koljenović, S.; Schut, T.B.; Vincent, A.; Kros, J.M.; Puppels, G.J. Detection of meningioma in dura mater by Raman spectroscopy. Anal. Chem. 2005, 77, 7958–7965. [Google Scholar] [CrossRef]
- Mizuno, A.; Hayashi, T.; Tashibu, K.; Maraishi, S.; Kawauchi, K.; Ozaki, Y. Near-infrared FT-Raman spectra of the rat brain tissues. Neurosci. Lett. 1992, 141, 47–52. [Google Scholar] [CrossRef]
- Mizuno, A.; Kitajima, H.; Kawauchi, K.; Muraishi, S.; Ozaki, Y. Near-infrared Fourier transform Raman spectroscopic study of human brain tissues and tumours. J. Raman Spectrosc. 1994, 25, 25–29. [Google Scholar] [CrossRef]
- Aarnoutse, P.J.; Westerhuis, J.A. Quantitative Raman reaction monitoring using the solvent as internal standard. Anal. Chem. 2005, 77, 1228–1236. [Google Scholar] [CrossRef]
- Tfayli, A.; Piot, O.; Pitre, F.; Manfait, M. Follow-up of drug permeation through excised human skin with confocal Raman microspectroscopy. Eur. Biophys. J. 2007, 36, 1049–1058. [Google Scholar] [CrossRef]
- Tippavajhala, V.K.; de Oliveira Mendes, T.; Martin, A.A. In Vivo Human Skin Penetration Study of Sunscreens by Confocal Raman Spectroscopy. AAPS Pharm. Sci. Tech. 2018, 19, 753–760. [Google Scholar] [CrossRef]
- Tfaili, S.; Josse, G.; Angiboust, J.F.; Manfait, M.; Piot, O. Monitoring caffeine and resveratrol cutaneous permeation by confocal Raman microspectroscopy. J. Biophotonics 2014, 7, 676–681. [Google Scholar] [CrossRef]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In vitro-in vivo correlation in skin permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Mateus, R.; Abdalghafor, H.; Oliveira, G.; Hadgraft, J.; Lane, M.E. A new paradigm in dermatopharmacokinetics-Confocal Raman spectroscopy. Int. J. Pharm. 2013, 444, 106–108. [Google Scholar] [CrossRef]
- Caspers, P.J.; Nico, C.; Bakker Schut, T.C.; Sterke, J.; Pudney, P.D.A.; Curto, P.R.; Illand, A.; Puppels, G.J. Method to quantify the in vivo skin penetration of topically applied materials based on confocal Raman spectroscopy. Transl. Biophotonics 2019, 1, e201900004. [Google Scholar] [CrossRef]
- Choe, C.S.; Ri, J.S.; Choe, S.H.; Kim, P.S.; Lademann, J.; Schleusener, J.; Darvin, M.E. tMCR-ALS method for the determination of water concentration profiles in the stratum corneum of untreated and treated skin in vivo. J. Raman Spectrosc. 2022, 1–8. [Google Scholar] [CrossRef]
- Jacobson, S.; Marcus, E.M.; Stanley, P. Neuroanatomy for the Neuroscientist, 3rd ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Walsh, D.R.; Ross, A.M.; Newport, D.T.; Zhou, Z.; Kearns, J.; Fearon, C.; Lorigan, J.; Mulvihill, J.J.E. Mechanical characterisation of the human dura mater, falx cerebri and superior sagittal sinus. Acta Biomater. 2021, 134, 388–400. [Google Scholar] [CrossRef]
- Cheshire, E.C.; Malcomson, R.D.G.; Joseph, S.; Biggs, M.J.B.; Adlam, D.; Rutty, G.N. Optical clearing of the dura mater using glycerol: A reversible process to aid the post-mortem investigation of infant head injury. Forensic. Sci. Med. Pathol. 2015, 11, 395–404. [Google Scholar] [CrossRef]
- Riemenschneider, M.J.; Perry, A.; Reifenberger, G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006, 5, 1045–1054. [Google Scholar] [CrossRef]
- Umana, G.E.; Scalia, G.; Vats, A.; Pompili, G.; Barone, F.; Passanisi, M.; Graziano, F.; Maugeri, R.; Tranchina, M.G.; Cosentino, S.; et al. Primary extracranial meningiomas of the head and neck. Life 2021, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Ferini, G.; Muhammad, N.; Ahmed, N.; Wakil, A.N.M.; Islam, K.M.A.; Arifin, M.S.; Al Mahbub, A.; Habib, R.; Mojumder, M.R.; et al. Skull Base Approaches for Tuberculum Sellae Meningiomas: Institutional Experience in a Series of 34 Patients. Life 2022, 12, 492. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.-H.; Wu, B.; Yu, X.; Cheng, G.; Zhu, K.; Wang, K.; Zhang, C.; Zhao, M.; Zong, R.; et al. Optical biopsy identification and grading of gliomas using label-free visible resonance Raman spectroscopy. J. Biomed. Opt. 2019, 24, 095001-12. [Google Scholar] [CrossRef]
- Jelke, F.; Mirizzi, G.; Borgmann, F.K.; Husch, A.; Slimani, R.; Klamminger, G.G.; Klein, K.; Mombaerts, L.; Gérardy, J.J.; Mittelbronn, M.; et al. Intraoperative discrimination of native meningioma and dura mater by Raman spectroscopy. Sci. Rep. 2021, 11, 23583. [Google Scholar] [CrossRef] [PubMed]
- Wolthuis, R.; van Aken, M.; Fountas, K.; Robinson, J.S.; Bruining, H.A.; Puppels, G.J. Determination of water concentration in brain tissue by Raman spectroscopy. Anal. Chem. 2001, 73, 3915–3920. [Google Scholar] [CrossRef]
- Huizinga, A.; Bot, A.C.C.; de Mul, F.F.M.; Vrensen, G.F.J.M.; Greve, J. Local variation in absolute water content of human and rabbit eye lenses measured by Raman microspectroscopy. Exp. Eye Res. 1989, 48, 487–496. [Google Scholar] [CrossRef]
- Calikoglu, C.; Cakir, M.; Tuzun, Y. Histopathological investigation of the effectiveness of collagen matrix in the repair of experimental spinal dura mater defects. Eurasian J. Med. 2019, 51, 133–137. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Su, J.; Jiang, Q.; Wang, J.; Xu, Y.; Zheng, Y.; Zhong, Z.; Lin, H. In vivo Evaluation of Fibrous Collagen Dura Substitutes. Front. Bioeng. Biotechnol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Zwirner, J.; Scholze, M.; Waddell, J.N.; Ondruschka, B.; Hammer, N. Mechanical Properties of Human Dura Mater in Tension–An Analysis at an Age Range of 2 to 94 Years. Sci. Rep. 2019, 9, 16655. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Tang, J.; Li, Z.; Zhou, X.; Zhang, R.; Chen, L.; Mao, Y.; Li, C. Noninvasively Imaging Subcutaneous Tumor Xenograft by a Handheld Raman Detector, with the Assistance of an Optical Clearing Agent. ACS Appl. Mater. Interfaces 2017, 9, 17769–17776. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Sinichkin, Y.P.; Korobov, A.A.; Lakodina, N.A.; Tuchin, V.V. In vitro study of control of human dura mater optical properties by acting of osmotical liquids. Control. Tissue Opt. Prop. Appl. Clin. Study 2000, 4162, 182–188. [Google Scholar] [CrossRef]
- Genina, E.A.; Bashkatov, A.N.; Kochubey, V.I.; Tuchin, V.V. Optical clearing of human dura mater. Opt. Spectrosc. 2005, 98, 470–476. [Google Scholar] [CrossRef]
- Genina, E.; Bashkatov, A.; Tuchin, V. Optical clearing of human dura mater by glucose solutions. J. Biomed. Photonics Eng. 2017, 3, 010309. [Google Scholar] [CrossRef][Green Version]
- Tuchin, V.V.; Maksimova, I.L.; Zimnyakov, D.A.; Kon, I.L.; Mavlutov, A.K.; Mishin, A.A. Light propagation in tissues with controlled optical properties. J. Biomed. Opt. 1997, 2, 401–417. [Google Scholar] [CrossRef]
- Sdobnov, A.; Darvin, M.; Genina, E.; Bashkatov, A.; Lademann, J.; Tuchin, V. Recent progress in tissue optical clearing for spectroscopic application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 216–229. [Google Scholar] [CrossRef]
- Oliveira, L.M.C.; Tuchin, V.V. The Optical Clearing Method-A New Tool for Clinical Practice and Biomedical Engineering; Springer: Basel, Switzerland, 2019; p. 177. [Google Scholar]
- Tuchin, V.V. Optical Clearing of Tissues and Blood; PM 154; SPIE Press: Bellingham, WA, USA, 2005; pp. 1–255. [Google Scholar]
- Schulmerich, M.V.; Cole, J.H.; Dooley, K.A.; Morris, M.D.; Kreider, J.M.; Goldstein, S.A. Optical clearing in transcutaneous Raman spectroscopy of murine cortical bone tissue. J. Biomed. Opt. 2008, 13, 021108. [Google Scholar] [CrossRef]
- Jaafar, A.; Holomb, R.; Sdobnov, A.Y.; Ocskay, Z.; Jakus, Z.; Tuchin, V.V.; Veres, M. Ex vivo confocal Raman microspectroscopy of porcine dura mater supported by optical clearing. J. Biophotonics 2022, 15, e202100332. [Google Scholar] [CrossRef]
- Zhernovaya, O.; Tuchin, V.V.; Leahy, M.J. Enhancement of OCT imaging by blood optical clearing in vessels-A feasibility study. Photonics Lasers Med. 2016, 5, 151–159. [Google Scholar] [CrossRef]
- Liang, Y.; Yuan, W.; Mavadia-Shukla, J.; Li, X. Optical clearing for luminal organ imaging with ultrahigh-resolution optical coherence tomography. J. Biomed. Opt. 2016, 21, 081211. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.Y.; Tang, S.C. Optical clearing facilitates integrated 3D visualization of mouse ileal microstructure and vascular network with high definition. Microvasc. Res. 2010, 80, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Nadiarnykh, O.; Campagnola, P.J. Retention of polarization signatures in SHG microscopy of scattering tissues through optical clearing. Opt. Express 2009, 17, 5794. [Google Scholar] [CrossRef] [PubMed]
- Larin, K.V.; Tuchin, V.V. Functional imaging and assessment of the glucose diffusion rate in epithelial tissues in optical coherence tomography. Quantum Electron. 2008, 38, 551–556. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, L.; Zheng, Y.; Song, Y.; Xu, Q.; Wang, J.; Si, K.; Duan, S.; Gong, W. Ultrafast optical clearing method for three-dimensional imaging with cellular resolution. Proc. Natl. Acad. Sci. USA 2019, 166, 11480–11489. [Google Scholar] [CrossRef]
- Pan, C.; Cai, R.; Quacquarelli, F.P.; Ghasemigharagoz, A.; Lourbopoulos, A.; Matryba, P.; Plesnila, N.; Dichgans, M.; Hellal, F.; Ertürk, A. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat. Methods 2016, 13, 859–867. [Google Scholar] [CrossRef]
- Hama, H.; Hioki, H.; Namiki, K.; Hoshida, T.; Kurokawa, H.; Ishidate, F.; Kaneko, T.; Akagi, T.; Saito, T.; Saido, T.; et al. ScaleS: An optical clearing palette for biological imaging. Nat. Neurosci. 2015, 18, 1518–1529. [Google Scholar] [CrossRef]
- Hama, H.; Kurokawa, H.; Kawano, H.; Ando, R.; Shimogori, T.; Noda, H.; Fukami, K.; Sakaue-Sawano, A.; Miyawaki, A. Scale: A chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat. Neurosci. 2011, 14, 1481–1488. [Google Scholar] [CrossRef]
- Tuchin, V.V.; Zhu, D.; Genina, E.A. Handbook of Tissue Optical Clearing New Prospects in Optical Imaging Edited; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Guo, X.; Guo, Z.Y.; Wei, H.J.; Yang, H.Q.; He, Y.H.; Xie, S.S.; Wu, G.Y.; Zhong, H.Q.; Li, L.Q.; Zhao, Q.L. In vivo quantification of propylene glycol, glucose and glycerol diffusion in human skin with optical coherence tomography. Laser Phys. 2010, 20, 1849–1855. [Google Scholar] [CrossRef]
- Sdobnov, A.Y.; Tuchin, V.V.; Lademann, J.; Darvin, M.E. Confocal Raman microscopy supported by optical clearing treatment of the skin-Influence on collagen hydration. J. Phys. D Appl. Phys. 2017, 50, 285401. [Google Scholar] [CrossRef]
- Egawa, M.; Yanai, M.; Maruyama, N.; Fukaya, Y.; Hirao, T. Visualization of water distribution in the facial epidermal layers of skin using high-sensitivity near-infrared (NIR) imaging. Appl. Spectrosc. 2015, 69, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Boireau-Adamezyk, E.; Baillet-Guffroy, A.; Stamatas, G.N. Mobility of water molecules in the stratum corneum: Effects of age and chronic exposure to the environment. J. Investig. Dermatol. 2014, 134, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, M.; Boeing, H.; Müller-Werdan, U.; Aykac, V.; Steffen, A.; Schell, M.; Blume-Peytavi, U.; Kottner, J. Effect of Fluid Intake on Hydration Status and Skin Barrier Characteristics in Geriatric Patients: An Explorative Study. Skin Pharmacol. Physiol. 2018, 31, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.; Carvalho, M.I.; Nogueira, E.M.; Tuchin, V.V. Skeletal muscle dispersion (400–1000 nm) and kinetics at optical clearing. J. Biophotonics 2018, 11, e201700094. [Google Scholar] [CrossRef]
- Carneiro, I.; Carvalho, S.; Henrique, R.; Oliveira, L.; Tuchin, V.V. Simple multimodal optical technique for evaluation of free/bound water and dispersion of human liver tissue. J. Biomed. Opt. 2017, 22, 125002. [Google Scholar] [CrossRef]
- Shi, R.; Guo, L.; Zhang, C.; Feng, W.; Li, P.; Ding, Z.; Zhu, D. A useful way to develop effective in vivo skin optical clearing agents. J. Biophotonics 2017, 10, 887–895. [Google Scholar] [CrossRef]
- Behm, P.; Hashemi, M.; Hoppe, S.; Wessel, S.; Hagens, R.; Jaspers, S.; Wenck, H.; Rübhausen, M. Confocal spectroscopic imaging measurements of depth dependent hydration dynamics in human skin in-vivo. AIP Adv. 2017, 7, 115004. [Google Scholar] [CrossRef]
- Sdobnov, A.Y.; Darvin, M.E.; Schleusener, J.; Lademann, J.; Tuchin, V.V. Hydrogen bound water profiles in the skin influenced by optical clearing molecular agents—Quantitative analysis using confocal Raman microscopy. J. Biophotonics 2019, 12, e201800283. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.; Ye, Y.S.; Kim, T.I. Materials and device design for advanced phototherapy systems. Adv. Drug Deliv. Rev. 2022, 186, 114339. [Google Scholar]
- Protasoni, M.; Sangiorgi, S.; Cividini, A.; Culuvaris, G.T.; Tomei, G.; Dell’Orbo, C.; Raspanti, M.; Balbi, S.; Reguzzoni, M. The collagenic architecture of human dura mater: Laboratory investigation. J. Neurosurg. 2011, 114, 1723–1730. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Sinichkin, Y.P.; Kochubey, V.I.; Lakodina, N.A.; Tuchin, V.V. Glucose and Mannitol Diffusion in Human Dura Mater. Biophys. J. 2003, 85, 3310–3318. [Google Scholar] [CrossRef]
- Rua, R.; McGavern, D.B. Advances in Meningeal Immunity. Trends Mol. Med. 2018, 24, 542–559. [Google Scholar] [CrossRef]
- Absinta, M.; Ha, S.-K.; Nair, G.; Sati, P.; Luciano, N.J.; Palisoc, M.; Louveau, A.; Zaghloul, K.A.; Pittaluga, S.; Kipnis, J.; et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 2017, 6, e29738. [Google Scholar] [CrossRef] [PubMed]
- Frink, M.; Andruszkow, H.; Zeckey, C.; Krettek, C.; Hildebrand, F. Experimental trauma models: An update. J. Biomed. Biotechnol. 2011, 2011, 797383. [Google Scholar] [CrossRef] [PubMed]
- Mazgajczyk, E.; Ścigała, K.; Czyż, M.; Jarmundowicz, W.; Będziński, R. Mechanical properties of cervical dura mater. Acta Bioeng. Biomech. 2012, 14, 51–58. [Google Scholar] [PubMed]
- Kinaci, A.; Bergmann, W.; Bleys, R.L.A.W.; van der Zwan, A.; van Doormaal, T.P.C. Histologic comparison of the dura mater among species. Comp. Med. 2020, 70, 170–175. [Google Scholar] [CrossRef]
- Lai, J.H.; Liao, E.Y.; Liao, Y.H.; Sun, C.K. Investigating the optical clearing effects of 50% glycerol in ex vivo human skin by harmonic generation microscopy. Sci. Rep. 2021, 11, 329. [Google Scholar] [CrossRef]
- Chang, C.Y.; Pan, P.H.; Li, J.R.; Ou, Y.C.; Liao, S.L.; Chen, W.Y.; Kuan, Y.H.; Chen, C.J. Glycerol improves intracerebral hemorrhagic brain injury and associated kidney dysfunction in rats. Antioxidants 2021, 10, 623. [Google Scholar] [CrossRef]
- Berger, C.; Sakowitz, O.W.; Kiening, K.L.; Schwab, S. Neurochemical monitoring of glycerol therapy in patients with ischemic brain edema. Stroke 2005, 36, 4–6. [Google Scholar] [CrossRef]
- Sakamaki, M.; Igarashi, H.; Nishiyama, Y.; Hagiwara, H.; Ando, J.; Chishiki, T.; Curran, B.C.; Katayama, Y. Effect of glycerol on ischemic cerebral edema assessed by magnetic resonance imaging. J. Neurol. Sci. 2003, 209, 69–74. [Google Scholar] [CrossRef]
- Iijima, K.; Oshima, T.; Kawakami, R.; Nemoto, T. Optical clearing of living brains with MAGICAL to extend in vivo imaging. iScience 2021, 24, 101888. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, A.; Mahmood, M.H.; Holomb, R.; Himics, L.; Váczi, T.; Sdobnov, A.Y.; Tuchin, V.V.; Veres, M. Ex-vivo confocal Raman microspectroscopy of porcine skin with 633/785-NM laser excitation and optical clearing with glycerol/water/DMSO solution. J. Innov. Opt. Health Sci. 2021, 14, 1–13. [Google Scholar] [CrossRef]
- Yanina, I.Y.; Schleusener, J.; Lademann, J.; Tuchin, V.V.; Darvin, M.E. The Effectiveness of Glycerol Solutions for Optical Clearing of the Intact Skin as Measured by Confocal Raman Microspectroscopy. Opt. Spectrosc. 2020, 128, 759–765. [Google Scholar] [CrossRef]
- Spectragryph-Optical Spectroscopy Software: Description, (n.d.). Available online: https://www.effemm2.de/spectragryph/about_descr.html (accessed on 17 October 2020).
- Mujica Ascencio, S.; Choe, C.S.; Meinke, M.C.; Müller, R.H.; Maksimov, G.V.; Wigger-Alberti, W.; Lademann, J.; Darvin, M.E. Confocal Raman microscopy and multivariate statistical analysis for determination of different penetration abilities of caffeine and propylene glycol applied simultaneously in a mixture on porcine skin ex vivo. Eur. J. Pharm. Biopharm. 2016, 104, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.; Lademann, J.; Darvin, M.E. A depth-dependent profile of the lipid conformation and lateral packing order of the stratum corneum in vivo measured using Raman microscopy. Analyst 2016, 141, 1981–1987. [Google Scholar] [CrossRef]
- Pezzotti, G.; Boffelli, M.; Miyamori, D.; Uemura, T.; Marunaka, Y.; Zhu, W.; Ikegaya, H. Raman spectroscopy of human skin: Looking for a quantitative algorithm to reliably estimate human age. J. Biomed. Opt. 2015, 20, 065008. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhang, W.; Zhong, H.; Xiong, H.; Guo, X.; Guo, Z. Optical clearing of porcine skin tissue in vitro studied by Raman microspectroscopy. J. Biomed. Opt. 2012, 17, 015004. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Y.; Guo, Z.; Wang, J.; Zhuang, Z.; Liu, S. Discrimination of dimethyl sulphoxide diffusion coefficient in the process of optical clearing by confocal micro-Raman spectroscopy. J. Biomed. Opt. 2013, 18, 020507. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, R.K. Theoretical model on optical clearing of biological tissue with semipermeable chemical agents. Complex Dyn. Fluct. Chaos Fractals Biomed. Photonics 2004, 5330, 215. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Tuchin, V.V.; Genina, E.A.; Stolnitz, M.M.; Zhestkov, D.M.; Altshuler, G.B.; Yaroslavsky, I.V. Monte Carlo study of skin optical clearing to enhance light penetration in the tissue. Complex Dyn. Fluct. Biomed. Photonics IV 2007, 6436, 64360Z. [Google Scholar] [CrossRef]
- Zhuo, S.; Chen, J.; Wu, G.; Xie, S.; Zheng, L.; Jiang, X.; Zhu, X. Quantitatively linking collagen alteration and epithelial tumor progression by second harmonic generation microscopy. Appl. Phys. Lett. 2010, 96, 94–97. [Google Scholar] [CrossRef]
- Takeuchi, M.; Hayakawa, S.; Ichikawa, A.; Hasegawa, A.; Hasegawa, Y.; Fukuda, T. Multilayered artificial dura-mater models for a minimally invasive brain surgery simulator. Appl. Sci. 2020, 10, 9000. [Google Scholar] [CrossRef]
- Akinkunmi, F.O.; Jahn, D.A.; Giovambattista, N. Effects of temperature on the thermodynamic and dynamical properties of glycerol-water mixtures: A computer simulation study of three different force fields. J. Phys. Chem. B 2015, 119, 6250–6261. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.H.; Heller, A.; Fröba, A.P. Binary Diffusion Coefficients of Glycerol-Water Mixtures for Temperatures from 323 to 448 K by Dynamic Light Scattering. J. Chem. Eng. Data 2017, 62, 4364–4370. [Google Scholar] [CrossRef]
- Rylander, C.G.; Stumpp, O.F.; Milner, T.E.; Kemp, N.J.; Mendenhall, J.M.; Diller, K.R.; Welch, A.J. Dehydration mechanism of optical clearing in tissue. J. Biomed. Opt. 2006, 11, 041117. [Google Scholar] [CrossRef]
- D’Errico, G.; Ortona, O.; Capuano, F.; Vitagliano, V. Diffusion coefficients for the binary system glycerol + water at 25 °C. A velocity correlation study. J. Chem. Eng. Data 2004, 49, 1665–1670. [Google Scholar] [CrossRef]
- Egorov, A.V.; Lyubartsev, A.P.; Laaksonen, A. Molecular dynamics simulation study of glycerol-water liquid mixtures. J. Phys. Chem. B 2011, 115, 14572–14581. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef]
- Tuchin, V.V. Coherent Optical Techniques for The Analysis Of Tissue Structure and Dynamics. J. Biomed. Opt. 1999, 4, 106–124. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Carvalho, M.I.; Nogueira, E.M.; Tuchin, V.V. Errata: Diffusion characteristics of ethylene glycol in skeletal muscle. J. Biomed. Opt. 2015, 20, 059801. [Google Scholar] [CrossRef][Green Version]
- Carvalho, S.; Gueiral, N.; Nogueira, E.; Henrique, R.; Oliveira, L.; Tuchin, V.V. Glucose diffusion in colorectal mucosa—a comparative study between normal and cancer tissues. J. Biomed. Opt. 2017, 22, 091506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirshburg, J.M.; Ravikumar, K.M.; Hwang, W.; Yeh, A.T. Molecular basis for optical clearing of collagenous tissues. J. Biomed. Opt. 2010, 15, 055002. [Google Scholar] [CrossRef]
- Weny, X.; Maoy, Z.; Han, Z.; Tuchin, V.V.; Zhu, D. In vivo skin optical clearing by glycerol solutions: Mechanism. J. Biophotonics 2010, 3, 44–52. [Google Scholar] [CrossRef]
- Matousek, P. Raman signal enhancement in deep spectroscopy of turbid media. Appl. Spectrosc. 2007, 61, 845–854. [Google Scholar] [CrossRef]
- Menyaev, Y.A.; Nedosekin, D.A.; Sarimollaoglu, M.; Juratli, M.A.; Galanzha, E.I.; Tuchin, V.V.; Zharov, V.P. Optical clearing in photoacoustic flow cytometry. Biomed. Opt. Express 2013, 4, 3030. [Google Scholar] [CrossRef]
- Sun, Q. The Raman OH stretching bands of liquid water. Vib. Spectrosc. 2009, 51, 213–217. [Google Scholar] [CrossRef]
- Vyumvuhore, R.; Tfayli, A.; Duplan, H.; Delalleau, A.; Manfait, M.; Baiilet-Guffroy, A. Effects of atmospheric relative humidity on Stratum Corneum structure at the molecular level: Ex vivo Raman spectroscopy analysis. Analyst 2013, 138, 4103–4111. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.; Lademann, J.; Darvin, M.E. Depth profiles of hydrogen bound water molecule types and their relation to lipid and protein interaction in the human stratum corneum: In vivo. Analyst 2016, 141, 6329–6337. [Google Scholar] [CrossRef]
- Mudalige, A.; Pemberton, J.E. Raman spectroscopy of glycerol/D2O solutions. Vib. Spectrosc. 2007, 45, 27–35. [Google Scholar] [CrossRef]
- Utz, S.R.; Tuchin, V.V.; Galkina, E.M. The dynamics of some human skin biophysical parameters in the process of optical clearing after hyperosmotic solutions topical application. Vestn. Dermatol. Venerol. 2015, 91, 60–68. [Google Scholar] [CrossRef]
- Choe, C.; Choe, S.; Schleusener, J.; Lademann, J.; Darvin, M.E. Modified normalization method in in vivo stratum corneum analysis using confocal Raman microscopy to compensate nonhomogeneous distribution of keratin. J. Raman Spectrosc. 2019, 50, 945–957. [Google Scholar] [CrossRef]
- Schulz, B.; Chan, D.; Bäckström, J.; Rübhausen, M. Spectroscopic ellipsometry on biological materials-Investigation of hydration dynamics and structural properties. Thin Solid Films 2004, 455, 731–734. [Google Scholar] [CrossRef]
- Choe, C.; Schleusener, J.; Choe, S.; Ri, J.; Lademann, J.; Darvin, M.E. Stratum corneum occlusion induces water transformation towards lower bonding state: A molecular level in vivo study by confocal Raman microspectroscopy. Int. J. Cosmet. Sci. 2020, 42, 482–493. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Happillon, T.; Feru, J.; Brassart-Passco, S.; Angiboust, J.F.; Manfait, M.; Piot, O. Raman Comparison of Skin Dermis of Different Ages: Focus on Spectral Markers of Collagen Hydration. J. Raman Spectrosc. 2013, 44, 1230–1237. [Google Scholar] [CrossRef]



| Depth 50 µm | Depth 150 µm | |||
|---|---|---|---|---|
| Raman bands | D (cm2/s) | C0 | D (cm2/s) | C0 |
| 938 cm−1 | 3.0 × 10−6 | 21 | 9.4 × 10−6 | 1.2 |
| 1003 cm−1 | 2.5 × 10−6 | 27 | 6.5 × 10−5 | 2.3 |
| 1247 cm−1 | 3.5 × 10−5 | 11 | 2.2 × 10−5 | 1.1 |
| 1270 cm−1 | 5.0 × 10−6 | 15 | 9.6 × 10−6 | 1.9 |
| 1665 cm−1 | 2.3 × 10−6 | 26 | 4.2 × 10−6 | 1.7 |
| Average | 9.6 × 10−6 | 20 | 2.2 × 10−5 | 1.6 |
| Depth 100 µm | Depth 200 µm | |||
| Raman bands | D (cm2/s) | C0 | D (cm2/s) | C0 |
| 938 cm−1 | 1.7 × 10−6 | 3.4 | 9.8 × 10−5 | 0.6 |
| 1003 cm−1 | 2.7 × 10−6 | 4.2 | 6.2 × 10−6 | 0.8 |
| 1247 cm−1 | 5.9 × 10−6 | 2.4 | 8.1 × 10−6 | 0.5 |
| 1270 cm−1 | 4.6 × 10−5 | 1.5 | 1.1 × 10−5 | 0.6 |
| 1665 cm−1 | 4.3 × 10−6 | 3.1 | 2.5 × 10−5 | 0.6 |
| Average | 1.2 × 10−5 | 2.9 | 3.0 × 10−5 | 0.6 |
| Depths | 50 µm | 100 µm | 150 µm | 200 µm | ||||
|---|---|---|---|---|---|---|---|---|
| Water Types | Before | After | Before | After | Before | After | Before | After |
| Tightly bound | 0.20 | 0.12 | 0.13 | 0.15 | 0.22 | 0.19 | 0.18 | 0.177 |
| Strongly bound | 6.00 | 4.12 | 4.57 | 3.42 | 4.60 | 3.58 | 6.53 | 4.44 |
| Weakly bound | 3.49 | 2.18 | 2.47 | 1.80 | 2.41 | 1.70 | 3.24 | 2.15 |
| Unbound water | 0.73 | 0.48 | 0.61 | 0.36 | 0.82 | 0.53 | 1.27 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaafar, A.; Darvin, M.E.; Tuchin, V.V.; Veres, M. Confocal Raman Micro-Spectroscopy for Discrimination of Glycerol Diffusivity in Ex Vivo Porcine Dura Mater. Life 2022, 12, 1534. https://doi.org/10.3390/life12101534
Jaafar A, Darvin ME, Tuchin VV, Veres M. Confocal Raman Micro-Spectroscopy for Discrimination of Glycerol Diffusivity in Ex Vivo Porcine Dura Mater. Life. 2022; 12(10):1534. https://doi.org/10.3390/life12101534
Chicago/Turabian StyleJaafar, Ali, Maxim E. Darvin, Valery V. Tuchin, and Miklós Veres. 2022. "Confocal Raman Micro-Spectroscopy for Discrimination of Glycerol Diffusivity in Ex Vivo Porcine Dura Mater" Life 12, no. 10: 1534. https://doi.org/10.3390/life12101534
APA StyleJaafar, A., Darvin, M. E., Tuchin, V. V., & Veres, M. (2022). Confocal Raman Micro-Spectroscopy for Discrimination of Glycerol Diffusivity in Ex Vivo Porcine Dura Mater. Life, 12(10), 1534. https://doi.org/10.3390/life12101534








