Post-Intensive Care Syndrome in Survivors from Critical Illness including COVID-19 Patients: A Narrative Review
Abstract
:1. Introduction
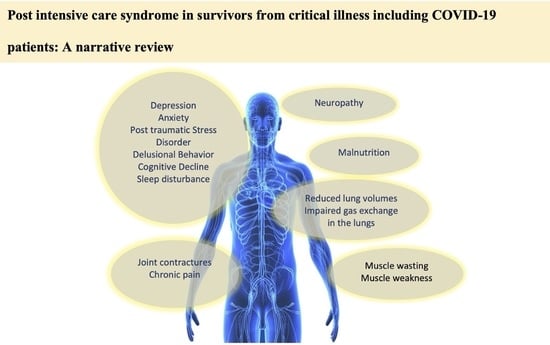
2. Physical Dysfunction
3. Cognitive Dysfunction
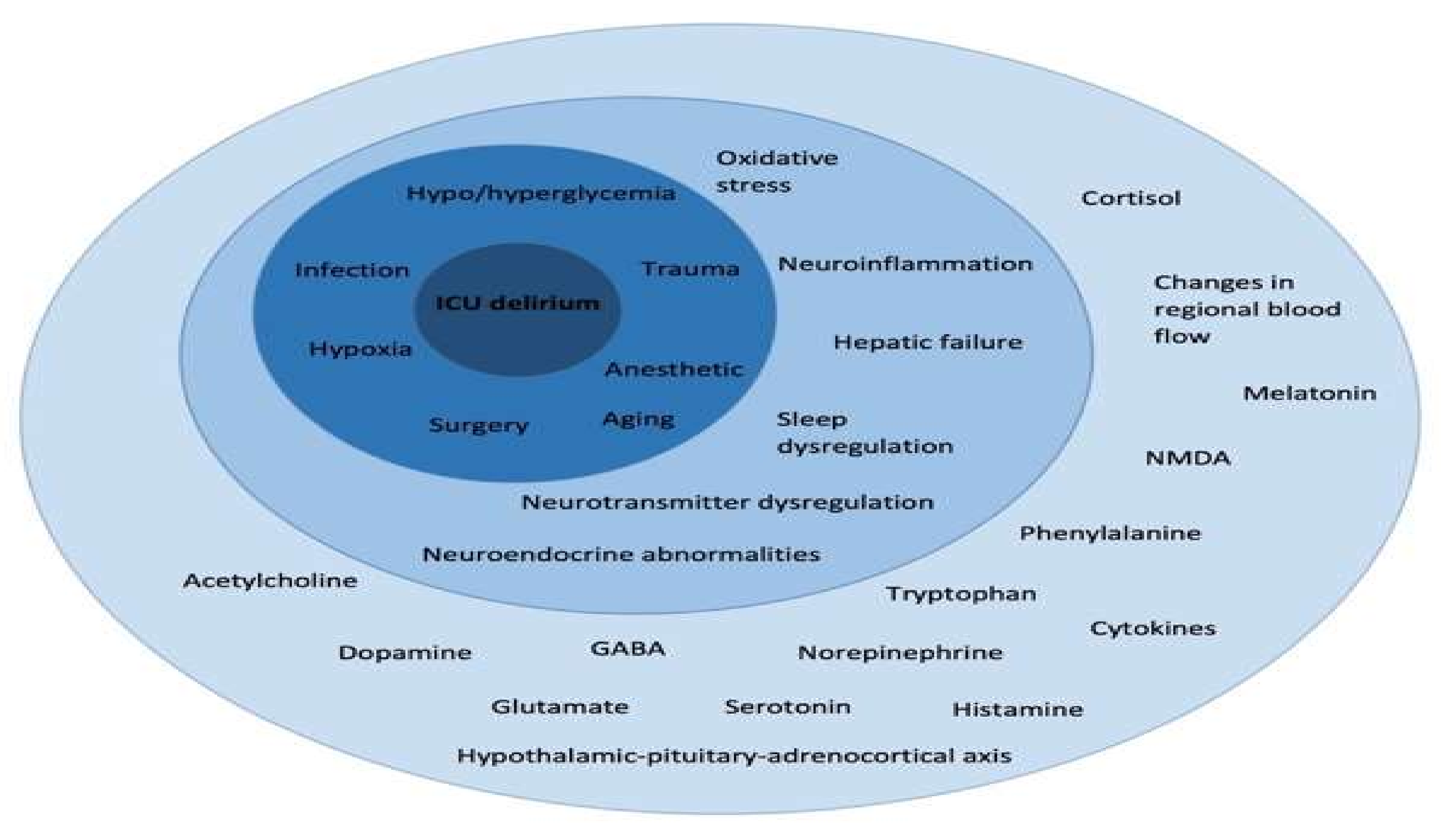
3.1. Risk Factors for Cognitive Decline in ICU Survivors
3.2. Persistence of Cognitive Impairment
4. Psychological Dysfunction
4.1. Depression
4.2. Anxiety
4.3. Post-Traumatic Stress Disorder (PTSD)
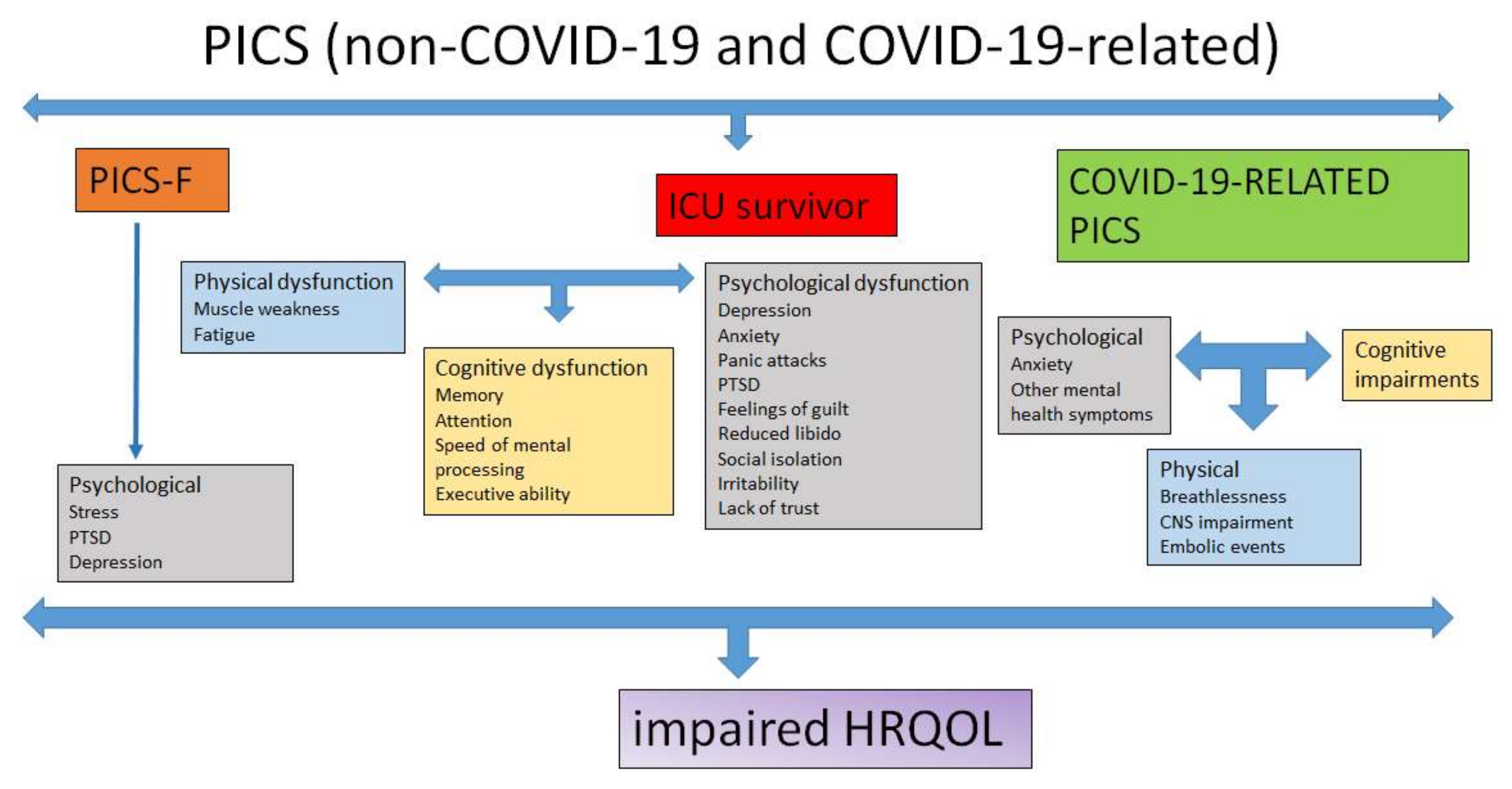
5. PICS Family
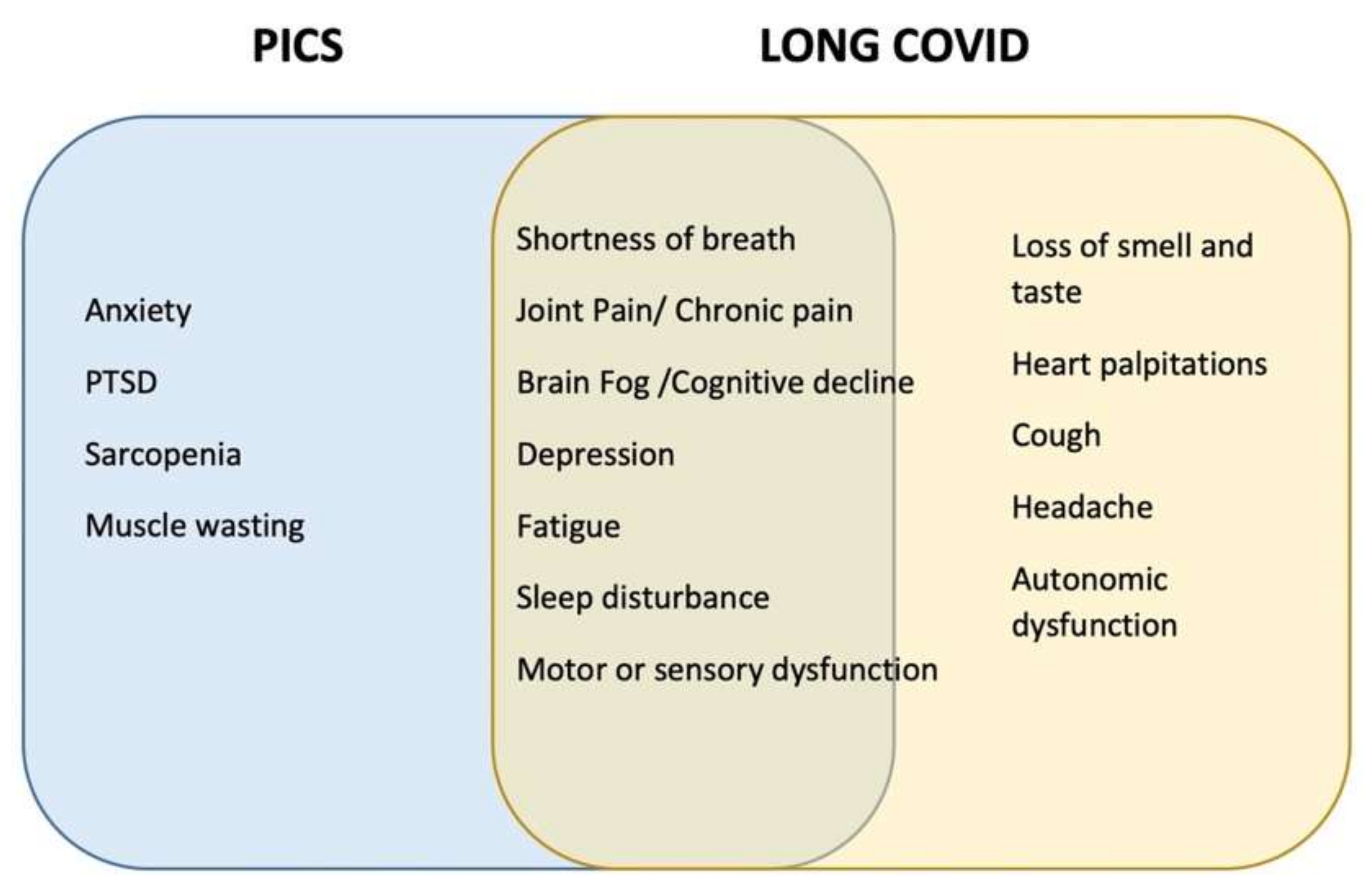
6. COVID-19-Related PICS
7. PICS Prevention and Treatment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackson, J.C.; Pandharipande, P.P.; Girard, T.D.; Brummel, N.E.; Thompson, J.L.; Hughes, C.G.; Pun, B.T.; Vasilevskis, E.E.; Morandi, A.; Shintani, A.K.; et al. Depression, Post-Traumatic Stress Disorder, and Functional Disability in Survivors of Critical Illness in the BRAIN-ICU Study: A Longitudinal Cohort Study. Lancet Respir. Med. 2014, 2, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving Long-Term Outcomes after Discharge from Intensive Care Unit: Report from a Stakeholders’ Conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Serrano, P.; Kheir, Y.N.P.; Wang, S.; Khan, S.; Scheunemann, L.; Khan, B. Aging and Postintensive Care Syndrome– Family: A Critical Need for Geriatric Psychiatry. Am. J. Geriatr. Psychiatry 2019, 27, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Dinglas, V.D.; Morris, P.E.; Jackson, J.C.; Hough, C.L.; Mendez-Tellez, P.A.; Wozniak, A.W.; Colantuoni, E.; Ely, E.W.; Rice, T.W.; et al. Physical and Cognitive Performance of Patients with Acute Lung Injury 1 Year after Initial Trophic versus Full Enteral Feeding EDEN Trial Follow-Up. Am. J. Respir. Crit. Care Med. 2013, 188, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. Long-Term Cognitive Impairment after Critical Illness. N. Engl. J. Med. 2013, 369, 1519–1544. [Google Scholar] [CrossRef] [Green Version]
- Marra, A.; Pandharipande, P.P.; Girard, T.D.; Patel, M.B.; Hughes, C.G.; Jackson, J.C.; Thompson, J.L.; Chandrasekhar, R.; Ely, E.W.; Brummel, N.E. Co-Occurrence of Post-Intensive Care Syndrome Problems among 406 Survivors of Critical Illness. Crit. Care Med. 2018, 46, 1393–1401. [Google Scholar] [CrossRef]
- Maley, J.H.; Brewster, I.; Mayoral, I.; Siruckova, R.; Adams, S.; McGraw, K.A.; Piech, A.A.; Detsky, M.; Mikkelsen, M.E. Resilience in Survivors of Critical Illness in the Context of the Survivors’ Experience and Recovery. Ann. Am. Thorac. Soc. 2016, 13, 1351–1360. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed Up and Go: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Spies, C.D.; Krampe, H.; Paul, N.; Denke, C.; Kiselev, J.; Piper, S.K.; Kruppa, J.; Grunow, J.J.; Steinecke, K.; Gülmez, T.; et al. Instruments to Measure Outcomes of Post-Intensive Care Syndrome in Outpatient Care Settings–Results of an Expert Consensus and Feasibility Field Test. J. Intensive Care Soc. 2021, 22, 159–174. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A Review of the Measurement of Grip Strength in Clinical and Epidemiological Studies: Towards a Standardised Approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Brooks, D.; Davis, A.M.; Naglie, G. Validity of 3 Physical Performance Measures in Inpatient Geriatric Rehabilitation. Arch. Phys. Med. Rehabil. 2006, 87, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. N. Engl. J. Med. 1995, 332, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyland, D.K.; Garland, A.; Bagshaw, S.M.; Cook, D.; Rockwood, K.; Stelfox, H.T.; Dodek, P.; Fowler, R.A.; Turgeon, A.F.; Burns, K.; et al. Recovery after Critical Illness in Patients Aged 80 Years or Older: A Multi-Center Prospective Observational Cohort Study. Intensive Care Med. 2015, 41, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Nanas, S.; Kritikos, K.; Angelopoulos, E.; Siafaka, A.; Tsikriki, S.; Poriazi, M.; Kanaloupiti, D.; Kontogeorgi, M.; Pratikaki, M.; Zervakis, D.; et al. Predisposing Factors for Critical Illness Polyneuromyopathy in a Multidisciplinary Intensive Care Unit. Acta Neurol. Scand. 2008, 118, 175–181. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and Preliminary Testing of the New Five-Level Version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, G.; Kemp, A.; Sunderland, M.; von Korff, M.; Ustun, T.B. Normative Data for the 12 Item WHO Disability Assessment Schedule 2.0. PLoS ONE 2009, 4, e8343. [Google Scholar] [CrossRef] [Green Version]
- Skevington, S.M.; Lotfy, M.; O’Connell, K.A. The World Health Organization’s WHOQOL-BREF Quality of Life Assessment: Psychometric Properties and Results of the International Field Trial a Report from the WHOQOL Group. Qual. Life Res. 2004, 13, 299–310. [Google Scholar] [CrossRef]
- Pettilä, V.; Kaarlola, A.; Mäkeläinen, A. Health-Related Quality of Life of Multiple Organ Dysfunction Patients One Year after Intensive Care. Intensive Care Med. 2000, 26, 1473–1479. [Google Scholar] [CrossRef]
- McKinley, S.; Fien, M.; Elliott, R.; Elliott, D. Sleep and Psychological Health during Early Recovery from Critical Illness: An Observational Study. J. Psychosom. Res. 2013, 75, 539–545. [Google Scholar] [CrossRef]
- Chelluri, L.; Im, K.A.; Belle, S.H.; Schulz, R.; Rotondi, A.J.; Donahoe, M.P.; Sirio, C.A.; Mendelsohn, A.B.; Pinsky, M.R. Long-Term Mortality and Quality of Life after Prolonged Mechanical Ventilation. Crit. Care Med. 2004, 32, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.; Tomlinson, G.; Chu, L.; Robles, P.; Matte, A.; Burns, S.; Thomas, C.; Lamontagne, F.; Adhikari, N.K.J.; Ferguson, N.; et al. Determinants of Depressive Symptoms at 1 Year Following ICU Discharge in Survivors of ≥ 7 Days of Mechanical Ventilation: Results From the RECOVER Program, a Secondary Analysis of a Prospective Multicenter Cohort Study. Chest 2019, 156, 466–476. [Google Scholar] [CrossRef]
- Wang, S.; Allen, D.; Perkins, A.; Monahan, P.; Khan, S.; Lasiter, S.; Boustani, M.; Khan, B. Validation of a New Clinical Tool for Post-Intensive Care Syndrome. Am. J. Crit. Care 2019, 28, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Borson, S.; Scanlan, J.M.; Chen, P.; Ganguli, M. The Mini-Cog as a Screen for Dementia: Validation in a Population-Based Sample. J. Am. Geriatr. Soc. 2003, 51, 1451–1454. [Google Scholar] [CrossRef]
- Sager, M.A.; Hermann, B.P.; la Rue, A.; Woodard, J.L. Screening for Dementia in Community-Based Memory Clinics. Wis. Med. J. 2006, 105, 25–29. [Google Scholar]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary Clinical Validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Hopkins, R.O.; Weaver, L.K.; Collingridge, D.; Parkinson, R.B.; Chan, K.J.; Orme, J.F. Two-Year Cognitive, Emotional, and Quality-of-Life Outcomes in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 300–304. [Google Scholar] [CrossRef]
- Teeters, D.A.; Moua, T.; Li, G.; Kashyap, R.; Biehl, M.; Kaur, R.; Gajic, O.; Boeve, B.F.; St Louis, E.K.; Petersen, R.C.; et al. Mild Cognitive Impairment and Risk of Critical Illness. Crit. Care Med. 2016, 44, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.E. Reliability of the CES-D Scale in Different Ethnic Contexts. Psychiatry Res. 1980, 2, 125–134. [Google Scholar] [CrossRef]
- Jackson, J.C.; Hart, R.P.; Gordon, S.M.; Shintani, A.; Truman, B.; May, L.; Ely, E.W. Six-Month Neuropsychological Outcome of Medical Intensive Care Unit Patients. Crit. Care Med. 2003, 31, 1226–1234. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W.; Löwe, B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: A Systematic Review. Gen. Hosp. Psychiatry 2010, 32, 345–359. [Google Scholar] [CrossRef]
- Gaudry, E.; Vagg, P.; Spielberger, C.D. Validation of the State-Trait Distinction in Anxiety Research. Multivar. Behav. Res. 1975, 10, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Creamer, M.; Bell, R.; Failla, S. Psychometric Properties of the Impact of Event Scale-Revised. Behav. Res. Ther. 2003, 41, 1489–1496. [Google Scholar] [CrossRef]
- Righy, C.; Rosa, R.G.; da Silva, R.T.A.; Kochhann, R.; Migliavaca, C.B.; Robinson, C.C.; Teche, S.P.; Teixeira, C.; Bozza, F.A.; Falavigna, M. Prevalence of Post-Traumatic Stress Disorder Symptoms in Adult Critical Care Survivors: A Systematic Review and Meta-Analysis. Crit. Care 2019, 23, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löwe, B.; Wahl, I.; Rose, M.; Spitzer, C.; Glaesmer, H.; Wingenfeld, K.; Schneider, A.; Brähler, E. A 4-Item Measure of Depression and Anxiety: Validation and Standardization of the Patient Health Questionnaire-4 (PHQ-4) in the General Population. J. Affect. Disord. 2010, 122, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Hatch, R.; Young, D.; Barber, V.; Griffiths, J.; Harrison, D.A.; Watkinson, P. Anxiety, Depression and Post Traumatic Stress Disorder after Critical Illness: A UK-Wide Prospective Cohort Study. Crit. Care 2018, 22, 310. [Google Scholar] [CrossRef] [Green Version]
- Weinert, C.; Meller, W. Epidemiology of Depression and Antidepressant Therapy after Acute Respiratory Failure. Psychosomatics 2006, 47, 399–407. [Google Scholar] [CrossRef]
- Fujinami, Y.; Inoue, S.; Ono, Y.; Miyazaki, Y.; Fujioka, K.; Yamashita, K.; Kotani, J. Sepsis Induces Physical and Mental Impairments in a Mouse Model of Post-Intensive Care Syndrome. J. Clin. Med. 2021, 10, 1593. [Google Scholar] [CrossRef]
- Stevens, R.D.; Dowdy, D.W.; Michaels, R.K.; Mendez-Tellez, P.A.; Pronovost, P.J.; Needham, D.M. Neuromuscular Dysfunction Acquired in Critical Illness: A Systematic Review. Intensive Care Med. 2007, 33, 1876–1891. [Google Scholar] [CrossRef] [PubMed]
- Appleton, R.T.D.; Kinsella, J.; Quasim, T. The Incidence of Intensive Care Unit-Acquired Weakness Syndromes: A Systematic Review. J. Intensive Care Soc. 2015, 16, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kress, J.P.; Hall, J.B. ICU-Acquired Weakness and Recovery from Critical Illness. N. Engl. J. Med. 2014, 370, 1626–1635. [Google Scholar] [CrossRef] [Green Version]
- Guarneri, B.; Bertolini, G.; Latronico, N. Long-Term Outcome in Patients with Critical Illness Myopathy or Neuropathy: The Italian Multicentre CRIMYNE Study. J. Neurol. Neurosurg. Psychiatry 2008, 79, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Wollersheim, T.; Bierbrauer, J.; Haas, K.; Mörgeli, R.; Deja, M.; Spies, C.D.; Spuler, S.; Krebs, M.; Weber-Carstens, S. Long-Term Recovery In Critical Illness Myopathy Is Complete, Contrary to Polyneuropathy. Muscle Nerve 2014, 50, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Clavet, H.; Doucette, S.; Trudel, G. Joint Contractures in the Intensive Care Unit: Quality of Life and Function 3.3 Years after Hospital Discharge. Disabil. Rehabil. 2015, 37, 207–213. [Google Scholar] [CrossRef]
- Carpentier, V.T.; Salga, M.; Gatin, L.; Genêt, F.; Paquereau, J. Early Diagnosis of Heterotopic Ossification among Patients Admitted to a Neurological Post-Intensive Care Rehabilitation Unit. Eur. J. Phys. Rehabil. Med. 2021, 57, 527–534. [Google Scholar] [CrossRef]
- De Jonghe, B.; Sharshar, T.; Lefaucheur, J.P.; Authier, F.J.; Durand-Zaleski, I.; Boussarsar, M.; Cerf, C.; Renaud, E.; Mesrati, F.; Carlet, J.; et al. Paresis Acquired in the Intensive Care Unit: A Prospective Multicenter Study. J. Am. Med Assoc. 2002, 288, 2859–2867. [Google Scholar] [CrossRef] [Green Version]
- Fan, E.; Dowdy, D.W.; Colantuoni, E.; Mendez-Tellez, P.A.; Sevransky, J.E.; Shanholtz, C.; Himmelfarb, C.R.D.; Desai, S.V.; Ciesla, N.; Herridge, M.S.; et al. Physical Complications in Acute Lung Injury Survivors: A Two-Year Longitudinal Prospective Study. Crit. Care Med. 2014, 42, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Schweickert, W.D.; Hall, J. ICU-Acquired Weakness. Chest 2007, 131, 1541–1549. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-Term Cognitive Impairment and Functional Disability among Survivors of Severe Sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef] [Green Version]
- Herridge, M.S.; Cheung, A.M.; Tansey, C.M.; Matte-Martyn, A.; Diaz-Granados, N.; Al-Saidi, F.; Cooper, A.B.; Guest, C.B.; Mazer, C.D.; Mehta, S.; et al. One-Year Outcomes in Survivors of the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2003, 348, 683–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional Disability 5 Years after Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef] [Green Version]
- Hermans, G.; Wilmer, A.; Meersseman, W.; Milants, I.; Wouters, P.J.; Bobbaers, H.; Bruyninckx, F.; van den Berghe, G. Impact of Intensive Insulin Therapy on Neuromuscular Complications and Ventilator Dependency in the Medical Intensive Care Unit. Am. J. Respir. Crit. Care Med. 2007, 175, 480–489. [Google Scholar] [CrossRef]
- Asfar, P.; Schortgen, F.; Boisramé-Helms, J.; Charpentier, J.; Guérot, E.; Megarbane, B.; Grimaldi, D.; Grelon, F.; Anguel, N.; Lasocki, S.; et al. Hyperoxia and Hypertonic Saline in Patients with Septic Shock (HYPERS2S): A Two-by-Two Factorial, Multicentre, Randomised, Clinical Trial. Lancet Respir. Med. 2017, 5, 180–190. [Google Scholar] [CrossRef]
- Wolfe, K.S.; Patel, B.K.; MacKenzie, E.L.; Giovanni, S.P.; Pohlman, A.S.; Churpek, M.M.; Hall, J.B.; Kress, J.P. Impact of Vasoactive Medications on ICU-Acquired Weakness in Mechanically Ventilated Patients. Chest 2018, 154, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Price, D.R.; Mikkelsen, M.E.; Umscheid, C.A.; Armstrong, E.J. Neuromuscular Blocking Agents and Neuromuscular Dysfunction Acquired in Critical Illness: A Systematic Review and Meta-Analysis. Crit. Care Med. 2016, 44, 2070–2078. [Google Scholar] [CrossRef] [Green Version]
- Altman, M.T.; Knauert, M.P.; Pisani, M.A. Sleep Disturbance after Hospitalization and Critical Illness: A Systematic Review. Ann. Am. Thorac. Soc. 2017, 14, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Kemp, H.I.; Corner, E.; Colvin, L.A. Chronic Pain after COVID-19: Implications for Rehabilitation. Br. J. Anaesth. 2020, 125, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Berlińska, A.; Świątkowska-Stodulska, R.; Sworczak, K. Old Problem, New Concerns: Hypercortisolemia in the Time of COVID-19. Front. Endocrinol. 2021, 12, 711612. [Google Scholar] [CrossRef]
- Nakamura, K.; Kawasaki, A.; Suzuki, N.; Hosoi, S.; Fujita, T.; Hachisu, S.; Nakano, H.; Naraba, H.; Mochizuki, M.; Takahashi, Y. Grip Strength Correlates with Mental Health and Quality of Life after Critical Care: A Retrospective Study in a Post-Intensive Care Syndrome Clinic. J. Clin. Med. 2021, 10, 3044. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; Puthucheary, Z.; Millán, I.S.; Butz, D.; Grocott, M.P.W. Muscle Mass and Physical Recovery in ICU: Innovations for Targeting of Nutrition and Exercise. Curr. Opin. Crit. Care 2017, 23, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Schroter-Noppe, D.; Drover, J.W.; Jain, M.; Keefe, L.; Dhaliwal, R.; Day, A. Nutrition Support in the Critical Care Setting: Current Practice in Canadian ICUs-Opportunities for Improvement? J. Parenter. Enter. Nutr. 2003, 27, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.C.; Mitchell, N.; Hopkins, R.O. Cognitive Functioning, Mental Health, and Quality of Life in ICU Survivors: An Overview. Crit. Care Clin. 2009, 25, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Wergin, R.; Modrykamien, A. Cognitive Impairment in ICU Survivors: Assessment and Therapy. Clevel. Clin. J. Med. 2012, 79, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Wolters, A.E.; Slooter, A.J.C.; van der Kooi, A.W.; van Dijk, D. Cognitive Impairment after Intensive Care Unit Admission: A Systematic Review. Intensive Care Med. 2013, 39, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.O.; Jackson, J.C. Long-Term Neurocognitive Function after Critical Illness. Chest 2006, 130, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Ambrosino, N.; Bruletti, G.; Scala, V.; Porta, R.; Vitacca, M. Cognitive and Perceived Health Status in Patient with Chronic Obstructive Pulmonary Disease Surviving Acute on Chronic Respiratory Failure: A Controlled Study. Intensive Care Med. 2002, 28, 170–177. [Google Scholar] [CrossRef]
- Schillerstrom, J.E.; Horton, M.S.; Schillerstrom, T.L.; Joshi, K.G.; Earthman, B.S.; Velez, A.M.; Royall, D.R. Prevalence, Course, and Risk Factors for Executive Impairment in Patients Hospitalized on a General Medicine Service. Psychosomatics 2005, 46, 411–417. [Google Scholar] [CrossRef]
- Sakusic, A.; O’Horo, J.C.; Dziadzko, M.; Volha, D.; Ali, R.; Singh, T.D.; Kashyap, R.; Farrell, A.M.; Fryer, J.D.; Petersen, R.; et al. Potentially Modifiable Risk Factors for Long-Term Cognitive Impairment After Critical Illness: A Systematic Review. Mayo Clin. Proc. 2018, 93, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Sakusic, A.; Rabinstein, A.A. Cognitive Outcomes after Critical Illness. Curr. Opin. Crit. Care 2018, 24, 410–414. [Google Scholar] [CrossRef]
- Denke, C.; Balzer, F.; Menk, M.; Szur, S.; Brosinsky, G.; Tafelski, S.; Wernecke, K.D.; Deja, M. Long-Term Sequelae of Acute Respiratory Distress Syndrome Caused by Severe Community-Acquired Pneumonia: Delirium-Associated Cognitive Impairment and Post-Traumatic Stress Disorder. J. Int. Med. Res. 2018, 46, 2265–2283. [Google Scholar] [CrossRef] [Green Version]
- Filatov, A.; Sharma, P.; Hindi, F.; Espinosa, P.S. Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus 2020, 12, e7352. [Google Scholar] [CrossRef] [Green Version]
- Kotfis, K.; Williams Roberson, S.; Wilson, J.E.; Dabrowski, W.; Pun, B.T.; Ely, E.W. COVID-19: ICU Delirium Management during SARS-CoV-2 Pandemic. Crit. Care 2020, 24, 176. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Roberson, S.W.; Wilson, J.E.; Pun, B.T.; Wesley Ely, E.; Jeżowska, I.; Jezierska, M.; Dabrowski, W. COVID-19: What Do We Need to Know about ICU Delirium during the SARS-CoV-2 Pandemic? Anaesthesiol. Intensive Ther. 2020, 52, 132–138. [Google Scholar] [CrossRef]
- Sasannejad, C.; Ely, E.W.; Lahiri, S. Long-Term Cognitive Impairment after Acute Respiratory Distress Syndrome: A Review of Clinical Impact and Pathophysiological Mechanisms. Crit. Care 2019, 23, 352. [Google Scholar] [CrossRef] [Green Version]
- Calsavara, A.J.C.; Costa, P.A.; Nobre, V.; Teixeira, A.L. Factors Associated with Short and Long Term Cognitive Changes in Patients with Sepsis. Sci. Rep. 2018, 8, 4509. [Google Scholar] [CrossRef] [PubMed]
- Rothenhäusler, H.B.; Ehrentraut, S.; Stoll, C.; Schelling, G.; Kapfhammer, H.P. The Relationship between Cognitive Performance and Employment and Health Status in Long-Term Survivors of the Acute Respiratory Distress Syndrome: Results of an Exploratory Study. Gen. Hosp. Psychiatry 2001, 23, 90–96. [Google Scholar] [CrossRef]
- Semmler, A.; Widmann, C.N.; Okulla, T.; Urbach, H.; Kaiser, M.; Widman, G.; Mormann, F.; Weide, J.; Fliessbach, K.; Hoeft, A.; et al. Persistent Cognitive Impairment, Hippocampal Atrophy and EEG Changes in Sepsis Survivors. J. Neurol. Neurosurg. Psychiatry 2013, 84, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Inoue, S.; Hatakeyama, J.; Kondo, Y.; Hifumi, T.; Sakuramoto, H.; Kawasaki, T.; Taito, S.; Nakamura, K.; Unoki, T.; Kawai, Y.; et al. Post-intensive Care Syndrome: Its Pathophysiology, Prevention, and Future Directions. Acute Med. Surg. 2019, 6, 233–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.B.; Jackson, J.C.; Morandi, A.; Girard, T.D.; Hughes, C.G.; Thompson, J.L.; Kiehl, A.L.; Elstad, M.R.; Wasserstein, M.L.; Goodman, R.B.; et al. Incidence and Risk Factors for Intensive Care Unit-Related Post-Traumatic Stress Disorder in Veterans and Civilians. Am. J. Respir. Crit. Care Med. 2016, 193, 1373–1381. [Google Scholar] [CrossRef] [Green Version]
- Asimakopoulou, E.; Madianos, M. Depression and Post-Traumatic Stress Disorder among Patients in Intensive Care Units. Psychiatr. Psychiatr. 2014, 25, 257–269. [Google Scholar]
- Desai, S.V.; Law, T.J.; Needham, D.M. Long-Term Complications of Critical Care. Crit. Care Med. 2011, 39, 371–379. [Google Scholar] [CrossRef]
- Griffiths, J.; Gager, M.; Alder, N.; Fawcett, D.; Waldmann, C.; Quinlan, J. A Self-Report-Based Study of the Incidence and Associations of Sexual Dysfunction in Survivors of Intensive Care Treatment. Intensive Care Med. 2006, 32, 501–510. [Google Scholar] [CrossRef]
- Broomhead, L.R.; Brett, S.J. Clinical Review: Intensive Care Follow-up-What Has It Told Us? Crit. Care 2002, 6, 411–417. [Google Scholar] [CrossRef]
- Kapfhammer, H.P.; Rothenhäusler, H.B.; Krauseneck, T.; Stoll, C.; Schelling, G. Posttraumatic Stress Disorder and Health-Related Quality of Life in Long-Term Survivors of Acute Respiratory Distress Syndrome. Am. J. Psychiatry 2004, 161, 376–386. [Google Scholar] [CrossRef]
- Weinert, C.R.; Gross, C.R.; Kangas, J.R.; Bury, C.L.; Marinelli, W.A. Health-Related Quality of Life after Acute Lung Injury. Am. J. Respir. Crit. Care Med. 1997, 156, 1120–1128. [Google Scholar] [CrossRef]
- Skodol, A.E. Anxiety in the Medically Ill: Nosology and Principles of Differential Diagnosis. Semin. Clin. Neuropsychiatry 1999, 4, 64–71. [Google Scholar]
- Martillo, M.A.; Dangayach, N.S.; Tabacof, L.; Spielman, L.A.; Dams-O’Connor, K.; Chan, C.C.; Kohli-Seth, R.; Cortes, M.; Escalon, M.X. Postintensive Care Syndrome in Survivors of Critical Illness Related to Coronavirus Disease 2019: Cohort Study from a New York City Critical Care Recovery Clinic. Crit. Care Med. 2021, 49, 1427–1438. [Google Scholar] [CrossRef]
- Zatzick, D.; Jurkovich, G.J.; Rivara, F.P.; Wang, J.; Fan, M.Y.; Joesch, J.; MacKenzie, E. A National US Study of Posttraumatic Stress Disorder, Depression, and Work and Functional Outcomes after Hospitalization for Traumatic Injury. Ann. Surg. 2008, 248, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.B.; Stewart, A.; Hays, R.D.; Burnam, M.A.; Rogers, W.; Daniels, M.; Berry, S.; Greenfield, S.; Ware, J. The Functioning and Well-Being of Depressed Patients: Results From the Medical Outcomes Study. JAMA J. Am. Med. Assoc. 1989, 262, 914–919. [Google Scholar] [CrossRef]
- Angus, D.C.; Musthafa, A.A.; Clermont, G.; Griffin, M.F.; Linde-Zwirble, W.T.; Dremsizov, T.T.; Pinsky, M.R. Quality-Adjusted Survival in the First Year after the Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2001, 163, 1389–1394. [Google Scholar] [CrossRef]
- Cheung, A.M.; Tansey, C.M.; Tomlinson, G.; Diaz-Granados, N.; Matté, A.; Barr, A.; Mehta, S.; Mazer, C.D.; Guest, C.B.; Stewart, T.E.; et al. Two-Year Outcomes, Health Care Use, and Costs of Survivors of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, D.W.; Dinglas, V.; Mendez-Tellez, P.A.; Bienvenu, O.J.; Sevransky, J.; Dennison, C.R.; Shanholtz, C.; Needham, D.M. Intensive Care Unit Hypoglycemia Predicts Depression during Early Recovery from Acute Lung Injury. Crit. Care Med. 2008, 36, 2726–2733. [Google Scholar] [CrossRef] [PubMed]
- Rattray, J.E.; Johnston, M.; Wildsmith, J.A.W. Predictors of Emotional Outcomes of Intensive Care. Anaesthesia 2005, 60, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Sukantarat, K.; Greer, S.; Brett, S.; Williamson, R. Physical and Psychological Sequelae of Critical Illness. Br. J. Health Psychol. 2007, 12, 65–74. [Google Scholar] [CrossRef]
- Jones, C.; Griffiths, R.D.; Humphris, G.; Skirrow, P.M. Memory, Delusions, and the Development of Acute Posttraumatic Stress Disorder-Related Symptoms after Intensive Care. Crit. Care Med. 2001, 29, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, K.A.M.; Lundberg, D.; Fridlund, B. Stressful Memories and Psychological Distress in Adult Mechanically Ventilated Intensive Care Patients? A 2-Month Follow-up Study. Acta Anaesthesiol. Scand. 2007, 51, 671–678. [Google Scholar] [CrossRef]
- Wade, D.M.; Howell, D.C.; Weinman, J.A.; Hardy, R.J.; Mythen, M.G.; Brewin, C.R.; Borja-Boluda, S.; Matejowsky, C.F.; Raine, R.A. Investigating Risk Factors for Psychological Morbidity Three Months after Intensive Care: A Prospective Cohort Study. Crit. Care 2012, 16, R192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davydow, D.S.; Gifford, J.M.; Desai, S.V.; Needham, D.M.; Bienvenu, O.J. Posttraumatic Stress Disorder in General Intensive Care Unit Survivors: A Systematic Review. Gen. Hosp. Psychiatry 2008, 30, 421–434. [Google Scholar] [CrossRef] [Green Version]
- Davydow, D.S.; Desai, S.V.; Needham, D.M.; Bienvenu, O.J. Psychiatric Morbidity in Survivors of the Acute Respiratory Distress Syndrome: A Systematic Review. Psychosom. Med. 2008, 70, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, N.K.J.; McAndrews, M.P.; Tansey, C.M.; Matté, A.; Pinto, R.; Cheung, A.M.; Diaz-Granados, N.; Barr, A.; Herridge, M.S. Self-Reported Symptoms of Depression and Memory Dysfunction in Survivors of ARDS. Chest 2009, 135, 678–687. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, M.E.; Christie, J.D.; Lanken, P.N.; Biester, R.C.; Thompson, B.T.; Bellamy, S.L.; Localio, A.R.; Demissie, E.; Hopkins, R.O.; Angus, D.C. The Adult Respiratory Distress Syndrome Cognitive Outcomes Study: Long-Term Neuropsychological Function in Survivors of Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2012, 185, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Myhren, H.; Ekeberg, Ø.; Tøien, K.; Karlsson, S.; Stokland, O. Posttraumatic Stress, Anxiety and Depression Symptoms in Patients during the First Year Post Intensive Care Unit Discharge. Crit. Care 2010, 14, R14. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, R.O.; Key, C.W.; Suchyta, M.R.; Weaver, L.K.; Orme, J.F. Risk Factors for Depression and Anxiety in Survivors of Acute Respiratory Distress Syndrome. Gen. Hosp. Psychiatry 2010, 32, 147–155. [Google Scholar] [CrossRef]
- Vrettou, C.S.; Mantziou, V.; Ilias, I.; Vassiliou, A.G.; Orfanos, S.E.; Kotanidou, A.; Dimopoulou, I. Quality of Life, Depression, and Anxiety in Survivors of Critical Illness from a Greek ICU. A Prospective Observational Study. Healthcare 2021, 9, 849. [Google Scholar] [CrossRef]
- Nikayin, S.; Rabiee, A.; Hashem, M.D.; Huang, M.; Bienvenu, O.J.; Turnbull, A.E.; Needham, D.M. Anxiety Symptoms in Survivors of Critical Illness: A Systematic Review and Meta-Analysis. Gen. Hosp. Psychiatry 2016, 43, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuthbertson, B.H.; Rattray, J.; Campbell, M.K.; Gager, M.; Roughton, S.; Smith, A.; Hull, A.; Breeman, S.; Norrie, J.; Jenkinson, D.; et al. The PRaCTICaL Study of Nurse Led, Intensive Care Follow-up Programmes for Improving Long Term Outcomes Fromcritical Illness: A Pragmatic Randomised Controlled Trial. BMJ 2009, 339, b3723. [Google Scholar] [CrossRef] [Green Version]
- Bienvenu, O.J.; Colantuoni, E.; Mendez-Tellez, P.A.; Shanholtz, C.; Dennison-Himmelfarb, C.R.; Pronovost, P.J.; Needham, D.M. Cooccurrence of and Remission from General Anxiety, Depression, and Posttraumatic Stress Disorder Symptoms after Acute Lung Injury: A 2-Year Longitudinal Study. Crit. Care Med. 2015, 43, 642–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javidi, H.M.Y. Post-Traumatic Stress Disorder. Int. J. Occup. Environ. Med. 2012, 3, 2–9. [Google Scholar]
- Bienvenu, O.J.; Williams, J.B.; Yang, A.; Hopkins, R.O.; Needham, D.M. Posttraumatic Stress Disorder in Survivors of Acute Lung Injury: Evaluating the Impact of Event Scale-Revised. Chest 2013, 144, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienvenu, O.J.; Gellar, J.; Althouse, B.M.; Colantuoni, E.; Sricharoenchai, T.; Mendez-Tellez, P.A.; Shanholtz, C.; Dennison, C.R.; Pronovost, P.J.; Needham, D.M. Post-Traumatic Stress Disorder Symptoms after Acute Lung Injury: A 2-Year Prospective Longitudinal Study. Psychol. Med. 2013, 43, 2657–2671. [Google Scholar] [CrossRef]
- Herridge, M.S. Long-Term Outcomes after Critical Illness: Past, Present, Future. Curr. Opin. Crit. Care 2007, 13, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Breslau, N.; Davis, G.C.; Andreski, P.; Peterson, E. Traumatic Events and Posttraumatic Stress Disorder in an Urban Population of Young Adults. Arch. Gen. Psychiatry 1991, 48, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.E.; Powers, K.; Hedayat, K.M.; Tieszen, M.; Kon, A.A.; Shepard, E.; Spuhler, V.; Todres, I.D.; Levy, M.; Barr, J.; et al. Clinical Practice Guidelines for Support of the Family in the Patient-Centered Intensive Care Unit: American College of Critical Care Medicine Task Force 2004–2005. Crit. Care Med. 2007, 35, 605–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gries, C.J.; Engelberg, R.A.; Kross, E.K.; Zatzick, D.; Nielsen, E.L.; Downey, L.; Curtis, J.R. Predictors of Symptoms of Posttraumatic Stress and Depression in Family Members after Patient Death in the ICU. Chest 2010, 137, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Azoulay, E.; Pochard, F.; Kentish-Barnes, N.; Chevret, S.; Aboab, J.; Adrie, C.; Annane, D.; Bleichner, G.; Bollaert, P.E.; Darmon, M.; et al. Risk of Post-Traumatic Stress Symptoms in Family Members of Intensive Care Unit Patients. Am. J. Respir. Crit. Care Med. 2005, 171, 987–994. [Google Scholar] [CrossRef]
- McAdam, J.L.; Dracup, K.A.; White, D.B.; Fontaine, D.K.; Puntillo, K.A. Symptom Experiences of Family Members of Intensive Care Unit Patients at High Risk for Dying. Crit. Care Med. 2010, 38, 1078–1085. [Google Scholar] [CrossRef] [Green Version]
- Anderson, W.G.; Arnold, R.M.; Angus, D.C.; Bryce, C.L. Posttraumatic Stress and Complicated Grief in Family Members of Patients in the Intensive Care Unit. J. Gen. Intern. Med. 2008, 23, 1871–1876. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.; Skirrow, P.; Griffiths, R.D.; Humphris, G.; Ingleby, S.; Eddleston, J.; Waldmann, C.; Gager, M. Post-Traumatic Stress Disorder-Related Symptoms in Relatives of Patients Following Intensive Care. Intensive Care Med. 2004, 30, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Paparrigopoulos, T.; Melissaki, A.; Efthymiou, A.; Tsekou, H.; Vadala, C.; Kribeni, G.; Pavlou, E.; Soldatos, C. Short-Term Psychological Impact on Family Members of Intensive Care Unit Patients. J. Psychosom. Res. 2006, 61, 459–468. [Google Scholar] [CrossRef]
- Cameron, J.I.; Chu, L.M.; Matte, A.; Tomlinson, G.; Chan, L.; Thomas, C.; Friedrich, J.O.; Mehta, S.; Lamontagne, F.; Levasseur, M.; et al. One-Year Outcomes in Caregivers of Critically Ill Patients. N. Engl. J. Med. 2016, 374, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Pochard, F.; Azoulay, E.; Chevret, S.; Lemaire, F.; Hubert, P.; Canoui, P.; Grassin, M.; Zittoun, R.; le Gall, J.R.; Dhainaut, J.F.; et al. Symptoms of Anxiety and Depression in Family Members of Intensive Care Unit Patients: Ethical Hypothesis Regarding Decision-Making Capacity. Crit. Care Med. 2001, 29, 1893–1897. [Google Scholar] [CrossRef]
- Lautrette, A.; Darmon, M.; Megarbane, B.; Joly, L.M.; Chevret, S.; Adrie, C.; Barnoud, D.; Bleichner, G.; Bruel, C.; Choukroun, G.; et al. A Communication Strategy and Brochure for Relatives of Patients Dying in the ICU. N. Engl. J. Med. 2007, 356, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.M.; Pérez San Gregorio, M.Á.; Rodríguez, A.G. Psychological Repercussions in Family Members of Hospitalised Critical Condition Patients. J. Psychosom. Res. 2005, 58, 447–458. [Google Scholar] [CrossRef]
- Davidson, J.E.; Jones, C.; Bienvenu, O.J. Family Response to Critical Illness: Postintensive Care Syndrome-Family. Crit. Care Med. 2012, 40, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Anesi, G.L.; Jablonski, J.; Harhay, M.O.; Atkins, J.H.; Bajaj, J.; Baston, C.; Brennan, P.J.; Candeloro, C.L.; Catalano, L.M.; Cereda, M.F.; et al. Characteristics, Outcomes, and Trends of Patients with Covid-19-Related Critical Illness at a Learning Health System in the United States. Ann. Intern. Med. 2021, 174, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Bangash, M.N.; Owen, A.; Alderman, J.E.; Chotalia, M.; Patel, J.M.; Parekh, D. COVID-19 Recovery: Potential Treatments for Post-Intensive Care Syndrome. Lancet Respir. Med. 2020, 8, 1071–1073. [Google Scholar] [CrossRef]
- Venkatesan, P. NICE Guideline on Long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef]
- The Lancet. Facing up to Long COVID. Lancet 2020, 396, 1861. [Google Scholar] [CrossRef]
- Stam, H.J.; Stucki, G.; Bickenbach, J. Covid-19 and Post Intensive Care Syndrome: A Call for Action. J. Rehabil. Med. 2020, 52, jrm00044. [Google Scholar] [CrossRef]
- Lutchmansingh, D.D.; Knauert, M.P.; Antin-Ozerkis, D.E. A Clinic Blueprint for Post-COVID-19 RECOVERY: Learning from the Past, Looking to the Future. Chest 2020, 159, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.F.; Minguet, P.; Colson, C.; Kellens, I.; Chaabane, S.; Delanaye, P.; Cavalier, E.; Chase, J.G.; Lambermont, B.; Misset, B. Post-Intensive Care Syndrome after a Critical COVID-19: Cohort Study from a Belgian Follow-up Clinic. Ann. Intensive Care 2021, 11, 118. [Google Scholar] [CrossRef]
- Daste, C.; Ficarra, S.; Dumitrache, A.; Cariou, A.; Lefèbvre, A.; Pène, F.; Roche, N.; Roren, A.; Thery, C.; Vidal, J.; et al. Post-Intensive Care Syndrome in Patients Surviving COVID-19. Ann. Phys. Rehabil. Med. 2021, 64, 101549. [Google Scholar] [CrossRef]
- Cadd, M.; Nunn, M. An A-E Assessment of Post-ICU COVID-19 Recovery. J. Intensive Care 2021, 9, 29. [Google Scholar] [CrossRef]
- Valent, A.; Dudoignon, E.; Ressaire, Q.; Dépret, F.; Plaud, B. Three-Month Quality of Life in Survivors of ARDS Due to COVID-19: A Preliminary Report from a French Academic Centre. Anaesth. Crit. Care Pain Med. 2020, 39, 740–741. [Google Scholar] [CrossRef] [PubMed]
- Peach, B.C.; Valenti, M.; Sole, M.L. A Call for the World Health Organization to Create International Classification of Disease Diagnostic Codes for Post-Intensive Care Syndrome in the Age of COVID-19. World Med. Health Policy 2021, 13, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Gensichen, J.; Gehrke-Beck, S.; Kosilek, R.P.; Kühne, F.; Heintze, C.; Baldwin, L.M.; Needham, D.M. Management of COVID-19 ICU-Survivors in Primary Care: A Narrative Review. BMC Fam. Pract. 2021, 22, 160. [Google Scholar] [CrossRef]
- Zante, B.; Erne, K.; Grossenbacher, J.; Camenisch, S.A.; Schefold, J.C.; Jeitziner, M.M. Symptoms of Post-Traumatic Stress Disorder (PTSD) in next of Kin during Suspension of ICU Visits during the COVID-19 Pandemic: A Prospective Observational Study. BMC Psychiatry 2021, 21, 477. [Google Scholar] [CrossRef]
- Li, Y.; Scherer, N.; Felix, L.; Kuper, H. Prevalence of Depression, Anxiety and Posttraumatic Stress Disorder in Health Care Workers during the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0246454. [Google Scholar] [CrossRef]
- Ilias, I.; Mantziou, V.; Vamvakas, E.; Kampisiouli, E.; Theodorakopoulou, M.; Vrettou, C.; Douka, E.; Vassiliou, A.G.; Orfanos, S.; Kotanidou, A.; et al. Post-Traumatic Stress Disorder and Burnout in Healthcare Professionals During the SARS-CoV-2 Pandemic: A Cross-Sectional Study. J. Crit. Care Med. 2021, 7, 14–20. [Google Scholar] [CrossRef]
- Pun, B.T.; Balas, M.C.; Barnes-Daly, M.A.; Thompson, J.L.; Aldrich, J.M.; Barr, J.; Byrum, D.; Carson, S.S.; Devlin, J.W.; Engel, H.J.; et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit. Care Med. 2019, 47, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Fuke, R.; Hifumi, T.; Kondo, Y.; Hatakeyama, J.; Takei, T.; Yamakawa, K.; Inoue, S.; Nishida, O. Early Rehabilitation to Prevent Postintensive Care Syndrome in Patients with Critical Illness: A Systematic Review and Meta-Analysis. BMJ Open 2018, 8, e019998. [Google Scholar] [CrossRef] [Green Version]
- Routsi, C.; Gerovasili, V.; Vasileiadis, I.; Karatzanos, E.; Pitsolis, T.; Tripodaki, E.; Markaki, V.; Zervakis, D.; Nanas, S. Electrical Muscle Stimulation Prevents Critical Illness Polyneuromyopathy: A Randomized Parallel Intervention Trial. Crit. Care 2010, 14, R74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, J.E.; Harvey, M.A.; Bemis-Dougherty, A.; Smith, J.M.; Hopkins, R.O. Implementation of the Pain, Agitation, and Delirium Clinical Practice Guidelines and Promoting Patient Mobility to Prevent Post-Intensive Care Syndrome. Crit. Care Med. 2013, 41, S136–S145. [Google Scholar] [CrossRef]
- Sasangohar, F.; Dhala, A.; Zheng, F.; Ahmadi, N.; Kash, B.; Masud, F. Use of Telecritical Care for Family Visitation to ICU during the COVID-19 Pandemic: An Interview Study and Sentiment Analysis. BMJ Qual. Saf. 2021, 30, 715–721. [Google Scholar] [CrossRef]
- Rose, L.; Yu, L.; Casey, J.; Cook, A.; Metaxa, V.; Pattison, N.; Rafferty, A.M.; Ramsay, P.; Saha, S.; Xyrichis, A.; et al. Communication and Virtual Visiting for Families of Patients in Intensive Care during the COVID-19 Pandemic: A UK National Survey. Ann. Am. Thorac. Soc. 2021, 18, 1685–1692. [Google Scholar] [CrossRef]
- Floris, L.; Madeddu, A.; Deiana, V.; Pasero, D.; Terragni, P. The Use of the ICU Diary during the COVID-19 Pandemic as a Tool to Enhance Critically Ill Patient Recovery. Minerva Anestesiol. 2021, 87, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Haines, K.J.; McPeake, J.; Hibbert, E.; Boehm, L.M.; Aparanji, K.; Bakhru, R.N.; Bastin, A.J.; Beesley, S.J.; Beveridge, L.; Butcher, B.W.; et al. Enablers and Barriers to Implementing ICU Follow-up Clinics and Peer Support Groups Following Critical Illness: The Thrive Collaboratives. Crit. Care Med. 2019, 47, 1194–1200. [Google Scholar] [CrossRef]
- Weiss, D.S. The impact of event scale-revised. In Assessing Psychological Trauma and PTSD, 2nd ed.; Wilson, J.P., Keane, T.M., Eds.; Guilford Press: New York, NY, USA, 2004. [Google Scholar]
- Gautam, R.; Sankalp, Y.; Raj, K. Post-Traumatic Stress Disorder: A Review from Clinical Perspective. Int. J. Indian Psychol. 2016, 3, 156–164. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, X.Y.; He, X.; Zhou, C.M.; Shen, J.C.; Tong, J.H. Parvalbumin Interneuron-Mediated Neural Disruption in an Animal Model of Postintensive Care Syndrome: Prevention by Fluoxetine. Aging 2021, 13, 8720–8736. [Google Scholar] [CrossRef]
- Knapp, P.; Beck, A.T. Cognitive Therapy: Foundations, Conceptual Models, Applications and Research. Rev. Bras. De Psiquiatr. 2008, 30 (Suppl. S2), s54–s64. [Google Scholar] [CrossRef] [Green Version]
- Petrinec, A.B.; Hughes, J.W.; Zullo, M.D.; Wilk, C.; George, R.L. Smartphone Delivery of Cognitive Behavioral Therapy for Postintensive Care Syndrome-Family: Protocol for a Pilot Study. JMIR Res. Protoc. 2021, 10, e30813. [Google Scholar] [CrossRef] [PubMed]
- Rawal, G.; Yadav, S.; Kumar, R. Post-Intensive Care Syndrome: An Overview. J. Transl. Intern. Med. 2017, 5, 90–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, J.C.; Millán, I.S. Validation of Musculoskeletal Ultrasound to Assess and Quantify Muscle Glycogen Content. A Novel Approach. Physician Sportsmed. 2014, 42, 45–52. [Google Scholar] [CrossRef] [PubMed]

| Physical Function |
|---|
| Timed Up-and-Go (TUG) [8,9] |
| Handgrip strength [10] |
| 2-Minute Walk Test (2-MWT) [11] |
| Short Physical Performance Battery (SPPB) [12] |
| Comprehensive Geriatric Assessment (CGA) [13] |
| Medical Research Council scale for muscle strength (MRC) [14] |
| Health-Related Quality of Life (HRQOL)/Subjective Health |
| European Quality of Life 5 Dimensions 5 Level (EQ-5D-5L) [15] |
| Subjective concern [9] |
| WHO Disability Assessment Schedule (WHODAS) 2.0 [16] |
| World Health Organization Quality of Life (WHOQOL)-100 and (WHOQOL)-Bref [17] |
| RAND corporation tool for HRQoL (RAND 36) [18] |
| 36-Item Short Form Survey (SF-36) [19] |
| Activities of Daily Living and Instrumental Activities of Daily Living (ADLs and IADLs) [20] |
| Functional Independence Measure [21] |
| Healthy Aging Brain Care Monitor Self Report version (HABC-M SR) [22] |
| Cognition |
| MiniCog [23] |
| Animal Naming [24] |
| Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [25] |
| Trail Making Test (TMT) A, B [26] |
| Mini-Mental State Exam (MMSE) [27] |
| Wechsler Adult Intelligence Test-Revised [28] |
| Wechsler Memory Scale-Revised [28] |
| Rey Auditory-Verbal Learning Test [28] |
| Rey–Osterrieth Complex Figure Test [28] |
| Oklahoma Premorbid Intelligence Estimation method (OPIE) [28] |
| Verbal Fluency test [28] |
| Logical memory, Visual Reproduction, and Adult Video Learning Test (AVLT) [29] |
| Short Test of Mental Status, Modified Hachinski Scale, Prime MD [29] |
| Picture Completion, Block Design [29] |
| Boston Naming Test, Category Fluency [29] |
| Mental Health |
| Depression |
| Center for Epidemiologic Studies Depression (CES-D) [30] |
| Beck Depression Inventory [28] |
| Geriatric Depression Rating Scale-Short Form (GDS-SF) [31] |
| Anxiety |
| Generalized Anxiety Disorder Scale (GAD) [32] |
| Beck Anxiety Inventory [28] |
| State-Trait Anxiety Inventory (STAI) [33] |
| Post-Traumatic Stress Disorder |
| Impact of Event Scale—revised (IES-R) [34] |
| Post-Traumatic Stress Disorder Checklist for a Specific event (PCL-S) [28] |
| Post-Traumatic Stress Disorder Check List—Civilian version (PCL-C) [28] |
| Clinician-Administered Post-Traumatic Stress Disorder Scale (CAPS) [35] |
| Davidson Trauma Scale (DTS) [35] |
| Posttraumatic Stress Diagnostic Scale (PDS) [35] |
| Post-Traumatic Stress Syndrome—Question Inventory (PTSS) [35] |
| Global |
| Patient Health Questionnaire (PHQ)—various versions [32,36] |
| Depression Anxiety and Stress Scales instrument (DASS-21) [28] |
| Hospital Anxiety and Depression Scale (HADS) [37] |
| Structured Clinical Interview for DSM-IV (SCID) [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrettou, C.S.; Mantziou, V.; Vassiliou, A.G.; Orfanos, S.E.; Kotanidou, A.; Dimopoulou, I. Post-Intensive Care Syndrome in Survivors from Critical Illness including COVID-19 Patients: A Narrative Review. Life 2022, 12, 107. https://doi.org/10.3390/life12010107
Vrettou CS, Mantziou V, Vassiliou AG, Orfanos SE, Kotanidou A, Dimopoulou I. Post-Intensive Care Syndrome in Survivors from Critical Illness including COVID-19 Patients: A Narrative Review. Life. 2022; 12(1):107. https://doi.org/10.3390/life12010107
Chicago/Turabian StyleVrettou, Charikleia S., Vassiliki Mantziou, Alice G. Vassiliou, Stylianos E. Orfanos, Anastasia Kotanidou, and Ioanna Dimopoulou. 2022. "Post-Intensive Care Syndrome in Survivors from Critical Illness including COVID-19 Patients: A Narrative Review" Life 12, no. 1: 107. https://doi.org/10.3390/life12010107
APA StyleVrettou, C. S., Mantziou, V., Vassiliou, A. G., Orfanos, S. E., Kotanidou, A., & Dimopoulou, I. (2022). Post-Intensive Care Syndrome in Survivors from Critical Illness including COVID-19 Patients: A Narrative Review. Life, 12(1), 107. https://doi.org/10.3390/life12010107