Chemosensory Dysfunction in Long-Term COVID-19 Assessed by Self-Reported and Direct Psychophysical Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. SCENTinel Smell Test
2.1.2. GCCR Smell and Taste Check
2.2. Procedures
2.3. Statistical Analyses
3. Results
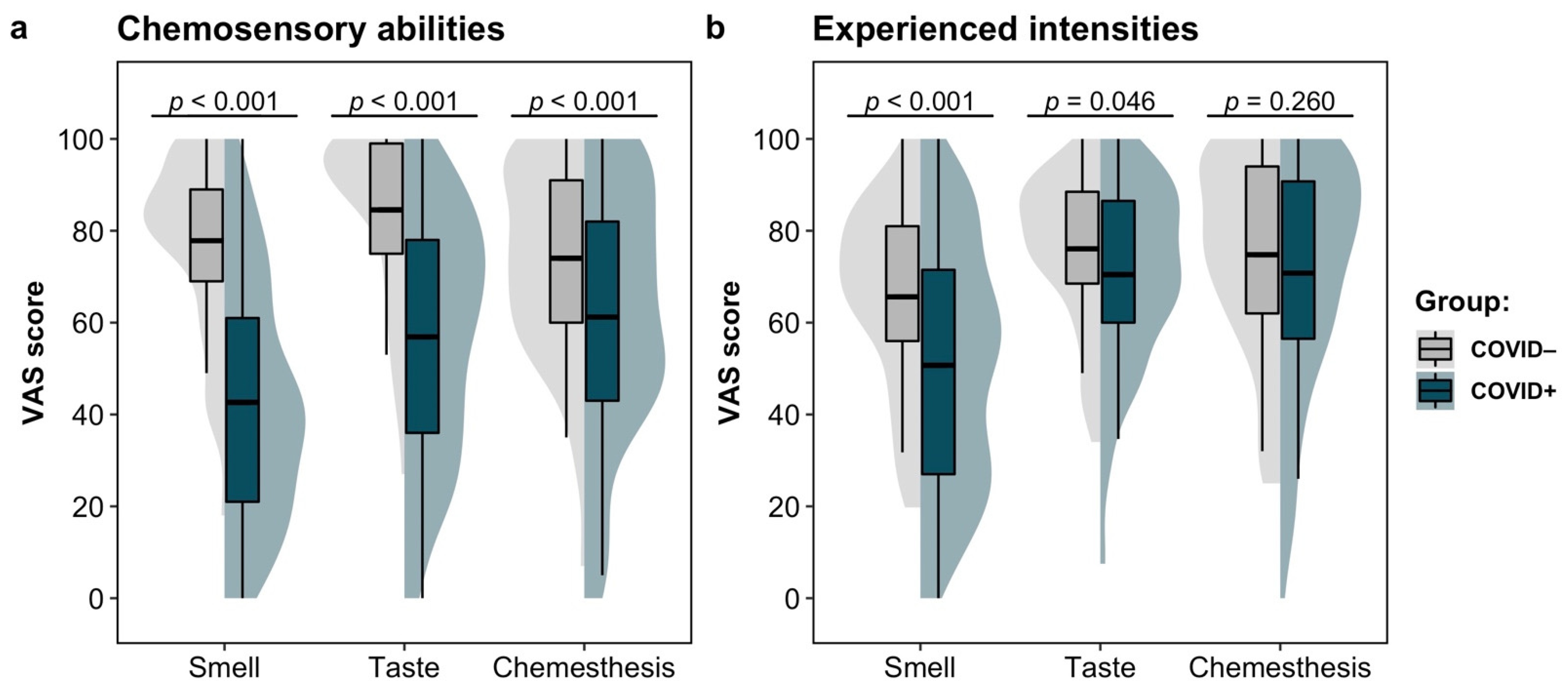
3.1. Chemosensory Abilities and Experienced Intensities
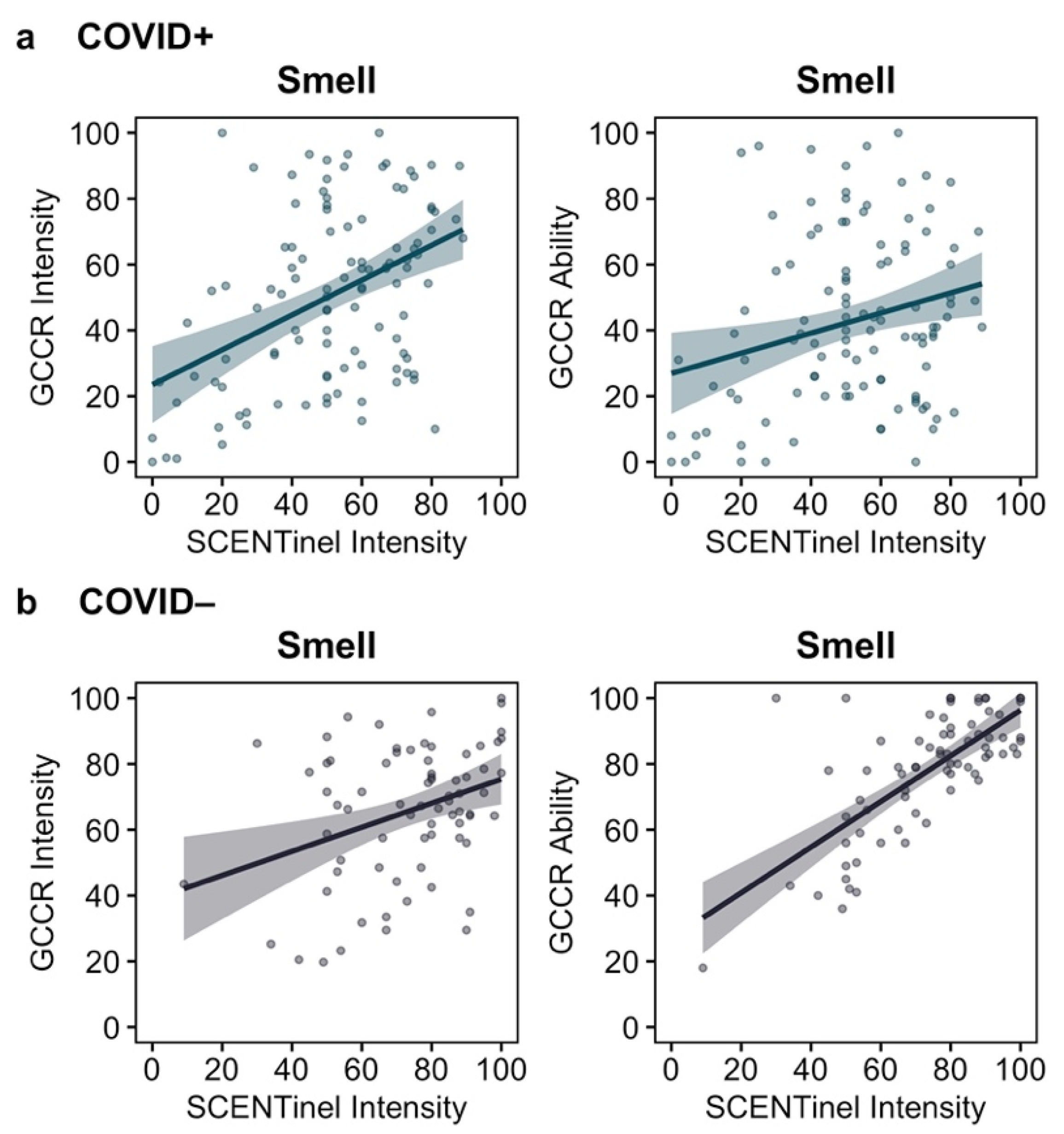
3.2. Correspondence between Self-Reported and Direct Psychophysical Chemosensory Measures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giacomelli, A.; Pezzati, L.; Conti, F.; Bernacchia, D.; Siano, M.; Oreni, L.; Rusconi, S.; Gervasoni, C.; Ridolfo, A.L.; Rizzardini, G.; et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: A cross-sectional study. Clin. Infect. Dis. 2020, 71, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Trecca, E.M.; Cassano, M.; Longo, F.; Petrone, P.; Miani, C.; Hummel, T.; Gelardi, M. Results from psychophysical tests of smell and taste during the course of SARS-CoV-2 infection: A review. Acta Otorhinolaryngol. Ital. 2022, 42, S20–S35. [Google Scholar] [CrossRef] [PubMed]
- Parma, V.; Ohla, K.; Veldhuizen, M.G.; Niv, M.Y.; Kelly, C.E.; Bakke, A.J.; Cooper, H.W.; Bouysset, C.; Pirastu, N.; Dibattista, M.; et al. More than smell—COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem. Senses 2020, 45, 609–622. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Neri, G.; D’Ardes, D.; De Luca, C.; Marinari, S.; Porreca, E.; Cipollone, F.; Vecchiet, J.; Falcicchia, C.; Panichi, V.; et al. Smell and taste in severe COVID-19: Self-reported vs. testing. Front. Med. 2020, 7, 589409. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Oppenheimer, J. The importance of considering olfactory dysfunction during the COVID-19 pandemic and in clinical practice. J. Allergy Clin. Immunol. Pract. 2021, 9, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gerkin, R.C.; Ohla, K.; Veldhuizen, M.G.; Joseph, P.V.; Kelly, C.E.; Bakke, A.J.; Steele, K.E.; Farruggia, M.C.; Pellegrino, R.; Pepino, M.Y.; et al. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem. Senses 2021, 46, bjaa081. [Google Scholar] [CrossRef]
- Coelho, D.H.; Reiter, E.R.; French, E.; Costanzo, R.M. Decreasing Incidence of Chemosensory Changes by COVID-19 Variant. Otolaryngol. Head Neck Surg. 2022, 1–3. [Google Scholar] [CrossRef]
- Cecchetto, C.; Di Pizio, A.; Genovese, F.; Calcinoni, O.; Macchi, A.; Dunkel, A.; Ohla, K.; Spinelli, S.; Farruggia, M.C.; Joseph, P.V.; et al. Assessing the extent and timing of chemosensory impairments during COVID-19 pandemic. Sci. Rep. 2021, 11, 17504. [Google Scholar] [CrossRef]
- Worldometer. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 24 September 2022).
- Herz, R.S.; Bajec, M.R. Your money or your sense of smell? A comparative analysis of the sensory and psychological value of olfaction. Brain Sci. 2022, 12, 299. [Google Scholar] [CrossRef]
- Stevenson, R.J. An initial evaluation of the functions of human olfaction. Chem. Senses 2010, 35, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Gessa, C.; Deiana, G.; Salzano, G.; Maglitto, F.; Lechien, J.R.; Saussez, S.; Piombino, P.; Biglio, A.; Biglioli, F.; et al. The effects of persistent olfactory and gustatory dysfunctions on quality of life in long-COVID-19 patients. Life 2022, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Ohla, K.; Veldhuizen, M.G.; Green, T.; Hannum, M.E.; Bakke, A.J.; Moein, S.T.; Tognetti, A.; Postma, E.M.; Pellegrino, R.; Hwang, D.L.D.; et al. A follow-up on quantitative and qualitative olfactory dysfunction and other symptoms in patients recovering from COVID-19 smell loss. Rhinology 2022, 60, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.J.; Han, R.; Zhao, J.J.; Tan, N.K.W.; Quah, E.S.H.; Tan, C.J.W.; Chan, Y.H.; Teo, N.W.Y.; Charn, T.C.; See, A.; et al. Prognosis and persistence of smell and taste dysfunction in patients with COVID-19: Meta-analysis with parametric cure modelling of recovery curves. BMJ 2022, 378, e069503. [Google Scholar] [CrossRef]
- Cao, A.C.; Nimmo, Z.M.; Mirza, N.; Cohen, N.A.; Brody, R.M.; Doty, R.L. Objective screening for olfactory and gustatory dysfunction during the COVID-19 pandemic: A prospective study in healthcare workers using self-administered testing. World J. Otorhinolaryngol. Head Neck Surg. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hannum, M.E.; Ramirez, V.A.; Lipson, S.J.; Herriman, R.D.; Toskala, A.K.; Lin, C.; Joseph, P.V.; Reed, D.R. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19–positive patients compared to subjective methods: A systematic review and meta-analysis. Chem. Senses 2020, 45, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Hannum, M.E.; Koch, R.J.; Ramirez, V.A.; Marks, S.S.; Toskala, A.K.; Herriman, R.D.; Lin, C.; Joseph, P.V.; Reed, D.R. Taste loss as a distinct symptom of COVID-19: A systematic review and meta-analysis. Chem. Senses 2022, 47, bjac001. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Hopkins, C.; Salzano, G.; Petrocelli, M.; Melis, A.; Cucurullo, M.; Ferrari, M.; Gagliardini, L.; Pipolo, C.; Deiana, G.; et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 2020, 42, 1560–1569. [Google Scholar] [CrossRef]
- Jang, S.S.; Choi, J.S.; Kim, J.H.; Kim, N.; Ference, E.H. Discordance between subjective and objective measures of smell and taste in US adults. Otolaryngol. Head Neck Surg. 2022, 166, 572–579. [Google Scholar] [CrossRef]
- Philpott, C.M.; Wolstenholme, C.R.; Goodenough, P.C.; Clark, A.; Murty, G.E. Comparison of subjective perception with objective measurement of olfaction. Otolaryngol. Head Neck Surg. 2006, 134, 488–490. [Google Scholar] [CrossRef]
- Rawal, S.; Hoffman, H.J.; Chapo, A.K.; Duffy, V.B. Sensitivity and specificity of self-reported olfactory function in a home-based study of independent-living, healthy older women. Chemosens. Percept. 2014, 7, 108–116. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Vaira, L.A.; De Riu, G.; Cammaroto, G.; Chekkoury-Idrissi, Y.; Circiu, M.; Distinguin, L.; Journe, F.; de Terwangne, C.; et al. Epidemiological, otolaryngological, olfactory and gustatory outcomes according to the severity of COVID-19: A study of 2579 patients. Eur. Arch. Oto-Rhino-Laringol. 2021, 278, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, D.P.; Shahrvini, B.; Said, M.; Srinivas, S.; DeConde, A.S.; Yan, C.H. Assessment of patient recognition of coronavirus disease 2019 (COVID-19)-associated olfactory loss and recovery: A longitudinal study. Int. Forum Allergy Rhinol. 2021, 11, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Shaman, P.; Dann, M. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function. Physiol. Behav. 1984, 32, 489–502. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. “Sniffin” sticks.’ Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Doty, R.L. Measurement of chemosensory function. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 11–28. [Google Scholar] [CrossRef]
- Parma, V.; Hannum, M.E.; O’Leary, M.; Pellegrino, R.; Rawson, N.E.; Reed, D.R.; Dalton, P.H. SCENTinel 1.0: Development of a rapid test to screen for smell loss. Chem. Senses 2021, 46, bjab012. [Google Scholar] [CrossRef]
- Duff, K.; McCaffrey, R.J.; Solomon, G.S. The Pocket Smell Test: Successfully discriminating probable Alzheimer’s dementia from vascular dementia and major depression. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 197–201. [Google Scholar] [CrossRef]
- Rawal, S.; Hoffman, H.J.; Honda, M.; Huedo-Medina, T.B.; Duffy, V.B. The taste and smell protocol in the 2011–2014 US National Health and Nutrition Examination Survey (NHANES): Test–retest reliability and validity testing. Chemosens. Percept. 2015, 8, 138–148. [Google Scholar] [CrossRef]
- World Medical Association. WMA Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Arslan, R.C.; Walther, M.P.; Tata, C.S. formr: A study framework allowing for automated feedback generation and complex longitudinal experience-sampling studies using R. Behav Res. Methods 2020, 52, 376–387. [Google Scholar] [CrossRef]
- Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef]
- Croy, I.; Buschhüter, D.; Seo, H.S.; Negoias, S.; Hummel, T. Individual significance of olfaction: Development of a questionnaire. Eur. Arch. Oto-Rhino-Laryngol. 2010, 267, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Trecca, E.M.; Fortunato, F.; Gelardi, M.; Petrone, P.; Cassano, M. Development of a questionnaire to investigate socio-cultural differences in the perception of smell, taste and flavour. Acta Otorhinolaryngol. Ital. 2021, 41, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Polesel, J.; Vaira, L.A. Smell and taste dysfunction after COVID-19. BMJ 2022, 378, o1653. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Tirelli, G.; Meloni, P.; Hopkins, C.; Lechien, J.R.; Madeddu, G.; Cancellieri, E.; Lazzarin, C.; Borsetto, D.; De Vito, A.; et al. Recovery from COVID-19 related olfactory and gustatory dysfunction following omicron BA. 1 subvariant infection: A six-month prospective study. Res. Sq. 2022, 1–15. [Google Scholar]
- Ciofalo, A.; Cavaliere, C.; Masieri, S.; Di Chicco, A.; Fatuzzo, I.; Lo Re, F.; Baroncelli, S.; Begvarfaj, E.; Adduci, A.; Mezzaroma, I.; et al. Long-Term Subjective and Objective Assessment of Smell and Taste in COVID-19. Cells 2022, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Beckers, E.; Mustin, V.; Ducarme, M.; Journe, F.; Marchant, A.; Jouffe, L.; Barillari, M.R.; Camarotto, G.; et al. Prevalence and 6-month recovery of olfactory dysfunction: A multicentre study of 1363 COVID-19 patients. J. Intern. Med. 2021, 290, 451–461. [Google Scholar] [CrossRef] [PubMed]
- von Bartheld, C.S.; Hagen, M.M.; Butowt, R. Prevalence of chemosensory dysfunction in COVID-19 patients: A systematic review and meta-analysis reveals significant ethnic differences. ACS Chem. Neurosci. 2020, 11, 2944–2961. [Google Scholar] [CrossRef]
- Doty, R.L.; Marcus, A.; Lee, W.W. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope 1996, 106, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.N.; Welge-Luessen, A.; Bramerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. “Taste Strips”—A rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J. Neurol. 2009, 256, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Shim, Y.J.; Rhee, C.S.; Kim, J.W. Correlation between olfactory severity ratings based on olfactory function test scores and self-reported severity rating of olfactory loss. Acta Otolaryngol. 2017, 137, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Bertlich, M.; Stihl, C.; Lüsebrink, E.; Hellmuth, J.C.; Scherer, C.; Freytag, S.; Spiegel, J.L.; Stoycheva, I.; Canis, M.; Weiss, B.G.; et al. The course of subjective and objective chemosensory dysfunction in hospitalized patients with COVID-19: A 6-month follow-up. Eur. Arch. Otorhinolaryngol. 2021, 278, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Lötsch, J.; Hummel, T. Clinical usefulness of self-rated olfactory performance—A data science-based assessment of 6000 patients. Chem. Senses 2019, 44, 357–364. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Tirelli, G.; Meloni, P.; Hopkins, C.; Madeddu, G.; De Vito, A.; Gardenal, N.; Valentinotti, R.; Tofanelli, M.; Borsetto, D.; et al. Coronavirus disease 2019 (COVID-19)–related smell and taste impairment with widespread diffusion of severe acute respiratory syndrome–coronavirus-2 (SARS-CoV-2) Omicron variant. Int. Forum Allergy Rhinol. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dehgani-Mobaraki, P.; Patel, Z.; Zaidi, A.K.; Giannandrea, D.; Hopkins, C. The Omicron Variant of SARS-CoV-2 and its Effect on the Olfactory System. Int. Forum Allergy Rhinol. 2022; in press. [Google Scholar] [CrossRef]
- Zazhytska, M.; Kodra, A.; Hoagland, D.A.; Frere, J.; Fullard, J.F.; Shayya, H.; McArthur, N.G.; Moeller, R.; Uhl, S.; Omer, D.A.; et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell 2022, 185, 1052–1064. [Google Scholar] [CrossRef]

| Variable | COVID+ (n = 133) | COVID− (n = 75) | ANOVA/Chi-Squared Test |
|---|---|---|---|
| Age (years), mean ± SD | 42.4 ± 13.9 | 38.6 ± 12.2 | F(1, 173) = 3.51, p = 0.063, η2p = 0.020 |
| Days since COVID-19 diagnosis, mean ± SD | 267.3 ± 113.9 | ||
| Gender, n (%) | χ2(1) = 7.9, p = 0.005, Cramér’s V = 0.195 | ||
| Female | 107 (80.4) | 47 (62.7) | |
| Male | 26 (19.6) | 28 (37.3) | |
| Education (years), n (%) | χ2(3) = 63, p < 0.001, Cramér’s V = 0.552 | ||
| 8–14 | 32 (24.1) | 2 (2.7) | |
| 15–19 | 56 (42.1) | 29 (38.7) | |
| ≥20 | 14 (10.5) | 42 (56.0) | |
| Not shared | 31 (23.3) | 2 (2.7) | |
| Socioeconomic status, n (%) | χ2(3) = 26, p < 0.001, Cramér’s V = 0.374 | ||
| Upper | 0 (0.0) | 6 (11.8) | |
| Upper middle | 45 (33.8) | 19 (37.3) | |
| Lower middle | 43 (32.3) | 19 (37.3) | |
| Lower | 1 (0.8) | 2 (3.9) | |
| Not shared | 44 (33.1) | 5 (9.8) | |
| Smoking status, n (%) | χ2(3) = 16, p = 0.001, Cramér’s V = 0.273 | ||
| Current smoker | 13 (9.8) | 11 (14.7) | |
| Former smoker | 23 (18.8) | 17 (22.7) | |
| Never smoker | 64 (48.1) | 45 (60.0) | |
| Not shared | 31 (23.3) | 2 (2,7) | |
| Vaccination status, n (%) | χ2(3) = 43, p < 0.001, Cramér’s V = 0.455 | ||
| One dose | 40 (30.1) | 10 (13.3) | |
| Two doses | 41 (30.8) | 58 (77.3) | |
| Not vaccinated | 11 (8.3) | 3 (4.0) | |
| Not shared | 41 (30.8) | 4 (5.3) |
| Type of Alteration, n (%) | COVID+ |
|---|---|
| Smell | |
| Smell loss | 105 (78.9) |
| Parosmia | 86 (64.7) |
| Fluctuations | 37 (27.8) |
| Phantosmia | 36 (27.1) |
| Taste | |
| Taste loss | 79 (59.4) |
| Dysgeusia | 55 (41.3) |
| Fluctuations | 21 (15.8) |
| Phantogeusia | 9 (6.8) |
| GCCR Smell and Taste Check Measure | COVID+ | COVID− | ANOVA | Effect Size (η2p) |
|---|---|---|---|---|
| Self-reports of ability, mean ± SD | ||||
| Smell | 42.6 ± 26.2 | 77.8 ± 18.7 | F(1, 180) = 98, p < 0.001 | 0.353 |
| Taste | 56.9 ± 26.6 | 84.5 ± 18.0 | F(1, 180) = 60.3, p < 0.001 | 0.251 |
| Chemesthesis | 61.2 ± 27.7 | 74.0 ± 20.5 | F(1, 180) = 11.4, p < 0.001 | 0.060 |
| Household item intensity, mean ± SD | ||||
| Smell | 50.7 ± 26.6 | 65.6 ± 20.2 | F(1, 180) = 16.5, p < 0.001 | 0.084 |
| Taste | 70.4 ± 20.0 | 76.1 ± 16.1 | F(1, 180) = 4.03, p = 0.046 | 0.022 |
| Chemesthesis | 70.8 ± 24.5 | 74.8 ± 21.5 | F(1, 177) = 1.27, p = 0.260 | 0.007 |
| SCENTinel Test, n (%) | COVID+ | COVID− | Chi-Squared Test | Effect Size (Cramér’s V) |
|---|---|---|---|---|
| Detection | 92 (69.2) | 68 (90.7) | χ2(1) = 12, p < 0.001 | 0.245 |
| Intensity | 117 (88.0) | 73 (97.3) | χ2(1) = 5, p = 0.020 | 0.160 |
| Identification (1st attempt) | 77 (57.9) | 65 (86.7) | χ2(1) = 18, p < 0.001 | 0.297 |
| Identification (2nd attempt) | 18 (32.1) | 9 (90) | χ2(1) = 12, p < 0.001 | 0.422 |
| Total score | 85 (63.9) | 68 (90.7) | χ2(1) = 18, p < 0.001 | 0.291 |
| Measure | β | SE | z Value | p Value | 95% CI | |
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||
| GCCR self-reports of ability | ||||||
| Smell | 0.0156 | 0.0244 | 0.64 | 0.520 | −0.0273 | 0.0586 |
| Taste | 0.0094 | 0.0235 | 0.40 | 0.690 | −0.0347 | 0.0534 |
| Chemesthesis | 0.0125 | 0.0243 | 0.52 | 0.610 | ||
| GCCR household item intensity | ||||||
| Smell | 0.0260 | 0.0214 | 1.21 | 0.230 | −0.0179 | 0.0699 |
| Taste | 0.0137 | 0.0201 | 0.68 | 0.500 | −0.0194 | 0.0468 |
| Chemesthesis | 0.0189 | 0.0223 | 0.85 | 0.400 | −0.0218 | 0.0596 |
| SCENTinel test | ||||||
| Detection | −0.0008 | 0.0005 | −1.59 | 0.110 | −0.0028 | 0.0010 |
| Intensity | 0.0004 | 0.0002 | 1.57 | 0.115 | −0.0012 | 0.0019 |
| Intensity rating 1 | 0.0036 | 0.0149 | 0.24 | 0.810 | −0.0302 | 0.0373 |
| Identification (1st attempt) | −0.0002 | 0.0007 | −0.25 | 0.800 | −0.0022 | 0.0018 |
| Identification (2nd attempt) | −0.0009 | 0.0018 | −0.51 | 0.612 | −0.0053 | 0.0029 |
| Total score | −0.0002 | 0.0006 | −0.39 | 0.699 | −0.0022 | 0.0016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albayay, J.; Fontana, L.; Parma, V.; Zampini, M. Chemosensory Dysfunction in Long-Term COVID-19 Assessed by Self-Reported and Direct Psychophysical Methods. Life 2022, 12, 1487. https://doi.org/10.3390/life12101487
Albayay J, Fontana L, Parma V, Zampini M. Chemosensory Dysfunction in Long-Term COVID-19 Assessed by Self-Reported and Direct Psychophysical Methods. Life. 2022; 12(10):1487. https://doi.org/10.3390/life12101487
Chicago/Turabian StyleAlbayay, Javier, Lara Fontana, Valentina Parma, and Massimiliano Zampini. 2022. "Chemosensory Dysfunction in Long-Term COVID-19 Assessed by Self-Reported and Direct Psychophysical Methods" Life 12, no. 10: 1487. https://doi.org/10.3390/life12101487
APA StyleAlbayay, J., Fontana, L., Parma, V., & Zampini, M. (2022). Chemosensory Dysfunction in Long-Term COVID-19 Assessed by Self-Reported and Direct Psychophysical Methods. Life, 12(10), 1487. https://doi.org/10.3390/life12101487







