Microbes from Brine Systems with Fluctuating Salinity Can Thrive under Simulated Martian Chemical Conditions
Abstract
:1. Introduction
2. Materials and Methods
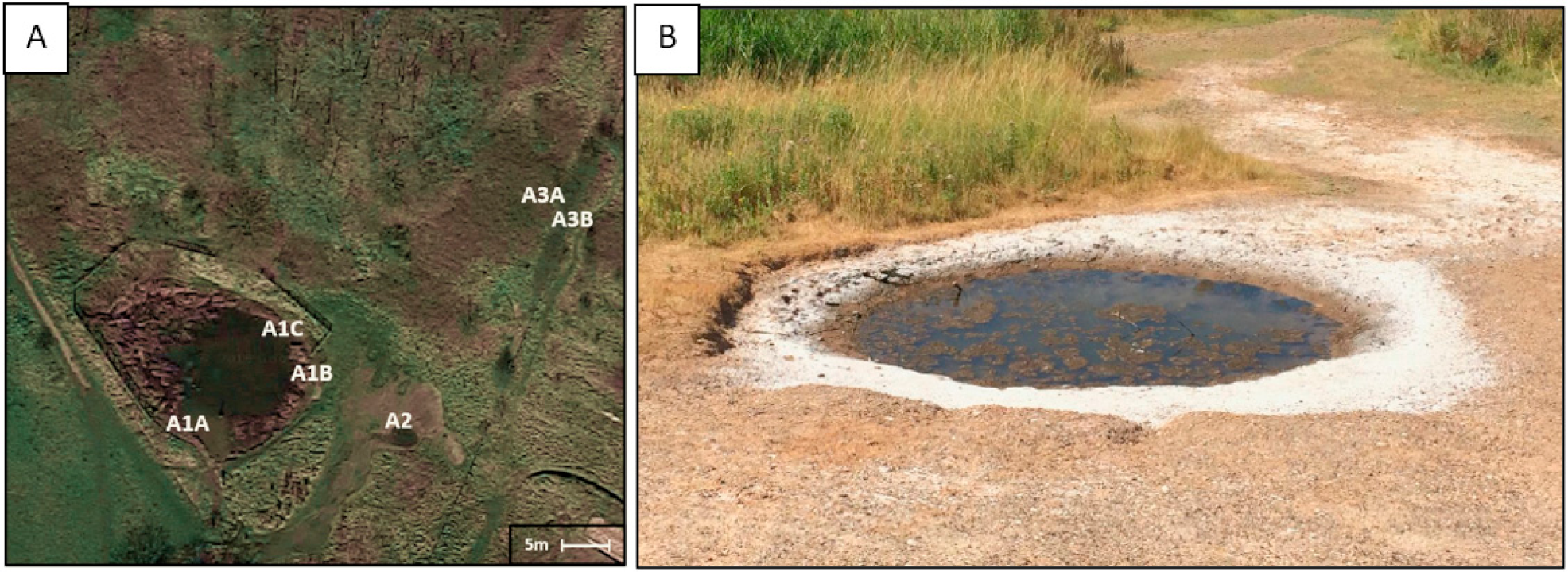
2.1. Sample Collection and Characterisation
2.2. Isolation and Identification of Microbial Strains from the Anderton Brine Springs
2.3. Preparation of Simulated Martian Fluids
2.4. Growth of Microbial Isolates from the Anderton Brine Springs in Rocknest Fluid Chemistry
2.5. Analysis of Fluid Chemistry by ICP-OES
3. Result
3.1. Chemical Analysis of Environmental and Simulated Martian Fluid Samples
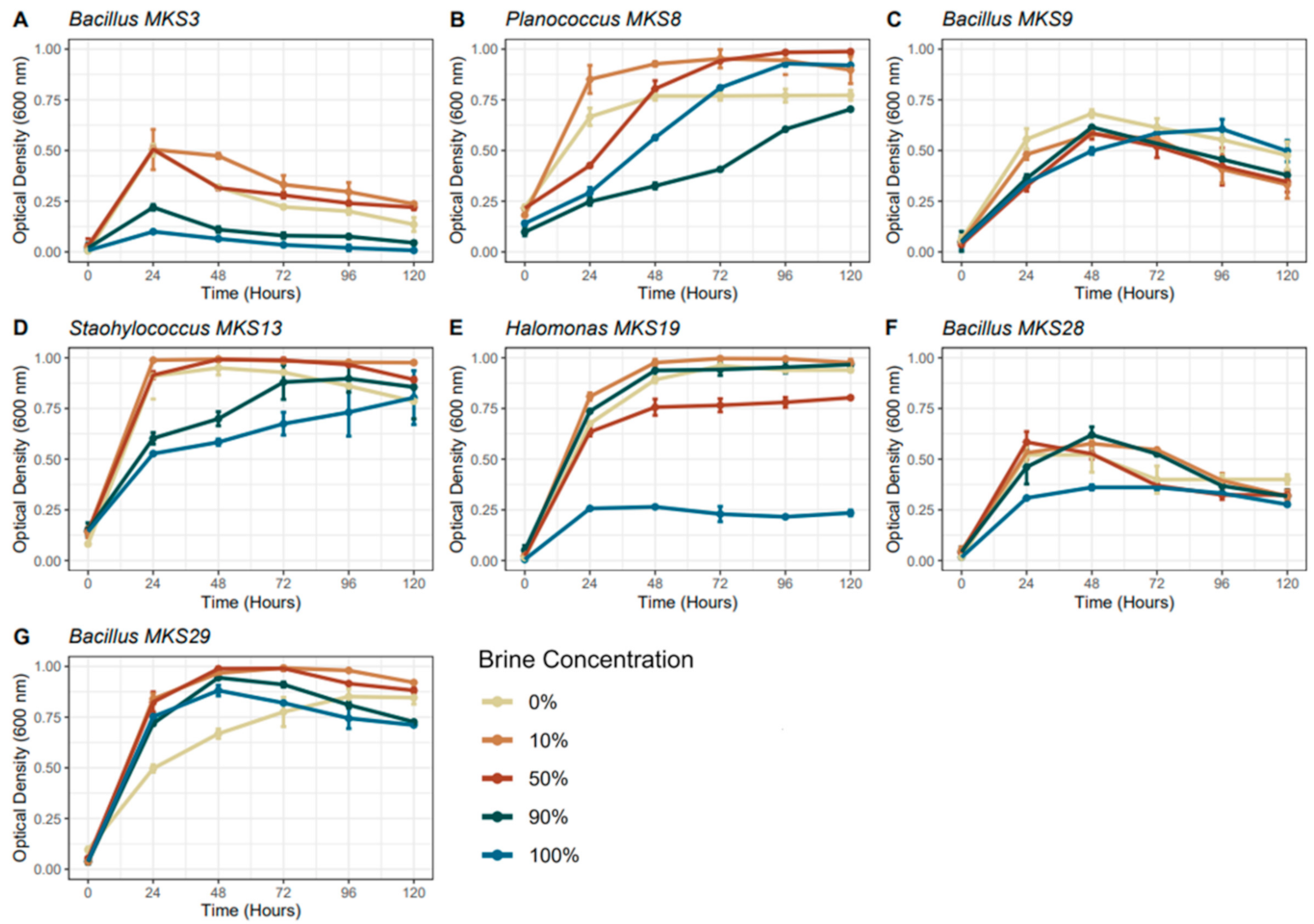
3.2. The Impact of the Simulated Martian Fluid Chemistries on the Microbes of Anderton Brine Springs
4. Discussion
4.1. Variation in Viability of the Anderton Brine Springs Isolates
4.2. Microbial Influence on Environmental Chemistry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schon, S.C.; Head, J.W.; Fassett, C.I. An overfilled lacustrine system and progradational delta in Jezero crater, Mars: Implications for Noachian climate. Planet Space Sci. 2012, 67, 28–45. [Google Scholar] [CrossRef]
- Wordsworth, R. The Climate of Early Mars. arXiv 2016, arXiv:1606.02813. [Google Scholar] [CrossRef] [Green Version]
- Carr, M.H.; Head, J.W. Geologic history of Mars. Earth Planet Sci. Lett. 2010, 294, 185–203. [Google Scholar] [CrossRef] [Green Version]
- Fastook, J.L.; Head, J.W.; Marchant, D.R.; Forget, F.; Madeleine, J.B. Early Mars climate near the Noachian-Hesperian boundary: Independent evidence for cold conditions from basal melting of the south polar ice sheet (Dorsa Argentea Formation) and implications for valley network formation. Icarus 2012, 219, 25–40. [Google Scholar] [CrossRef]
- Abramov, O.; Mojzsis, S.J. Thermal effects of impact bombardments on Noachian Mars. Earth Planet Sci. Lett. 2016, 442, 108–120. [Google Scholar] [CrossRef]
- Lasue, J.; Clifford, S.M.; Conway, S.J.; Mangold, N.; Butcher, F.E. The Hydrology of Mars Including a Potential Cryosphere. In Volatiles in the Martian Crust; Filiberto, J., Schwenzer, S.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 185–246. [Google Scholar]
- Kite, E.S. Geologic Constraints on Early Mars Climate. Space Sci. Rev. 2019, 215. [Google Scholar] [CrossRef] [Green Version]
- Carter, J.; Poulet, F.; Bibring, J.P.; Mangold, N.; Murchie, S. Hydrous minerals on Mars as seen by the CRISM and OMEGA imaging spectrometers: Updated global view. J. Geophys. Res. Planets 2013, 118, 831–858. [Google Scholar] [CrossRef]
- Rampe, E.B.; Blake, D.F.; Bristow, T.F.; Ming, D.W.; Vaniman, D.T.; Morris, R.V.; Achilles, C.N.; Chipera, S.J.; Morrison, S.M.; Tu, V.M.; et al. Mineralogy and geochemistry of sedimentary rocks and eolian sediments in Gale crater, Mars: A review after six Earth years of exploration with Curiosity. Geochemistry 2020, 80, 125605. [Google Scholar] [CrossRef]
- Yen, A.S.; Ming, D.W.; Vaniman, D.T.; Gellert, R.; Blake, D.F.; Morris, R.V.; Morrison, S.M.; Bristow, T.F.; Chipera, S.J.; Edgett, K.S.; et al. Multiple stages of aqueous alteration along fractures in mudstone and sandstone strata in Gale Crater, Mars. Earth Planet Sci. Lett. 2017, 471, 186–198. [Google Scholar] [CrossRef]
- Dong, C.; Lee, Y.; Ma, Y.; Lingam, M.; Bougher, S.; Luhmann, J.; Curry, S.; Toth, G.; Nagy, A.; Tenishev, V.; et al. Modeling Martian Atmospheric Losses over Time: Implications for Exoplanetary Climate Evolution and Habitability. Astrophys. J. Lett. 2018, 859, L14. [Google Scholar] [CrossRef] [Green Version]
- Halevy, I.; Head, J.W. Episodic warming of early Mars by punctuated volcanism. Nat. Geosci. 2014, 7, 865–868. [Google Scholar] [CrossRef]
- Halevy, I.; Zuber, M.T.; Schrag, D.P. A Sulfur Dioxide Climate Feedback on Early Mars. Science 2007, 318, 1903–1908. [Google Scholar] [CrossRef] [Green Version]
- Rapin, W.; Ehlmann, B.L.; Dromart, G.; Schieber, J.; Thomas, N.H.; Fischer, W.W.; Fox, V.K.; Stein, N.T.; Nachon, M.; Clark, B.C.; et al. An interval of high salinity in ancient Gale crater. Nat. Geosci. 2019, 12, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Ventosa, A.; Nieto, J.J.; Oren, A. Biology of Moderately Halophilic Aerobic Bacteria. Microbiol. Mol. Biol Rev. 1998, 62, 504–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox-Powell, M.G.; Cockell, C.S. Building a geochemical view of microbial salt tolerance: Halophilic adaptation of Marinococcus in a natural magnesium sulfate brine. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Lageard, J.G.A.; Drew, I.B. Evaporating legacies: Industrial heritage and salt in Cheshire, UK. Ind. Archaeol. Rev. 2015, 37, 48–61. [Google Scholar] [CrossRef]
- Kelbrick, M.; Abed, R.M.M.; Antunes, A. Motilimonas cestriensis sp. nov., isolated from an inland brine spring in Northern England. Int. J. Syst. Evol. Microbiol. 2021, 71, 004763. [Google Scholar] [CrossRef] [PubMed]
- Pontefract, A.; Zhu, T.F.; Walker, V.K.; Hepburn, H.; Lui, C.; Zuber, M.T.; Ruvkun, G.; Carr, C.E. Microbial diversity in a hypersaline sulfate lake: A terrestrial analog of ancient mars. Front. Microbiol. 2017, 8, 1819. [Google Scholar] [CrossRef] [Green Version]
- Sapers, H.M.; Ronholm, J.; Raymond-Bouchard, I.; Comrey, R.; Osinski, G.R.; Whyte, L.G. Biological characterization of microenvironments in a hypersaline cold spring Mars analog. Front. Microbiol. 2017, 8, 2527. [Google Scholar] [CrossRef]
- Lay, C.Y.; Mykytczuk, N.C.S.; Niederberger, T.D.; Martineau, C.; Greer, C.W.; Whyte, L.G. Microbial diversity and activity in hypersaline high Arctic spring channels. Extremophiles 2012, 16, 177–191. [Google Scholar] [CrossRef]
- Crisler, J.D.; Newville, T.M.; Chen, F.; Clark, B.C.; Schneegurt, M.A. Bacterial growth at the high concentrations of magnesium sulfate found in martian soils. Astrobiology 2012, 12, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.H.; Childers, D.; Fox-Powell, M.; Nicholson, N.; Jhoti, E.; Cockell, C.S. Growth, Viability, and Death of Planktonic and Biofilm Sphingomonas desiccabilis in Simulated Martian Brines. Astrobiology 2019, 19, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Davila, A.F.; Duport, L.G.; Melchiorri, R.; Jänchen, J.; Valea, S.; De Los Rios, A.; Fairén, A.G.; Möhlmann, D.; McKay, C.P.; Ascaso, C.; et al. Hygroscopic salts and the potential for life on mars. Astrobiology 2010, 10, 617–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirmack, J.; Alawi, M.; Wagner, D. Influence of Martian regolith analogs on the activity and growth of methanogenic archaea, with special regard to long-term desiccation. Front. Microbiol. 2015, 6, 210. [Google Scholar] [CrossRef]
- Serrano, P.; Alawi, M.; De Vera, J.P.; Wagner, D. Response of Methanogenic Archaea from Siberian Permafrost and Non-permafrost Environments to Simulated Mars-like Desiccation and the Presence of Perchlorate. Astrobiology 2019, 19, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, R.I.; Whiteley, A.S.; Donnell, A.G.O.; Bailey, M.J. Rapid Method for Coextraction of DNA and RNA from Natural Environments for Analysis of Ribosomal DNA- and rRNA-Based Microbial Community Composition. Appl. Environ. Microbiol. 2000, 66, 5488–5491. [Google Scholar] [CrossRef] [Green Version]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Altschup, S.F.; Gish, W.; Pennsylvania, T.; Park, U. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kim, O.S.; Cho, Y.J.; Lee, K.; Yoon, S.H.; Kim, M.; Na, H.; Park, S.C.; Jeon, Y.S.; Lee, J.H.; Yi, H.; et al. Introducing EzTaxon-e: A prokaryotic 16s rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef]
- Blake, D.F.; Morris, R.V.; Kocurek, G.; Morrison, S.M.; Downs, R.T.; Bish, D.; Ming, D.W.; Edgett, K.S.; Rubin, D.; Goetz, W.; et al. Curiosity at Gale Crater, Mars: Characterization and analysis of the rocknest sand shadow. Science 2013, 341, 1239505. [Google Scholar] [CrossRef] [Green Version]
- Ramkissoon, N.K.; Pearson, V.K.; Schwenzer, S.P.; Schr, C.; Kirnbauer, T.; Wood, D.; Seidel, R.G.W.; Miller, M.A.; Olsson-Francis, K. New simulants for martian regolith: Controlling iron variability. Planet Space Sci. 2019, 179, 104722. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Gupta, S.; Malin, M.C.; Rubin, D.M.; Schieber, J.; Siebach, K.; Sumner, D.Y.; Stack, K.M.; Vasavada, A.R.; Arvidson, R.E.; et al. Deposition, exhumation, and paleoclimate of an ancient lake deposit, Gale crater, Mars. Science 2015, 350, aac7575. [Google Scholar] [CrossRef] [PubMed]
- O’Connell-Cooper, C.D.; Spray, J.G.; Thompson, L.M.; Gellert, R.; Berger, J.A.; Boyd, N.I.; Desouza, E.D.; Perrett, G.M.; Schmidt, M.; VanBommel, S.J. APXS-derived chemistry of the Bagnold dune sands: Comparisons with Gale Crater soils and the global Martian average. J. Geophys. Res. Planets 2017, 122, 2623–2643. [Google Scholar] [CrossRef] [Green Version]
- Gellert, R.; Berger, J.A.; Boyd, N.; Brunet, C.; Campbell, J.L.; Curry, M.; Elliott, B.; Fulford, P.; Grotzinger, J.; Hipkin, V.; et al. Initial MSL APXS Activities and Observations at Gale Crater, Mars. In Proceedings of the 44th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 18–22 March 2013. [Google Scholar]
- Wilson, E.H.; Atreya, S.K.; Kaiser, R.I.; Mahaffy, P.R. Perchlorate formation on Mars through surface radiolysis-initiated atmospheric chemistry: A potential mechanism. Nature 2016, 121, 1472–1487. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.L.; Claire, M.W.; Catling, D.C.; Zahnle, K.J. The formation of sulfate, nitrate and perchlorate salts in the martian atmosphere. Icarus 2014, 231, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.O.; Markt, R.; Mutschlechner, M.; Lackner, N.; Prem, E.M.; Praeg, N.; Illmer, P. Medium preparation for the cultivation of microorganisms under strictly anaerobic/anoxic conditions. J. Vis. Exp. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Fox-Powell, M.G.; Hallsworth, J.E.; Cousins, C.R.; Cockell, C.S. Ionic Strength Is a Barrier to the Habitability of Mars. Astrobiology 2016, 16, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.H.; McDonald, A.; de Koning, C.; Riedo, A.; Preston, L.J.; Ehrenfreund, P.; Wurz, P.; Cockell, C.S. Detectability of biosignatures in a low-biomass simulation of martian sediments. Sci. Rep. 2019, 9, 9706. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.A.; Merrison, J.; Nørnberg, P.; Lomstein, B.A.; Finster, K. Activity and stability of a complex bacterial soil community under simulated Martian conditions. Int. J. Astrobiol. 2005, 4, 135–144. [Google Scholar] [CrossRef]
- Tian, F.; Kasting, J.F.; Solomon, S.C. Thermal escape of carbon from the early Martian atmosphere. Geophys. Res. Lett. 2009, 36, 1–5. [Google Scholar] [CrossRef]
- Carter, J.; Loizeau, D.; Mangold, N.; Poulet, F.; Bibring, J.P. Widespread surface weathering on early Mars: A case for a warmer and wetter climate. Icarus 2015, 248, 373–382. [Google Scholar] [CrossRef]
- Ramirez, R.M.; Craddock, R.A. The geological and climatological case for a warmer and wetter early Mars. Nat. Geosci. 2018, 11, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Babeyko, A.Y.; Zharkov, V.N. Martian crust: A modeling approach. Phys. Earth Planet Inter. 2000, 117, 421–435. [Google Scholar] [CrossRef]
- Macey, M.C.; Fox-Powell, M.; Ramkissoon, N.K.; Stephens, B.P.; Barton, T.; Schwenzer, S.P.; Pearson, V.K.; Cousins, C.R.; Olsson-Francis, K. The identification of sulfide oxidation as a potential metabolism driving primary production on late Noachian Mars. Sci. Rep. 2020, 10, 10941. [Google Scholar] [CrossRef]
- Dini-Andreote, F.; Elsas, J.D. Back to the basics: The need for ecophysiological insights to enhance our understanding of microbial behaviour in the rhizosphere. Plant Soil 2013, 373, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Prosser, J.I. Ecosystem processes and interactions in a morass of diversity. FEMS Microbiol. Ecol. 2012, 81, 507–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuerger, A.C.; Ming, D.W.; Golden, D.C. Biotoxicity of Mars soils: 2. Survival of Bacillus subtilis and Enterococcus faecalis in aqueous extracts derived from six Mars analog soils. Icarus 2017, 290, 215–223. [Google Scholar] [CrossRef]
- Wadsworth, J.; Cockell, C.S. Perchlorates on Mars enhance the bacteriocidal effects of UV light. Sci. Rep. 2017, 7, 4662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benardini, J.N.; Sawyer, J.; Venkateswaran, K.; Nicholson, W.L. Isolates obtained from sonoran desert basalt: Implications for lithopanspermia. Astrobiology 2003, 3, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, P.A.; Rabbow, E.; Horneck, G.; Venkateswaran, K.J. Survival of bacillus pumilus spores for a prolonged period of time in real space conditions. Astrobiology 2012, 12, 487–497. [Google Scholar] [CrossRef]
- Fajardo-Cavazos, P.; Link, L.; Melosh, H.J.; Nicholson, W.L. Bacillus subtilis spores on artificial meteorites survive hypervelocity atmospheric entry: Implications for lithopanspermia. Astrobiology 2005, 5, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Rettberg, P.; Antunes, A.; Brucato, J.; Cabezas, P.; Collins, G.; Haddaji, A.; Kminek, G.; Leuko, S.; McKenna-Lawlor, S.; Moissl-Eichinger, C. Biological contamination prevention for outer solar system moons of astrobiological interest: What do we need to know? Astrobiology 2019, 19, 951–974. [Google Scholar] [CrossRef]
- Sarrafzadeh, M.H.; Belloy, L.; Esteban, G.; Navarro, J.M.; Ghommidh, C. Dielectric monitoring of growth and sporulation of Bacillus thuringiensis. Biotechnol. Lett. 2005, 27, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Sarrafzadeh, M.H.; Guiraud, J.P.; Lagneau, C.; Gaven, B.; Navarro, A.C.; Navarro, J.-M. Growth, Sporulation, d-Endotoxins Synthesis, and Toxicity During Culture of Bacillus thuringiensis H14. Curr. Microbiol. 2005, 51, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Reder, A.; Albrecht, D.; Gerth, U.; Hecker, M. Cross-talk between the general stress response and sporulation initiation in Bacillus subtilis—The σB promoter of spo0E represents an AND-gate. Environ. Microbiol. 2012, 14, 2741–2756. [Google Scholar] [CrossRef] [PubMed]
- Nagler, K.; Krawczyk, A.O.; De Jong, A.; Madela, K.; Hoffmann, T.; Laue, M.; Kuipers, O.P.; Bremer, E.; Moeller, R. Identification of differentially expressed genes during Bacillus subtilis spore outgrowth in high-salinity environments using RNA sequencing. Front. Microbiol. 2016, 7, 1564. [Google Scholar] [CrossRef]
- Zammuto, V.; Rizzo, M.G.; De Plano, L.M.; Franco, D.; Guglielmino, S.; Caccamo, M.T.; Magazù, S.; Fujimori, A.; Giudice, A.L.; Guglielmin, M.; et al. Effects of heavy ion particle irradiation on spore germination of bacillus spp. from extremely hot and cold environments. Life 2020, 10, 264. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Halophilic archaea on Earth and in space: Growth and survival under extreme conditions. Philos. Trans. A Math. Phys. Eng. Sci. 2014, 372, 20140194. [Google Scholar] [CrossRef]
- Musilova, M.; Wright, G.; Ward, J.M.; Dartnell, L.R. Isolation of Radiation-Resistant Bacteria from Mars Analog Antarctic Dry Valleys by Preselection, and the Correlation between Radiation and Desiccation Resistance. Astrobiology 2015, 15, 1076–1090. [Google Scholar] [CrossRef] [Green Version]
- van Heereveld, L.; Merrison, J.; Nørnberg, P.; Finster, K. Assessment of the Forward Contamination Risk of Mars by Clean Room Isolates from Space-Craft Assembly Facilities through Aeolian Transport—A Model Study. Orig. Life Evol. Biosph. 2017, 47, 203–214. [Google Scholar] [CrossRef]
- Berry, B.J.; Jenkins, D.G.; Schuerger, A.C. Effects of simulated mars conditions on the survival and growth of Escherichia coli and Serratia liquefaciens. Appl. Environ. Microbiol. 2010, 76, 2377–2386. [Google Scholar] [CrossRef] [Green Version]
- Burchell, M.J. Panspermia today. Int. J. Astrobiol. 2004, 3, 73–80. [Google Scholar] [CrossRef]
- Lingam, M.; Loeb, A. CHAPTER 10 The Propagation of Life in the Universe. In Life in the Cosmos: From Biosignatures to Technosignatures; Harvard University Press: Cambridge, MA, USA, 2021; pp. 797–888. [Google Scholar]
- Corbett, G.; Leach, T. Controls on hydrothermal alteration and mineralization. In Southwest Pacific Rim Gold-Copper Systems: Structure, Alteration and Mineralization; Society of the Economic Geologists, Littleton, USA; 1998; pp. 69–82.
- Vaniman, D.T.; Bish, D.L.; Ming, D.W.; Bristow, T.F.; Morris, R.V.; Blake, D.F.; Chipera, S.J.; Morrison, S.M.; Treiman, A.H.; Rampe, E.B.; et al. Mineralogy of a Mudstone at Yellowknife Bay, Gale Crater, Mars Mineralogical Analysis and Quantitative Mineralogy. Science 2014, 343, 1243480. [Google Scholar] [CrossRef] [PubMed]
- Olsson-Francis, K.; Pearson, V.K.; Steer, E.D.; Schwenzer, S.P. Determination of geochemical bio-signatures in Mars-like basaltic environments. Front. Microbiol. 2017, 8, 1668. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, N.K.; Turner, S.M.R.; Macey, M.C.; Schwenzer, S.P.; Reed, M.H.; Pearson, V.K.; Olsson-Francis, K. Exploring the environments of martian impact-generated hydrothermal systems and their potential to support life. Earth Planet Sci. Lett. 2021, 1–19. [Google Scholar] [CrossRef]
- Westall, F.; Foucher, F.; Bost, N.; Bertrand, M.; Loizeau, D.; Vago, J.L.; Kminek, G.; Gaboyer, F.; Campbell, K.A.; Bréhéret, J.G.; et al. Biosignatures on Mars: What, Where, and How? Implications for the Search for Martian Life. Astrobiology 2015, 15, 998–1029. [Google Scholar] [CrossRef]
- Nishida, I.; Shimada, Y.; Saito, T.; Okaue, Y.; Yokoyama, T. Effect of aluminum on the deposition of silica scales in cooling water systems. J. Colloid Interface Sci. 2009, 335, 18–23. [Google Scholar] [CrossRef]
- Spinthaki, A.; Kamaratou, M.; Matheis, J.; Disci, D.; Hater, W.; Demadis, K.D. The precipitation of “aluminum silicate” under geothermal stresses: Identifying its idiosyncrasies. Geothermics 2021, 92, 102060. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; La Rosa, A.F.; Mason, K.E.; Whitaker, J.; McNamara, N.P.; Grant, H.K.; Ostlea, N.J. Sticky dead microbes: Rapid abiotic retention of microbial necromass in soil. Soil Biol. Biochem. 2020, 149, 107929. [Google Scholar] [CrossRef]
- Martín-Torres, F.J.; Zorzano, M.P.; Valentín-Serrano, P.; Harri, A.M.; Genzer, M.; Kemppinen, O.; Rivera-Valentin, E.G.; Jun, I.; Wray, J.; Madsen, M.B.; et al. Transient liquid water and water activity at Gale crater on Mars. Nat. Geosci. 2015, 8, 357–361. [Google Scholar] [CrossRef]
- Martínez, G.M.; Renno, N.O. Water and brines on mars: Current evidence and implications for MSL. Space Sci. Rev. 2013, 175, 29–51. [Google Scholar] [CrossRef] [Green Version]
- Canino-Koning, R.; Wiser, M.; Ofria, C. Fluctuating environments select for short-term phenotypic variation leading to long-term exploration. PLoS Comput. Biol. 2019, 15, e1006445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccazzo, S.; Esposito, A.; Borruso, L.; Brusetti, L. Microbial communities and primary succession in high altitude mountain environments. Ann. Microbiol. 2016, 66, 43–60. [Google Scholar] [CrossRef]
- Anderson, K.L.; Apolinario, E.E.; Sowers, K.R. Desiccation as a long-term survival mechanism for the archaeon Methanosarcina barkeri. Appl. Environ. Microbiol. 2012, 78, 1473–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapin, W.; Dromart, G.; Rubin, D.; Le Deit, L.; Mangold, N.; Edgar, L.A.; Gasnault, O.; Herkenhoff, K.; Le Mouélic, S.; Anderson, R.B.; et al. Alternating wet and dry depositional environments recorded in the stratigraphy of Mount Sharp at Gale crater, Mars. Geology 2021, 49, 842–846. [Google Scholar] [CrossRef]
| Strain | Isolation Site | Isolate Accession | Genus with Highest Sequence Similarity | Closed Relative Accession | Similarity to Closest Relative | Growth at 0% NaCl on Yeast Extract Agar (+ Represents Growth and—Represents no Growth) |
|---|---|---|---|---|---|---|
| MKS3 | A1C | MW132413 | Bacillus sp. | MK712419.1 | 100% MK712419.1 | + |
| MKS8 | A1B | MW130959 | Planococcus sp. | MK696244.1 | 100% MK696244.1 | + |
| MKS9 | A3B | MW132410 | Bacillus sp. | MK618601.1 | 100% MK618601.1 | + |
| MKS13 | A3B | MW131453 | Staphylococcus sp. | MK120203.1 | 100% MK120203.1 | + |
| MKS15 | A1B | MW130887 | Halomonas sp. | HF678757.1 | 99% HF678757.1 | - |
| MKS16 | A2 | MW130884 | Salinivibrio sp. | NR_042255.1 | 100% NR_042255.1 | - |
| MKS19 | A2 | MW131523 | Halomonas sp. | CP024811.1 | 100% CP024811.1 | + |
| MKS20 | A1B | MW130885 | Motilimonas sp. | NR_156090.1 | 97.5% NR_156090.1 | - |
| MKS21 | A1B | MW131554 | Planococcus sp. | MK696244.1 | 100% MK696244.1 | - |
| MKS22 | A1B | MW134719 | Photobacterium sp. | JN791338.1 | 99% JN791338.1 | - |
| MKS23 | A1B | MW132418 | Marinobacter sp. | MH266164.1 | 99% MH266164.1 | - |
| MKS24 | A1B | MW132419 | Pseudoalteromonas sp. | LT601323.2 | 99% LT601323.2 | - |
| MKS28 | A2 | MW131455 | Bacillus sp. | MK618601.1 | 100% MK618601.1 | + |
| MKS29 | A1C | MW130923 | Bacillus sp. | MG575987.1 | 99% MG575987.1 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelbrick, M.; Oliver, J.A.W.; Ramkissoon, N.K.; Dugdale, A.; Stephens, B.P.; Kucukkilic-Stephens, E.; Schwenzer, S.P.; Antunes, A.; Macey, M.C. Microbes from Brine Systems with Fluctuating Salinity Can Thrive under Simulated Martian Chemical Conditions. Life 2022, 12, 12. https://doi.org/10.3390/life12010012
Kelbrick M, Oliver JAW, Ramkissoon NK, Dugdale A, Stephens BP, Kucukkilic-Stephens E, Schwenzer SP, Antunes A, Macey MC. Microbes from Brine Systems with Fluctuating Salinity Can Thrive under Simulated Martian Chemical Conditions. Life. 2022; 12(1):12. https://doi.org/10.3390/life12010012
Chicago/Turabian StyleKelbrick, Matthew, James A. W. Oliver, Nisha K. Ramkissoon, Amy Dugdale, Ben P. Stephens, Ezgi Kucukkilic-Stephens, Susanne P. Schwenzer, André Antunes, and Michael C. Macey. 2022. "Microbes from Brine Systems with Fluctuating Salinity Can Thrive under Simulated Martian Chemical Conditions" Life 12, no. 1: 12. https://doi.org/10.3390/life12010012
APA StyleKelbrick, M., Oliver, J. A. W., Ramkissoon, N. K., Dugdale, A., Stephens, B. P., Kucukkilic-Stephens, E., Schwenzer, S. P., Antunes, A., & Macey, M. C. (2022). Microbes from Brine Systems with Fluctuating Salinity Can Thrive under Simulated Martian Chemical Conditions. Life, 12(1), 12. https://doi.org/10.3390/life12010012







