Elevated Systemic Inflammation Is Associated with Reduced Corticolimbic White Matter Integrity in Depression
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Behavioral Data
2.3. C-Reactive Protein
2.4. MRI Data Acquisition and Preprocessing
2.5. Connectometry Analysis
2.6. Exploratory Analysis and Sensitivity Analysis
3. Results
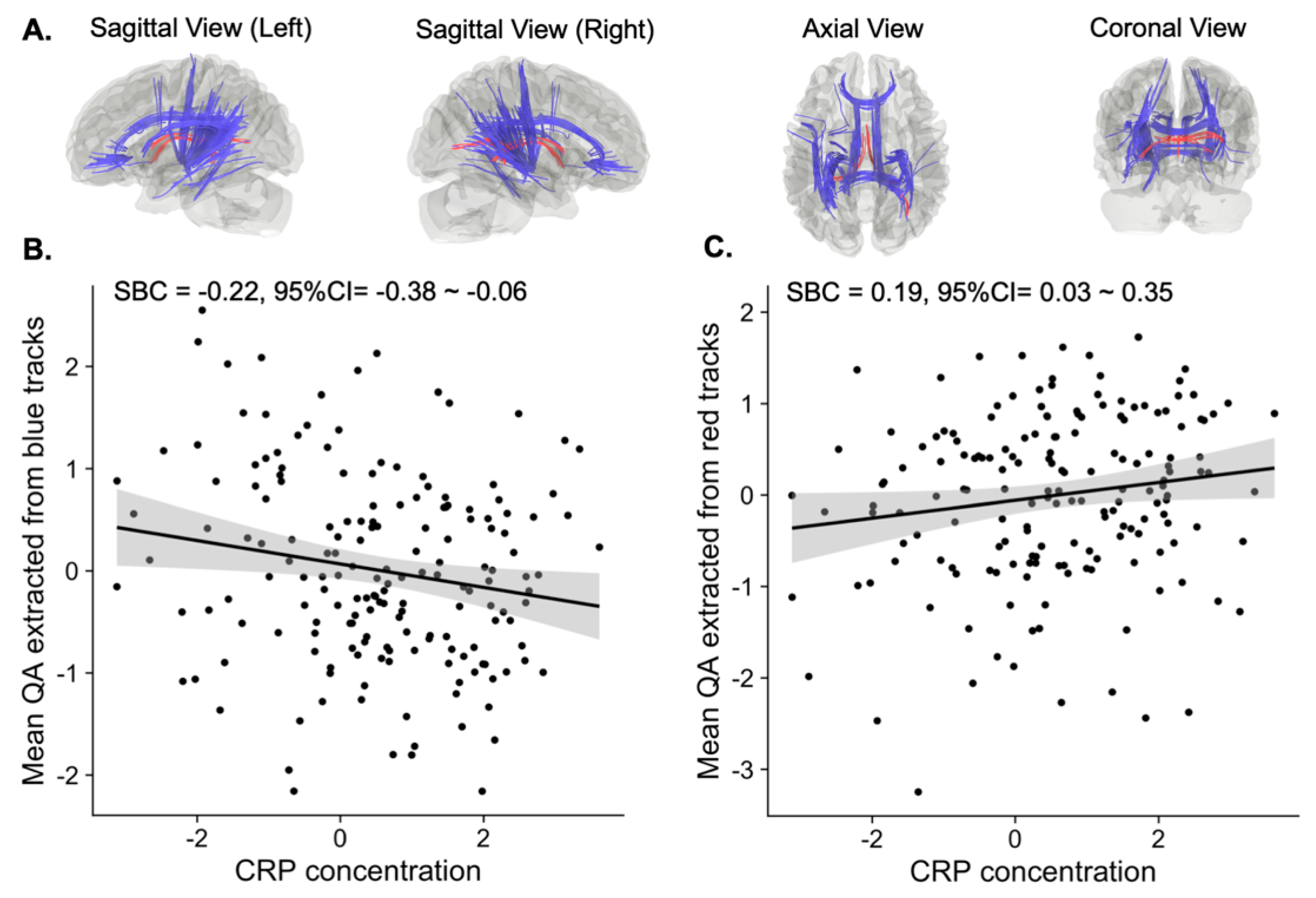
3.1. Tracts Correlated with CRP Concentration
3.2. Association between QA and Depressive Symptoms
3.3. Sensitivity to Potential Confounders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Dantzer, R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Han, K.M.; Ham, B.J. How Inflammation Affects the Brain in Depression: A Review of Functional and Structural MRI Studies. J. Clin. Neurol. 2021, 17, 503–515. [Google Scholar] [CrossRef]
- Ironside, M.; Admon, R.; Maddox, S.A.; Mehta, M.; Douglas, S.; Olson, D.P.; Pizzagalli, D.A. Inflammation and depressive phenotypes: Evidence from medical records from over 12,000 patients and brain morphology. Psychol. Med. 2020, 50, 2790–2798. [Google Scholar] [CrossRef]
- Byrne, M.L.; Whittle, S.; Allen, N.B. The Role of Brain Structure and Function in the Association between Inflammation and Depressive Symptoms: A Systematic Review. Psychosom. Med. 2016, 78, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.P.; Müller, V.I.; Eickhoff, S.B.; Fox, P.T. Multimodal Abnormalities of Brain Structure and Function in Major Depressive Disorder: A Meta-Analysis of Neuroimaging Studies. Am. J. Psychiatry 2020, 177, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Fornito, A.; Pantelis, C.; Yücel, M. Gray matter abnormalities in Major Depressive Disorder: A meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012, 138, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Wu, M.; Chen, Z.; Hu, X.; Chen, Y.; Zhu, H.; Jia, Z.; Gong, Q. Brain gray matter alterations in first episodes of depression: A meta-analysis of whole-brain studies. Neurosci. Biobehav. Rev. 2016, 60, 43–50. [Google Scholar] [CrossRef]
- Felger, J.C.; Li, Z.; Haroon, E.; Woolwine, B.J.; Jung, M.Y.; Hu, X.; Miller, A.H. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 2016, 21, 1358–1365. [Google Scholar] [CrossRef]
- Kitzbichler, M.G.; Aruldass, A.R.; Barker, G.J.; Wood, T.C.; Dowell, N.G.; Hurley, S.A.; McLean, J.; Correia, M.; Clarke, C.; Pointon, L.; et al. Peripheral inflammation is associated with micro-structural and functional connectivity changes in depression-related brain networks. Mol. Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Aruldass, A.R.; Kitzbichler, M.G.; Morgan, S.E.; Lim, S.; Lynall, M.E.; Turner, L.; Vertes, P.; Cavanagh, J.; Cowen, P.; Pariante, C.M.; et al. Dysconnectivity of a brain functional network was associated with blood inflammatory markers in depression. Brain Behav. Immun. 2021, 98, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Boorman, E.D.; O’Shea, J.; Sebastian, C.; Rushworth, M.F.; Johansen-Berg, H. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Curr. Biol. 2007, 17, 1426–1431. [Google Scholar] [CrossRef]
- Hermundstad, A.M.; Bassett, D.S.; Brown, K.S.; Aminoff, E.M.; Clewett, D.; Freeman, S.; Frithsen, A.; Johnson, A.; Tipper, C.M.; Miller, M.B.; et al. Structural foundations of resting-state and task-based functional connectivity in the human brain. Proc. Natl. Acad. Sci. USA 2013, 110, 6169–6174. [Google Scholar] [CrossRef]
- Hurlimann, J.; Thorbecke, G.J.; Hochwald, G.M. The liver as the site of C-reactive protein formation. J. Exp. Med. 1966, 123, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.; Gauldie, J. The acute phase response. Immunol. Today 1994, 15, 74–80. [Google Scholar] [CrossRef]
- Rhodes, B.; Furnrohr, B.G.; Vyse, T.J. C-reactive protein in rheumatology: Biology and genetics. Nat. Rev. Rheumatol. 2011, 7, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Haroon, E.; Patel, T.A.; Goldsmith, D.R.; Wommack, E.C.; Woolwine, B.J.; Le, N.A.; Feinberg, R.; Tansey, M.G.; Miller, A.H. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry 2020, 25, 1301–1311. [Google Scholar] [CrossRef]
- Horn, S.R.; Long, M.M.; Nelson, B.W.; Allen, N.B.; Fisher, P.A.; Byrne, M.L. Replication and reproducibility issues in the relationship between C-reactive protein and depression: A systematic review and focused meta-analysis. Brain Behav. Immun. 2018, 73, 85–114. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychol. Med. 2019, 49, 1958–1970. [Google Scholar] [CrossRef]
- Valkanova, V.; Ebmeier, K.P.; Allan, C.L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013, 150, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimaki, M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Moriarity, D.P.; Giollabhui, N.M.; Ellman, L.M.; Klugman, J.; Coe, C.L.; Abramson, L.Y.; Alloy, L.B. Inflammatory Proteins Predict Change in Depressive Symptoms in Male and Female Adolescents. Clin. Psychol. Sci. 2019, 7, 754–767. [Google Scholar] [CrossRef]
- Savitz, J.; Drevets, W.C.; Smith, C.M.; Victor, T.A.; Wurfel, B.E.; Bellgowan, P.S.; Bodurka, J.; Teague, T.K.; Dantzer, R. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 2015, 40, 463–471. [Google Scholar] [CrossRef]
- Meier, T.B.; Drevets, W.C.; Wurfel, B.E.; Ford, B.N.; Morris, H.M.; Victor, T.A.; Bodurka, J.; Teague, T.K.; Dantzer, R.; Savitz, J. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav. Immun. 2016, 53, 39–48. [Google Scholar] [CrossRef]
- Sugimoto, K.; Kakeda, S.; Watanabe, K.; Katsuki, A.; Ueda, I.; Igata, N.; Igata, R.; Abe, O.; Yoshimura, R.; Korogi, Y. Relationship between white matter integrity and serum inflammatory cytokine levels in drug-naive patients with major depressive disorder: Diffusion tensor imaging study using tract-based spatial statistics. Transl. Psychiatry 2018, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Sohn, H.; Kwon, M.S.; Kim, B. White Matter Alterations Associated with Pro-inflammatory Cytokines in Patients with Major Depressive Disorder. Clin. Psychopharmacol. Neurosci. 2021, 19, 449–458. [Google Scholar] [CrossRef]
- Green, C.; Shen, X.; Stevenson, A.J.; Conole, E.L.S.; Harris, M.A.; Barbu, M.C.; Hawkins, E.L.; Adams, M.J.; Hillary, R.F.; Lawrie, S.M.; et al. Structural brain correlates of serum and epigenetic markers of inflammation in major depressive disorder. Brain Behav. Immun. 2021, 92, 39–48. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Edden, R.A.; Jones, D.K. Spatial and orientational heterogeneity in the statistical sensitivity of skeleton-based analyses of diffusion tensor MR imaging data. J. Neurosci. Methods 2011, 201, 213–219. [Google Scholar] [CrossRef]
- Keihaninejad, S.; Ryan, N.S.; Malone, I.B.; Modat, M.; Cash, D.; Ridgway, G.R.; Zhang, H.; Fox, N.C.; Ourselin, S. The importance of group-wise registration in tract based spatial statistics study of neurodegeneration: A simulation study in Alzheimer’s disease. PLoS ONE 2012, 7, e45996. [Google Scholar] [CrossRef] [PubMed]
- Zalesky, A. Moderating registration misalignment in voxelwise comparisons of DTI data: A performance evaluation of skeleton projection. Magn. Reson. Imaging 2011, 29, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.; Laun, F.B.; Leemans, A.; Tax, C.M.; Biessels, G.J.; Stieltjes, B.; Maier-Hein, K.H. Methodological considerations on tract-based spatial statistics (TBSS). Neuroimage 2014, 100, 358–369. [Google Scholar] [CrossRef]
- Yeh, F.C.; Badre, D.; Verstynen, T. Connectometry: A statistical approach harnessing the analytical potential of the local connectome. Neuroimage 2016, 125, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 22–33. [Google Scholar] [PubMed]
- Victor, T.A.; Khalsa, S.S.; Simmons, W.K.; Feinstein, J.S.; Savitz, J.; Aupperle, R.L.; Yeh, H.W.; Bodurka, J.; Paulus, M.P. Tulsa 1000: A naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open 2018, 8, e016620. [Google Scholar] [CrossRef]
- Gershon, R.C.; Rothrock, N.; Hanrahan, R.; Bass, M.; Cella, D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J. Appl. Meas. 2010, 11, 304–314. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Brown, S.A.; Myers, M.G.; Lippke, L.; Tapert, S.F.; Stewart, D.G.; Vik, P.W. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. J. Stud. Alcohol. 1998, 59, 427–438. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016, 125, 1063–1078. [Google Scholar] [CrossRef]
- Bastiani, M.; Cottaar, M.; Fitzgibbon, S.P.; Suri, S.; Alfaro-Almagro, F.; Sotiropoulos, S.N.; Jbabdi, S.; Andersson, J.L.R. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. Neuroimage 2019, 184, 801–812. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Graham, M.S.; Zsoldos, E.; Sotiropoulos, S.N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 2016, 141, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.C.; Tseng, W.Y. NTU-90: A high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage 2011, 58, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.C.; Wedeen, V.J.; Tseng, W.Y. Generalized q-sampling imaging. IEEE Trans. Med. Imaging 2010, 29, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.L.; Hasan, K.M.; Lazar, M.; Tsuruda, J.S.; Parker, D.L. Analysis of partial volume effects in diffusion-tensor MRI. Magn. Reson. Med. 2001, 45, 770–780. [Google Scholar] [CrossRef]
- Alexander, D.C.; Barker, G.J.; Arridge, S.R. Detection and modeling of non-Gaussian apparent diffusion coefficient profiles in human brain data. Magn. Reson. Med. 2002, 48, 331–340. [Google Scholar] [CrossRef]
- Oouchi, H.; Yamada, K.; Sakai, K.; Kizu, O.; Kubota, T.; Ito, H.; Nishimura, T. Diffusion anisotropy measurement of brain white matter is affected by voxel size: Underestimation occurs in areas with crossing fibers. AJNR Am. J. Neuroradiol. 2007, 28, 1102–1106. [Google Scholar] [CrossRef]
- Tuch, D.S.; Reese, T.G.; Wiegell, M.R.; Makris, N.; Belliveau, J.W.; Wedeen, V.J. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn. Reson. Med. 2002, 48, 577–582. [Google Scholar] [CrossRef]
- Yeh, F.C.; Verstynen, T.D.; Wang, Y.; Fernandez-Miranda, J.C.; Tseng, W.Y. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE 2013, 8, e80713. [Google Scholar] [CrossRef]
- Maier-Hein, K.H.; Neher, P.F.; Houde, J.C.; Cote, M.A.; Garyfallidis, E.; Zhong, J.; Chamberland, M.; Yeh, F.C.; Lin, Y.C.; Ji, Q.; et al. The challenge of mapping the human connectome based on diffusion tractography. Nat. Commun. 2017, 8, 1349. [Google Scholar] [CrossRef] [PubMed]
- Zalesky, A.; Fornito, A.; Cocchi, L.; Gollo, L.L.; van den Heuvel, M.P.; Breakspear, M. Connectome sensitivity or specificity: Which is more important? Neuroimage 2016, 142, 407–420. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J. Principles of confounder selection. Eur. J. Epidemiol. 2019, 34, 211–219. [Google Scholar] [CrossRef]
- Van der Knaap, L.J.; van der Ham, I.J. How does the corpus callosum mediate interhemispheric transfer? A review. Behav. Brain Res. 2011, 223, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Raybaud, C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: Anatomy, development, and malformation. Neuroradiology 2010, 52, 447–477. [Google Scholar] [CrossRef] [PubMed]
- Cyprien, F.; Courtet, P.; Malafosse, A.; Maller, J.; Meslin, C.; Bonafé, A.; Le Bars, E.; de Champfleur, N.M.; Ritchie, K.; Artero, S. Suicidal behavior is associated with reduced corpus callosum area. Biol. Psychiatry 2011, 70, 320–326. [Google Scholar] [CrossRef]
- Won, E.; Choi, S.; Kang, J.; Kim, A.; Han, K.M.; Chang, H.S.; Tae, W.S.; Son, K.R.; Joe, S.H.; Lee, M.S.; et al. Association between reduced white matter integrity in the corpus callosum and serotonin transporter gene DNA methylation in medication-naive patients with major depressive disorder. Transl. Psychiatry 2016, 6, e866. [Google Scholar] [CrossRef]
- Kelly, S.; Jahanshad, N.; Zalesky, A.; Kochunov, P.; Agartz, I.; Alloza, C.; Andreassen, O.A.; Arango, C.; Banaj, N.; Bouix, S.; et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA Schizophrenia DTI Working Group. Mol. Psychiatry 2018, 23, 1261–1269. [Google Scholar] [CrossRef]
- Jackowski, A.P.; Douglas-Palumberi, H.; Jackowski, M.; Win, L.; Schultz, R.T.; Staib, L.W.; Krystal, J.H.; Kaufman, J. Corpus callosum in maltreated children with posttraumatic stress disorder: A diffusion tensor imaging study. Psychiatry Res. Neuroimaging 2008, 162, 256–261. [Google Scholar] [CrossRef]
- Wise, T.; Radua, J.; Nortje, G.; Cleare, A.J.; Young, A.H.; Arnone, D. Voxel-Based Meta-Analytical Evidence of Structural Disconnectivity in Major Depression and Bipolar Disorder. Biol. Psychiatry 2016, 79, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Yeh, P.-H.; Bellani, M.; Radaelli, D.; Nicoletti, M.A.; Poletti, S.; Falini, A.; Dallaspezia, S.; Colombo, C.; Scotti, G.; et al. Disruption of White Matter Integrity in Bipolar Depression as a Possible Structural Marker of Illness. Biol. Psychiatry 2011, 69, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Hynd, G.W.; Semrud-Clikeman, M.; Lorys, A.R.; Novey, E.S.; Eliopulos, D.; Lyytinen, H. Corpus Callosum Morphology in Attention Deficit-Hyperactivity Disorder: Morphometric Analysis of MRI. J. Learn. Disabil. 1991, 24, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, C.; Rocca, M.A.; Pagani, E.; Riccitelli, G.C.; Pravatà, E.; Radaelli, M.; Martinelli-Boneschi, F.; Falini, A.; Copetti, M.; Comi, G.; et al. Forceps minor damage and co-occurrence of depression and fatigue in multiple sclerosis. Mult. Scler. 2014, 20, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Van Velzen, L.S.; Kelly, S.; Isaev, D.; Aleman, A.; Aftanas, L.I.; Bauer, J.; Baune, B.T.; Brak, I.V.; Carballedo, A.; Connolly, C.G.; et al. White matter disturbances in major depressive disorder: A coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatry 2020, 25, 1511–1525. [Google Scholar] [CrossRef]
- Chen, G.; Hu, X.; Li, L.; Huang, X.; Lui, S.; Kuang, W.; Ai, H.; Bi, F.; Gu, Z.; Gong, Q. Disorganization of white matter architecture in major depressive disorder: A meta-analysis of diffusion tensor imaging with tract-based spatial statistics. Sci. Rep. 2016, 6, 21825. [Google Scholar] [CrossRef]
- Murphy, M.L.; Frodl, T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol. Mood Anxiety Disord. 2011, 1, 3. [Google Scholar] [CrossRef]
- Liao, Y.; Huang, X.; Wu, Q.; Yang, C.; Kuang, W.; Du, M.; Lui, S.; Yue, Q.; Chan, R.; Kemp, G.; et al. Is depression a disconnection syndrome? Meta- analysis of diffusion tensor imaging studies in patients with MDD. J. Psychiatry Neurosci. 2013, 38, 49–56. [Google Scholar] [CrossRef]
- Kieseppä, T.; Eerola, M.; Mäntylä, R.; Neuvonen, T.; Poutanen, V.-P.; Luoma, K.; Tuulio-Henriksson, A.; Jylhä, P.; Mantere, O.; Melartin, T. Major depressive disorder and white matter abnormalities: A diffusion tensor imaging study with tract-based spatial statistics. J. Affect. Disord. 2010, 120, 240–244. [Google Scholar] [CrossRef]
- Lai, C.H.; Wu, Y.T. Alterations in white matter micro-integrity of the superior longitudinal fasciculus and anterior thalamic radiation of young adult patients with depression. Psychol. Med. 2014, 44, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- Heilbronner, S.R.; Haber, S.N. Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: Implications for neuroimaging and psychiatric disorders. J. Neurosci. 2014, 34, 10041–10054. [Google Scholar] [CrossRef]
- Bubb, E.J.; Metzler-Baddeley, C.; Aggleton, J.P. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018, 92, 104–127. [Google Scholar] [CrossRef]
- Bora, E.; Harrison, B.J.; Davey, C.G.; Yücel, M.; Pantelis, C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol. Med. 2012, 42, 671–681. [Google Scholar] [CrossRef]
- Yang, J.; Yin, Y.; Svob, C.; Long, J.; He, X.; Zhang, Y.; Xu, Z.; Li, L.; Liu, J.; Dong, J.; et al. Amygdala Atrophy and Its Functional Disconnection with the Cortico-Striatal-Pallidal-Thalamic Circuit in Major Depressive Disorder in Females. PLoS ONE 2017, 12, e0168239. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I. 3D MRI studies of neuroanatomic changes in unipolar major depression: The role of stress and medical comorbidity. Biol. Psychiatry 2000, 48, 791–800. [Google Scholar] [CrossRef]
- Herbet, G.; Zemmoura, I.; Duffau, H. Functional Anatomy of the Inferior Longitudinal Fasciculus: From Historical Reports to Current Hypotheses. Front. Neuroanat. 2018, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pathak, S.; Stefaneanu, L.; Yeh, F.-C.; Li, S.; Fernandez-Miranda, J.C. Subcomponents and connectivity of the superior longitudinal fasciculus in the human brain. Brain Struct. Funct. 2016, 221, 2075–2092. [Google Scholar] [CrossRef]
- Wu, F.; Tang, Y.; Xu, K.; Kong, L.; Sun, W.; Wang, F.; Kong, D.; Li, Y.; Liu, Y. Whiter matter abnormalities in medication-naive subjects with a single short-duration episode of major depressive disorder. Psychiatry Res. Neuroimaging 2011, 191, 80–83. [Google Scholar] [CrossRef][Green Version]
- Jia, Z.; Huang, X.; Wu, Q.; Zhang, T.; Lui, S.; Zhang, J.; Amatya, N.; Kuang, W.; Chan, R.C.K.; Kemp, G.J.; et al. High-Field Magnetic Resonance Imaging of Suicidality in Patients with Major Depressive Disorder. Am. J. Psychiatry 2010, 167, 1381–1390. [Google Scholar] [CrossRef]
- Huang, H.; Fan, X.; Williamson, D.E.; Rao, U. White Matter Changes in Healthy Adolescents at Familial Risk for Unipolar Depression: A Diffusion Tensor Imaging Study. Neuropsychopharmacology 2011, 36, 684–691. [Google Scholar] [CrossRef]
- Bettcher, B.M.; Yaffe, K.; Boudreau, R.M.; Neuhaus, J.; Aizenstein, H.; Ding, J.; Kritchevsky, S.B.; Launer, L.J.; Liu, Y.; Satterfield, S.; et al. Declines in inflammation predict greater white matter microstructure in older adults. Neurobiol. Aging 2015, 36, 948–954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dudek, K.A.; Dion-Albert, L.; Lebel, M.; LeClair, K.; Labrecque, S.; Tuck, E.; Ferrer Perez, C.; Golden, S.A.; Tamminga, C.; Turecki, G.; et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc. Natl. Acad. Sci. USA 2020, 117, 3326–3336. [Google Scholar] [CrossRef]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016, 17, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.R.; Marsh, S.E.; Stevens, B. Immune Signaling in Neurodegeneration. Immunity 2019, 50, 955–974. [Google Scholar] [CrossRef]
- Mechawar, N.; Savitz, J. Neuropathology of mood disorders: Do we see the stigmata of inflammation? Transl. Psychiatry 2016, 6, e946. [Google Scholar] [CrossRef]
- Cumberworth, S.L.; Barrie, J.A.; Cunningham, M.E.; de Figueiredo, D.P.G.; Schultz, V.; Wilder-Smith, A.J.; Brennan, B.; Pena, L.J.; de Oliveira Franca, R.F.; Linington, C.; et al. Zika virus tropism and interactions in myelinating neural cell cultures: CNS cells and myelin are preferentially affected. Acta Neuropathol. Commun. 2017, 5, 50. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Xu, E.; Tu, G.; Guo, M.; Liang, S.; Xiong, H. Human immunodeficiency virus protein Tat induces oligodendrocyte injury by enhancing outward K(+) current conducted by KV1.3. Neurobiol. Dis. 2017, 97, 1–10. [Google Scholar] [CrossRef]
- Myung, W.; Han, C.E.; Fava, M.; Mischoulon, D.; Papakostas, G.I.; Heo, J.Y.; Kim, K.W.; Kim, S.T.; Kim, D.J.; Kim, D.K.; et al. Reduced frontal-subcortical white matter connectivity in association with suicidal ideation in major depressive disorder. Transl. Psychiatry 2016, 6, e835. [Google Scholar] [CrossRef]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Kimura-Ohba, S.; Yang, Y.; Thompson, J.; Kimura, T.; Salayandia, V.M.; Cosse, M.; Yang, Y.; Sillerud, L.O.; Rosenberg, G.A. Transient increase of fractional anisotropy in reversible vasogenic edema. J. Cereb. Blood Flow. Metab. 2016, 36, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Vorburger, R.; Scarmeas, N.; Luchsinger, J.A.; Manly, J.J.; Schupf, N.; Mayeux, R.; Brickman, A.M. Circulating inflammatory biomarkers in relation to brain structural measurements in a non-demented elderly population. Brain Behav. Immun. 2017, 65, 150–160. [Google Scholar] [CrossRef] [PubMed]
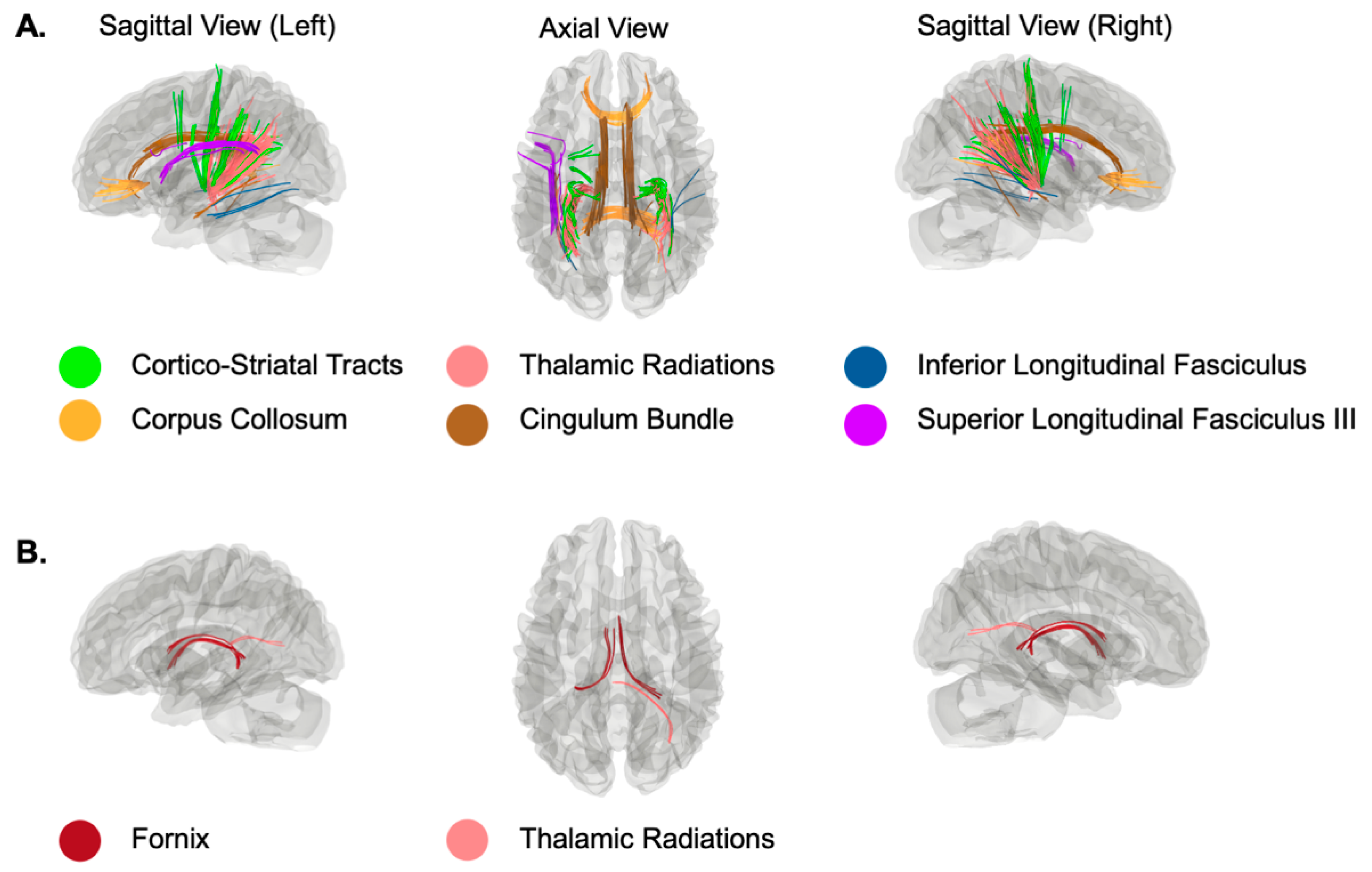
| Mean | SD | |
|---|---|---|
| Age | 34.00 | 10.68 |
| Sex (Male %) | 33.50 | - |
| BMI a | 28.86 | 5.22 |
| Education b | 6.64 | 1.52 |
| Income c | 9.76 | 2.89 |
| CTQ d | 46.69 | 18.37 |
| Medicated (%) e | 64.80 | - |
| Current smoker (%) | 13.00 | - |
| Depression severity f | 61.62 | 7.17 |
| Anxiety severity g | 62.32 | 6.69 |
| Number of episodes h | 3.88 | 3.27 |
| Alcohol use i | 4.99 | 2.54 |
| Log CRP j | 0.53 | 1.44 |
| Depressive symptoms k | Mean | SD |
| PHQ-9 total score | 13.16 | 4.94 |
| Anhedonia | 1.58 | 0.84 |
| Depressed mood | 1.53 | 0.82 |
| Sleep problems | 2.07 | 0.94 |
| Tiredness | 2.10 | 0.86 |
| Changes in appetite | 1.51 | 1.05 |
| Feelings of inadequacy | 1.79 | 1.00 |
| Concentration problems | 1.43 | 1.00 |
| Psychomotor changes | 0.72 | 0.83 |
| Suicidality | 0.44 | 0.69 |
| Ethnicity | % | |
| Asian | 1.14 | - |
| Black | 9.71 | - |
| Hispanic | 4.57 | - |
| Native American | 15.43 | - |
| White | 65.71 | - |
| Other | 3.43 | - |
| QA Extracted from Blue Tracts a | QA Extracted from Red Tracts b | |||||
|---|---|---|---|---|---|---|
| Symptoms | SBC | 95%CI | puncorrected | SBC | 95%CI | puncorrected |
| Anhedonia | 0.00 | −0.15–0.16 | 0.96 | −0.06 | −0.21–0.10 | 0.48 |
| Depressed mood | −0.01 | −0.17–0.14 | 0.85 | −0.01 | −0.17–0.14 | 0.87 |
| Sleep problems | −0.05 | −0.21–0.11 | 0.52 | −0.07 | −0.23–0.08 | 0.36 |
| Tiredness | −0.11 | −0.27–0.05 | 0.19 | −0.05 | −0.20–0.11 | 0.55 |
| Changes in appetite | −0.11 | −0.26–0.05 | 0.20 | −0.11 | −0.27–0.04 | 0.16 |
| Feelings of inadequacy | −0.06 | −0.22–0.10 | 0.48 | 0.05 | −0.10–0.21 | 0.51 |
| Concentration problems | −0.12 | −0.27–0.04 | 0.14 | −0.01 | −0.16–0.14 | 0.89 |
| Psychomotor changes | −0.09 | −0.25–0.06 | 0.25 | 0.03 | −0.13–0.18 | 0.72 |
| Suicidality | −0.04 | −0.20–0.12 | 0.66 | 0.04 | −0.11–0.20 | 0.59 |
| PHQ-9 total score | −0.11 | −0.27–0.05 | 0.18 | −0.04 | −0.19–0.12 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, M.; Savitz, J.; Zhang, Y.; Burrows, K.; Smith, R.; Figueroa-Hall, L.; Kuplicki, R.; Khalsa, S.S.; Taki, Y.; Teague, T.K.; et al. Elevated Systemic Inflammation Is Associated with Reduced Corticolimbic White Matter Integrity in Depression. Life 2022, 12, 43. https://doi.org/10.3390/life12010043
Thomas M, Savitz J, Zhang Y, Burrows K, Smith R, Figueroa-Hall L, Kuplicki R, Khalsa SS, Taki Y, Teague TK, et al. Elevated Systemic Inflammation Is Associated with Reduced Corticolimbic White Matter Integrity in Depression. Life. 2022; 12(1):43. https://doi.org/10.3390/life12010043
Chicago/Turabian StyleThomas, MacGregor, Jonathan Savitz, Ye Zhang, Kaiping Burrows, Ryan Smith, Leandra Figueroa-Hall, Rayus Kuplicki, Sahib S. Khalsa, Yasuyuki Taki, Tracy Kent Teague, and et al. 2022. "Elevated Systemic Inflammation Is Associated with Reduced Corticolimbic White Matter Integrity in Depression" Life 12, no. 1: 43. https://doi.org/10.3390/life12010043
APA StyleThomas, M., Savitz, J., Zhang, Y., Burrows, K., Smith, R., Figueroa-Hall, L., Kuplicki, R., Khalsa, S. S., Taki, Y., Teague, T. K., Irwin, M. R., Yeh, F.-C., Paulus, M. P., Zheng, H., & on behalf of Tulsa 1000 Investigators. (2022). Elevated Systemic Inflammation Is Associated with Reduced Corticolimbic White Matter Integrity in Depression. Life, 12(1), 43. https://doi.org/10.3390/life12010043






