Photobiomodulation for the Treatment of Primary Headache: Systematic Review of Randomized Clinical Trials
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.1.1. Types of Included Studies
2.1.2. Types of Participants
2.1.3. Types of Interventions
2.1.4. Types of Outcome Measures
Primary Outcomes
- Pain intensity during a headache episode, measured by any validated scale or questionnaire, such as visual analogue scale (VAS), numeric rating scale, among others.
- Severity, duration, and frequency of episodes of headache.
- Serious adverse events during or after treatment that could lead to hospitalization or death.
Secondary Outcomes
- Minor adverse events, measured as the proportion of participants with at least one adverse event during or after treatment (e.g., exacerbation of pain, discomfort).
- Analgesics needed.
- Quality of life (measured by valid questionnaires, such as the SF-36).
- Patient acceptability.
2.2. Search Strategies
2.3. Study Selection and Data Extraction
2.4. Methodological Quality Assessment of the Included Studies (Risk of Bias)
2.5. Data Synthesis
2.6. Certainty of the Evidence
3. Results
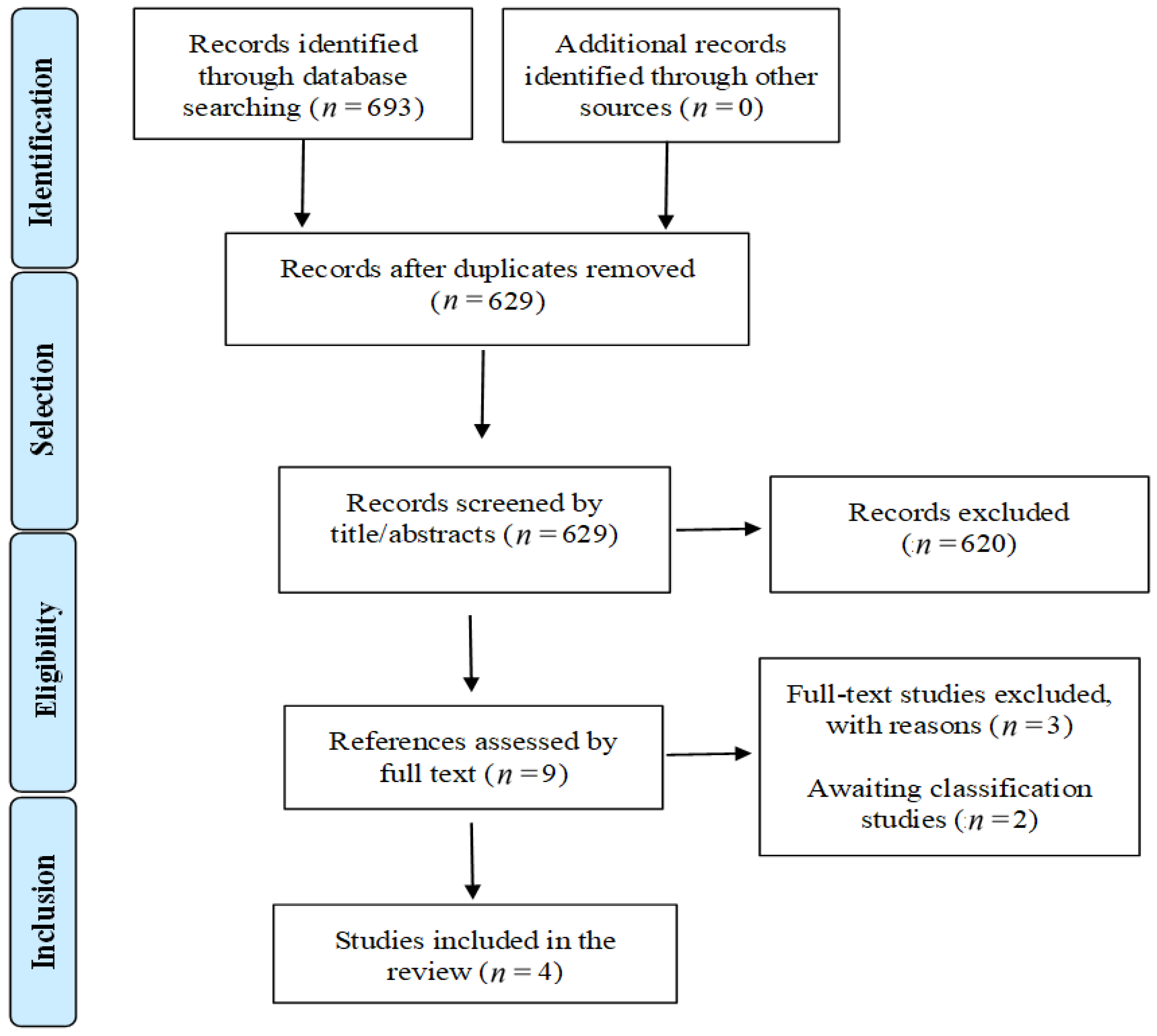
3.1. Search Results
3.2. Characteristics of Studies Included in the Review
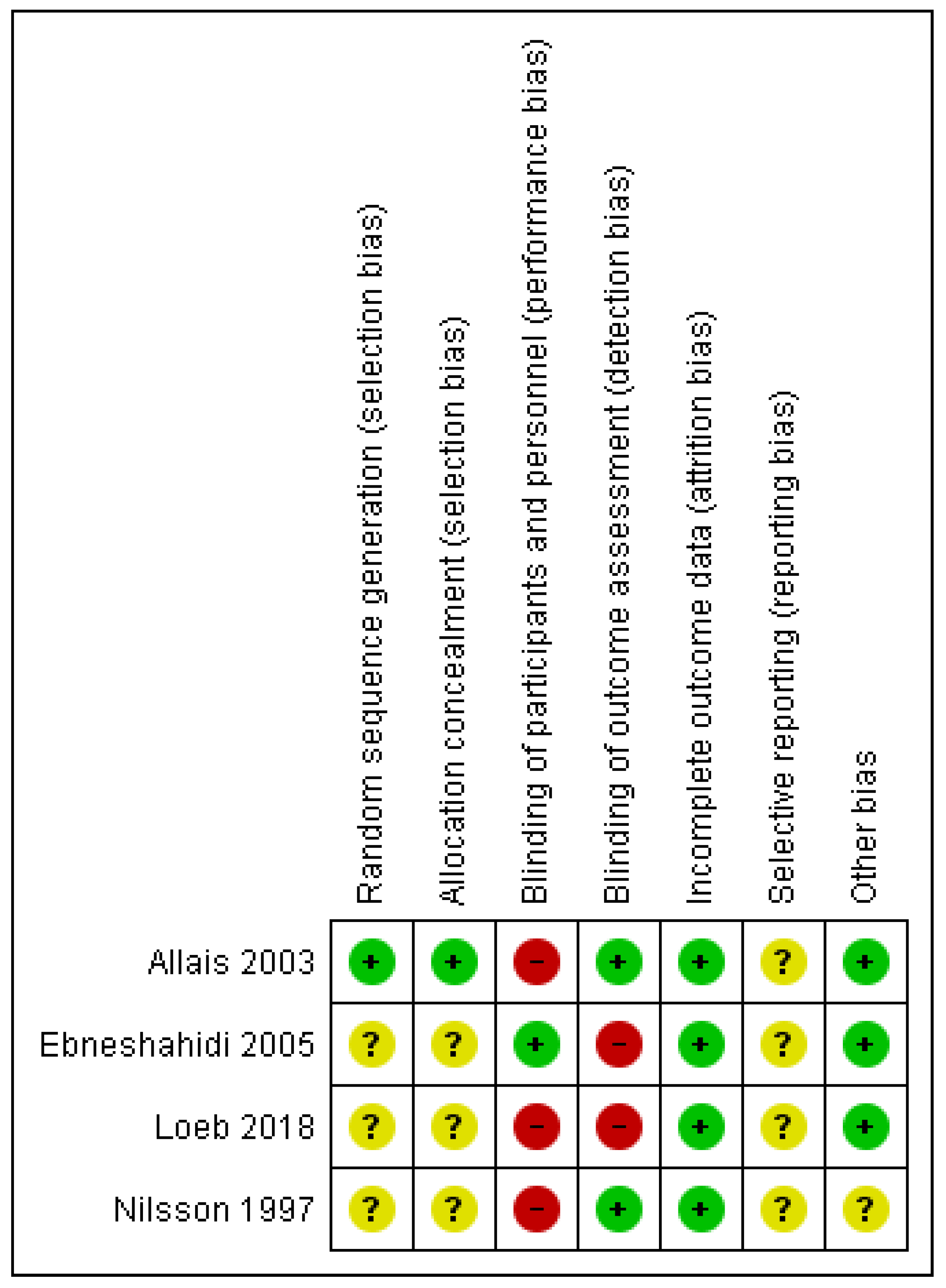
3.3. Methodological Quality Assessment of the Included Studies (Risk of Bias)
3.4. Effects of Intervention
3.4.1. Comparison 1. Low-Level Laser Therapy (LLLT) versus Sham
- Improvement in pain: Pain was assessed using the visual analogue scale (VAS). An improvement in pain was found favouring the LLLT group (mean −2.0 ± 6.3 versus 0 ± 0; p < 0.001).
- Frequency of headache episodes per day: an important reduction in headache episodes was found in the LLLT group compared to the sham group (−8.0 ± 21.5 versus 0.0 ± 0; p < 0.001).
- Duration of episodes (in hours): an improvement was found favoring the LLLT group (−4.0 ± 7.5 versus 0.0 ± 0.0; p < 0.001).
3.4.2. Comparison 2: LLLT versus Botulinum Toxin Type A (BTX-A)
- Frequency of headache episodes: no significant difference between groups was found regarding the reduction in episodes (p = 0.22).
- Adverse events: some patients in the LLLT group described a burning sensation at the application points, which disappeared at the end of the application.
- Use of analgesics: no significant difference between groups was found at the end of treatment (p = 0.12).
3.4.3. Comparison 3. LLLT versus Acupuncture
- Immediately after treatment: no significant difference in the frequency of episodes of headache (days/month) (mean difference (MD): 0.38; 95% confidence interval [CI]: −2.43 to 3.19; p = 0.79).
- One month after treatment: a significant reduction in the frequency of episodes was found in the LLLT group compared to the acupuncture group (MD −6.79; 95% CI: −9.79 to −3.79; p < 0.00001).
- Four months after treatment: an increase in the number of episodes was found in the LLLT group, and a significant reduction was found in the acupuncture group (MD: 5.39; 95% CI: 2.96 to 7.82; p < 0.00001).
3.4.4. Comparison 4. LLLT versus Transcutaneous Electrical Nerve Stimulation (TENS)
- Immediately after treatment: (MD: −0.01; 95% CI: −2.97 to 2.95; p = 0.99);
- One month after treatment (short term) (MD: 0.18; 95% CI: −3.43 to 3.79; p = 0.92);
- Four months after treatment (long term) (MD: −0.89; 95% CI: −3.30 to 1.52; p = 0.47).
3.5. Certainty of the Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barry, L.H.; Matheson, E.M. Approach to Acute Headache in Adults. Am. Fam. Physician 2013, 87, 682–687. [Google Scholar]
- Sheng, P. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004, 24 (Suppl. S1), 9–160. [Google Scholar]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Lombard, L.; Farrar, M.; Ye, W.; Kim, Y.; Cotton, S.; Buchanan, A.S.; Jackson, J.; Joshi, S. A global real-world assessment of the impact on health-related quality of life and work productivity of migraine in patients with insufficient versus good response to triptan medication. J. Headache Pain 2020, 21, 41. [Google Scholar] [CrossRef]
- Liebert, M.A.; Bjordal, J.A.N.M.; Johnson, M.I.; Iversen, V.; Lopes-Martins, R.A.B. Low-Level Laser Therapy in Acute Pain: A Systematic Review of Possible Mechanisms of Action and Clinical Effects in Randomized Placebo-Controlled Trials. Photomed. Laser Surg. 2006, 24, 158–168. [Google Scholar]
- da Silva, J.P.; da Silva, M.A.; Almeida, A.P.F.; Lombardi Junior, I.; Matos, A.P. Laser therapy in the tissue repair process: A literature review. Photomed. Laser Surg. 2010, 28, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Luke, A.M.; Mathew, S.; Altawash, M.M.; Madan, B.M. Lasers: A review with their applications in oral medicine. J. Lasers Med. Sci. 2019, 10, 324–329. [Google Scholar] [CrossRef] [Green Version]
- Cotler, H.B.; Chow, R.T.; Hamblin, M.R.; Carroll, J. The Use of Low Level Laser Therapy (LLLT) For Musculoskeletal Pain. MOJ Orthop. Rheumatol. 2015, 2, 68. [Google Scholar] [CrossRef]
- Wang, X.; Tian, F.; Soni, S.S.; Gonzalez-Lima, F.; Liu, H. Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci. Rep. 2016, 6, 30540. [Google Scholar] [CrossRef] [Green Version]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-eteghad, S.; Rasta, H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2019, 55, 6601–6636. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.; Hamblin, M.R. Photobiomodulation and the brain: A new paradigm. J. Opt. 2017, 19, 13003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Thoma, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.W.V. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torelli, P.; Jensen, R.; Olesen, J. Physiotherapy for tension-type headache: A controlled study. Cephalalgia 2004, 24, 29–36. [Google Scholar] [CrossRef]
- Amoils, S.; Krues, J. The effect of low level laser therapy on acute headache syndromes. Laser Ther. 1991, 3, 155–157. [Google Scholar] [CrossRef] [Green Version]
- Lavies, N.G. Laser acupuncture for migraine and muscle tension headache: A double-blind controlled trial. Acupunct. Med. 1998, 16, 73–76. [Google Scholar] [CrossRef]
- Allais, G.; De Lorenzo, C.; Quirico, P.; Lupi, G.; Airola, G.; Mana, O.; Benedetto, C. Non-pharmacological approaches to chronic headaches: Transcutaneous electrical nerve stimulation, lasertherapy and acupuncture in transformed migraine treatment. Neurol. Sci. 2003, 24 (Suppl. S2), s138–s142. [Google Scholar] [CrossRef]
- Ebneshahidi, N.S.; Heshmatipour, M.; Moghaddami, A.; Eghtesadi-Araghi, P. The effects of laser acupuncture on chronic tension headache—A randomised controlled trial. Acupunct. Med. 2005, 23, 13–18. [Google Scholar] [CrossRef]
- Loeb, L.M.; Amorim, R.P.; Mazzacoratti, M.d.G.N.; Scorza, F.A.; Peres, M.F.P. Botulinum toxin A (BT-A) versus low-level laser therapy (LLLT) in chronic migraine treatment: A comparison TT—Toxina botulínica A (BT-A) versus laser terapia de baixa potência (LLLT) em enxaqueca crônica: Uma triagem comparativa. Arq. Neuropsiquiatr. 2018, 76, 663–667. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0004-282X2018001000663 (accessed on 24 December 2021). [CrossRef] [Green Version]
- Nilsson, N.; Christensen, H.W.H.J. The effect of spinal manipulation in the treatment of cervicogenic headache. J. Manip. Physiol. Ther. 1997, 20, 326–330. [Google Scholar]
- Xu, S.; Yu, L.; Luo, X.; Wang, M.; Chen, G.; Zhang, Q.; Liu, W.; Zhou, Z.; Song, J.; Jing, H.; et al. Manual acupuncture versus sham acupuncture and usual care for prophylaxis of episodic migraine without aura: Multicentre, randomised clinical trial. BMJ 2020, 368, m697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Dai, Q.; Shi, Z.; Chen, H.; Hu, Y.; Wang, X.; Zhang, X.; Tian, G. Clinical Efficacy and Safety of Electroacupuncture in Migraine Treatment: A Systematic Review and Network Meta-Analysis. Am. J. Chin. Med. 2019, 47, 1755–1780. [Google Scholar] [CrossRef]
- World Association for Laser Therapy (WALT). Dosage Recommendations. Available online: https://waltza.co.za (accessed on 24 February 2021).
- Xu, J.; Zhang, F.-Q.; Pei, J.; Ji, J. Acupuncture for migraine without aura: A systematic review and meta-analysis. J. Integr. Med. 2018, 16, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. Consort 2010 statement: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2011, 9, 672–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Author/Year and Country | Population | Intervention | Comparators | Outcome Measure and Follow-Up | Main Results | Funding Sources |
|---|---|---|---|---|---|---|
| Allais et al., 2003, Italy [19] | n = 60 Age 21.6 years 100% women Headache more than 15 days/months, in previous six months | Infra LLLT 904 nm (n = 20) Intensity: 27 mV Time: 60 s/point Mode: NR 10 sessions, 25 min each Acupuncture points: GB20, GV20, GB14, Ex-HN5 | 10 sessions of acupuncture (n = 20) 10 sessions of TENS (n = 20) Points: GB20, GV20, GB14, Ex-HN5 | Number of headache episodes (days/month) Assessed at 1 and 2 months | Compared to acupuncture, LLLT may result in frequency of headache episodes improvement after the following: 1 month (MD −6.79; 95% CI −9.79 to −3.79) 4 months (MD: 5.39; 95% CI 2.96 to 7.82) Compared to TENS, no differences were observed. | NR |
| Ebneshahidi et al., 2005, Iran [20] | n = 50 Age: 25–54 years 90% women Headache more than 15 days/months | LLLT 830 nm (n = 25) low energy laser radiation Intensity: 39 mV/cm2 Time: 43 s/point Continuous mode Dose: 1.3 J 10 sessions Acupuncture points: GB14, GB20, L14, and LU7 | 10 sessions of sham (n = 25) Energy output: zero Acupuncture points: GB14, GB20, L14, and LU7 | Pain intensity (VAS) Duration of episodes (hours) Number of headache episodes (days/month) Assessed every 1 month, for 3 months | LLLT may result in a marginal improvement in the following: pain (−2.0 versus 0.0 VAS points) frequency of headache episodes (−8.0 versus 0.0 days) duration of headache episodes (−4.0 versus 0.0 h) | NR |
| Loeb et al., 2018, Brazil [21] | n = 36 Age: 20–65 years 90% women Headache more than 15 days/months | LLLT 808 nm (n = 18) Intensity: 100 mV Time: 33 s/point Continuous mode Dose: 120 J/cm2 10 sessions, twice a week Cranial points | BTX-A (n = 18) Same cranial points | Number of headache episodes (days/month) Adverse events Use of medication Assessed every 1 month, for 3 months | LLLT may result in a non-significant difference in the following: frequency of headache episodes (p = 0.22) use of medication (p = 0.12) | CNPq, FAPESP, CAPES, Instituto Nacional de Neurociência Translacional (INNT) |
| Nilsson et al., 1997, Country NR [22] | n = 53 Age NR Sex NR Frequency NR | 6 sessions of LLLT (type and parameters NR) Trigger points in the upper cervical region | Cervical manipulation Deep friction massage | Number of headache episodes (days) Assessed at 5 weeks | LLLT may result in a decreased number of headache hours per day (p = 0.03) | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, A.O.; Martimbianco, A.L.C.; Brugnera Junior, A.; Horliana, A.C.R.T.; da Silva, T.; Santos, E.M.; Fragoso, Y.D.; Fernandes, K.P.S.; Nammour, S.; Bussadori, S.K. Photobiomodulation for the Treatment of Primary Headache: Systematic Review of Randomized Clinical Trials. Life 2022, 12, 98. https://doi.org/10.3390/life12010098
Gomes AO, Martimbianco ALC, Brugnera Junior A, Horliana ACRT, da Silva T, Santos EM, Fragoso YD, Fernandes KPS, Nammour S, Bussadori SK. Photobiomodulation for the Treatment of Primary Headache: Systematic Review of Randomized Clinical Trials. Life. 2022; 12(1):98. https://doi.org/10.3390/life12010098
Chicago/Turabian StyleGomes, Andréa Oliver, Ana Luiza Cabrera Martimbianco, Aldo Brugnera Junior, Anna Carolina Ratto Tempestini Horliana, Tamiris da Silva, Elaine Marcílio Santos, Yara Dadalti Fragoso, Kristianne Porta Santos Fernandes, Samir Nammour, and Sandra Kalil Bussadori. 2022. "Photobiomodulation for the Treatment of Primary Headache: Systematic Review of Randomized Clinical Trials" Life 12, no. 1: 98. https://doi.org/10.3390/life12010098
APA StyleGomes, A. O., Martimbianco, A. L. C., Brugnera Junior, A., Horliana, A. C. R. T., da Silva, T., Santos, E. M., Fragoso, Y. D., Fernandes, K. P. S., Nammour, S., & Bussadori, S. K. (2022). Photobiomodulation for the Treatment of Primary Headache: Systematic Review of Randomized Clinical Trials. Life, 12(1), 98. https://doi.org/10.3390/life12010098








