The Procoagulant Activity of Emoxilane®: A New Appealing Therapeutic Use in Epistaxis of the Combination of Sodium Hyaluronate, Silver Salt, α-tocopherol and D-panthenol
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Preparation of Sample Solutions
- SA: 100 µg/mL; 200 µg/mL;
- Ag: 15 µg/mL; 30 µg/mL;
- Toc: 20 µM; 40 µM;
- Pant: 1% w/v: 2% w/v.
2.3. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.4. 1,1-diphenyl-2-picrylhydrazyl (DPPH) Assay
2.5. 2′,7′-Dichlorodihydrofluorescein Diacetate (DCHF-DA) Assay
2.6. Antimicrobial Activity
2.7. Plasmin Activity Assay
2.8. Thrombin Levels Measurement
2.9. Statistical Analysis
3. Results
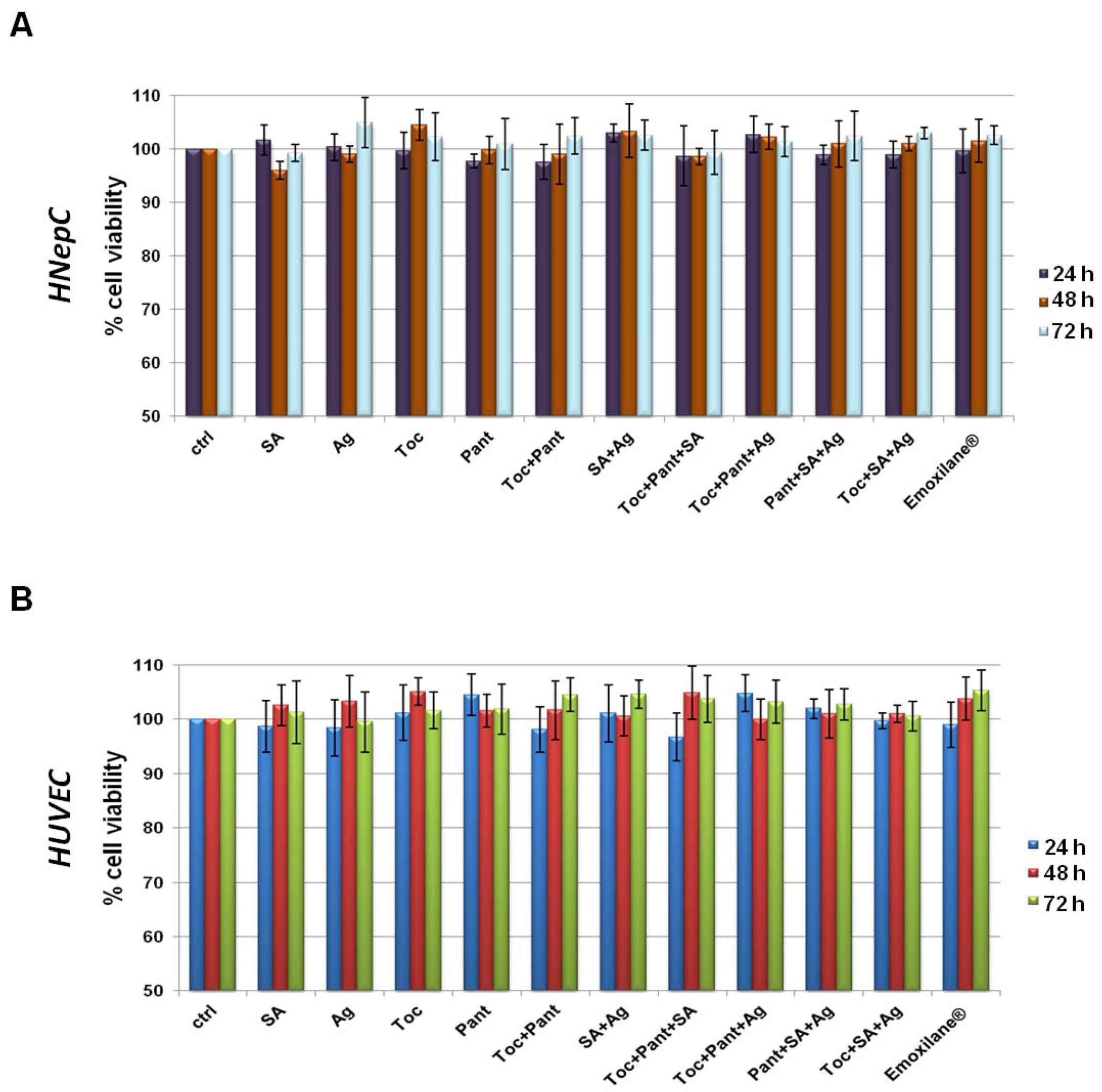
3.1. All the Substances of Our Interest, Alone or in Different Kind of Combination, Did Not Result Cytotoxic
3.2. The Combination Emoxilane® Showed Marked Antioxidant Effects
3.3. Emoxilane® Presented a Significant Antimicrobial Activity Thanks to the Presence of Silver Compound
3.4. Emoxilane® Mediates Anti-Fibrinolytic Effects by Inhibiting Plasmin Activity
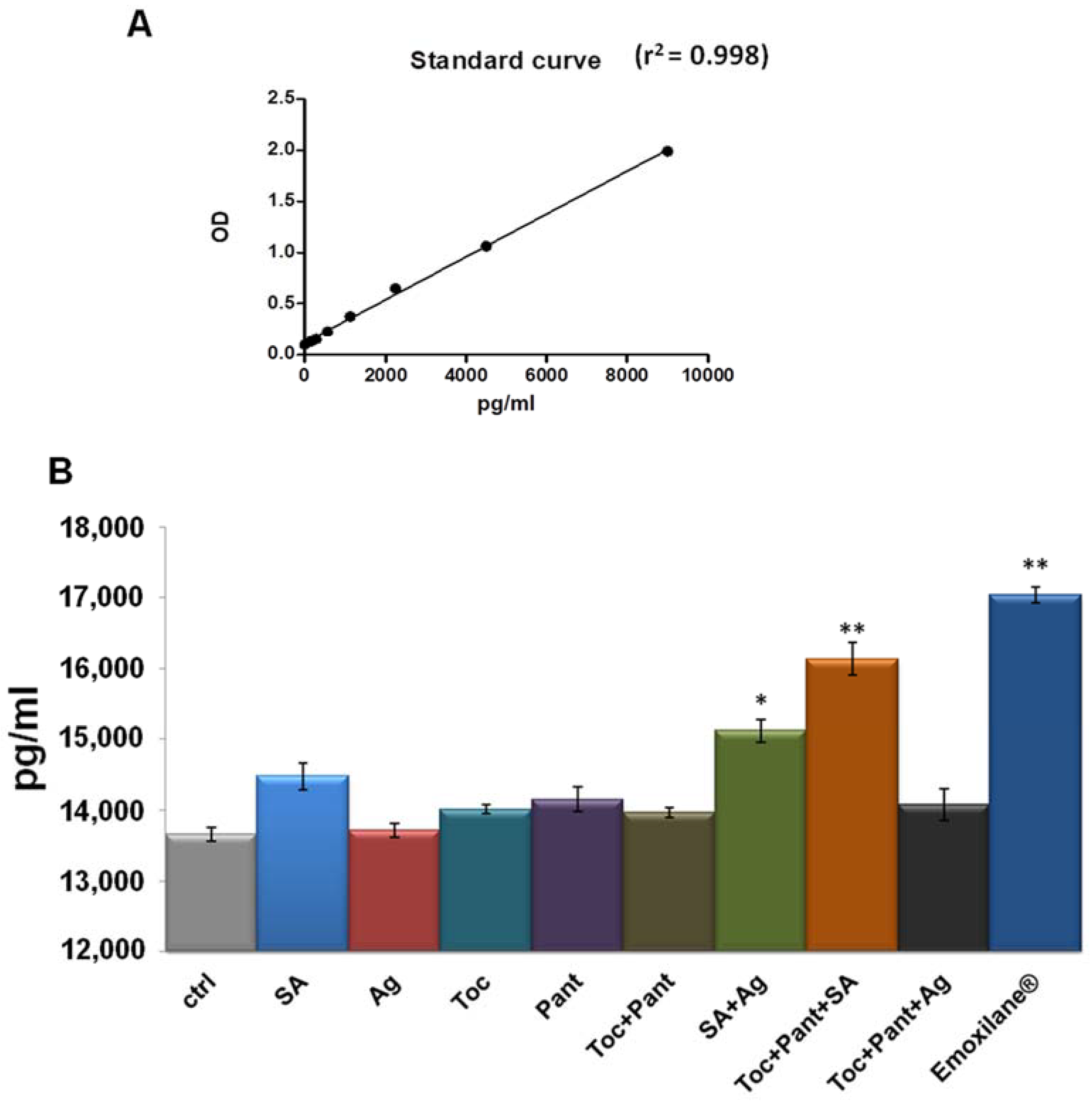
3.5. Thrombin Levels Increased in Human Plasma in Presence of Emoxilane®
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kullar, P.; Weerakkody, R.; Cathcart, R.; Yates, P. Locally applied haemostatic agents in the management of acute epistaxis (nosebleeds). Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
- Beck, R.; Sorge, M.; Schneider, A.; Dietz, A. Current Approaches to Epistaxis Treatment in Primary and Secondary Care. Dtsch. Aerzteblatt Online 2018, 115, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.J.; Kellerman, R. Epistaxis. Prim. Care: Clin. Off. Pr. 2014, 41, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Zhao, M.; Yang, S.; Chen, F.; Chen, J.; Yang, G.; Yuan, D. A new double-lumen hemostatic device for treatment of intractable traumatic epistaxis induced by craniofacial basicranial fractures. Int. J. Clin. Exp. Med. 2017, 10, 5832–5839. [Google Scholar]
- Kucik, C.J.; Clenney, T. Management of epistaxis. Am. Fam. Physician 2005, 71, 305–311. [Google Scholar]
- Varadharaj, S.; Kelly, O.; Khayat, R.N.; Kumar, P.; Ahmed, N.; Zweier, J.L. Role of Dietary Antioxidants in the Preservation of Vascular Function and the Modulation of Health and Disease. Front. Cardiovasc. Med. 2017, 4, 64. [Google Scholar] [CrossRef]
- Müller, L.; Theile, K.; Böhm, V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol. Nutr. Food Res. 2010, 54, 731–742. [Google Scholar] [CrossRef]
- Camargo, F.B., Jr.; Gaspar, L.R.; Campos, P.M.B.G.M. Skin moisturizing effects of panthenol-based formulations. J. Cosmet. Sci. 2011, 62, 361–370. [Google Scholar] [PubMed]
- Gouteva, I.; Shah-Hosseini, K.; Meiser, P. Clinical Efficacy of a Spray Containing Hyaluronic Acid and Dexpanthenol after Surgery in the Nasal Cavity (Septoplasty, Simple Ethmoid Sinus Surgery, and Turbinate Surgery). J. Allergy 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Chen, J.; Shen, Q.; Chan, D.; Li, J.; Tanguay, A.P.; Schmidt, T.A.; Niazi, F.; Plaas, A. Addition of High Molecular Weight Hyaluronic Acid to Fibroblast-Like Stromal Cells Modulates Endogenous Hyaluronic Acid Metabolism and Enhances Proteolytic Processing and Secretion of Versican. Cells 2020, 9, 1681. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kang, Y.-G.; Hwang, S.-H.; Kim, J.K.; Hong, Y.D.; Park, W.-S.; Kim, D.; Kim, E.; Cho, J.Y. In Vitro Effects of Dehydrotrametenolic Acid on Skin Barrier Function. Molecules 2019, 24, 4583. [Google Scholar] [CrossRef]
- Belvedere, R.; Bizzarro, V.; Parente, L.; Petrella, F.; Petrella, A. The Pharmaceutical Device Prisma® Skin Promotes in Vitro Angiogenesis through Endothelial to Mesenchymal Transition during Skin Wound Healing. Int. J. Mol. Sci. 2017, 18, 1614. [Google Scholar] [CrossRef]
- Belvedere, R.; Bizzarro, V.; Parente, L.; Petrella, F.; Petrella, A. Effects of Prisma® Skin dermal regeneration device containing glycosaminoglycans on human keratinocytes and fibroblasts. Cell Adhes. Migr. 2017, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De Torre, M.P.; Cavero, R.Y.; Calvo, M.I.; Vizmanos, J.L. A Simple and a Reliable Method to Quantify Antioxidant Activity In Vivo. Antioxidants 2019, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Bizzarro, V.; Fontanella, B.; Carratù, A.; Belvedere, R.; Marfella, R.; Parente, L.; Petrella, A. Annexin A1 N-Terminal Derived Peptide Ac2-26 Stimulates Fibroblast Migration in High Glucose Conditions. PLoS ONE 2012, 7, e45639. [Google Scholar] [CrossRef] [PubMed]
- Belvedere, R.; Morretta, E.; Pessolano, E.; Novizio, N.; Tosco, A.; Porta, A.; Whiteford, J.; Perretti, M.; Filippelli, A.; Monti, M.C.; et al. Mesoglycan exerts its fibrinolytic effect through the activation of annexin A2. J. Cell. Physiol. 2021, 236, 4926–4943. [Google Scholar] [CrossRef]
- Wolberg, A.S.; Campbell, R.A. Thrombin generation, fibrin clot formation and hemostasis. Transfus. Apher. Sci. 2008, 38, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, M.R.; Zhang, N.; Resto, V.; Goodwin, J.S. Demographic, Seasonal, and Geographic Differences in Emergency Department Visits for Epistaxis. Otolaryngol. Neck Surg. 2016, 156, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Tunkel, D.E.; Anne, S.; Payne, S.C.; Ishman, S.L.; Rosenfeld, R.M.; Abramson, P.J.; Alikhaani, J.D.; Benoit, M.M.; Bercovitz, R.; Brown, M.D.; et al. Clinical Practice Guideline: Nosebleed (Epistaxis). Otolaryngol. Neck Surg. 2020, 162, S1–S38. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zou, M.-H. Redox regulation of endothelial cell fate. Cell. Mol. Life Sci. 2014, 71, 3219–3239. [Google Scholar] [CrossRef]
- Biro, K.; Thaçi, D.; Ochsendorf, F.R.; Kaufmann, R.; Boehncke, W.-H. Efficacy of dexpanthenol in skin protection against irritation: A double-blind, placebo-controlled study. Contact Dermat. 2003, 49, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Ebner, F.; Heller, A.; Rippke, F.; Tausch, I. Topical Use of Dexpanthenol in Skin Disorders. Am. J. Clin. Dermatol. 2002, 3, 427–433. [Google Scholar] [CrossRef]
- Chang, X.; Yamada, R.; Yamamoto, K. Inhibition of antithrombin by hyaluronic acid may be involved in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2005, 7, R268–R273. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Jeon, E.J.; Jeona, J.; Cho, S.W. A serotonin-modified hyaluronic acid hydrogel for multifunctional hemostatic ad-hesives inspired by a platelet coagulation mediator. Mater. Horiz. 2019, 6, 1169. [Google Scholar] [CrossRef]
- Weigel, P.H.; Fuller, G.M.; LeBoeuf, R.D. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J. Theor. Biol. 1986, 119, 219–234. [Google Scholar] [CrossRef]
- Gong, F.; Lu, Y.; Guo, H.; Cheng, S.; Gao, Y. Hyaluronan Immobilized Polyurethane as a Blood Contacting Material. Int. J. Polym. Sci. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Walker, F.D.L.; Baring, D.E.C. Nasal bacterial carriage in adult epistaxis: Is neomycin the answer? J. Laryngol. Otol. 2008, 123, 623–625. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belvedere, R.; Novizio, N.; Eletto, D.; Porta, A.; Bagnulo, A.; Cerciello, A.; Di Maio, U.; Petrella, A. The Procoagulant Activity of Emoxilane®: A New Appealing Therapeutic Use in Epistaxis of the Combination of Sodium Hyaluronate, Silver Salt, α-tocopherol and D-panthenol. Life 2021, 11, 992. https://doi.org/10.3390/life11090992
Belvedere R, Novizio N, Eletto D, Porta A, Bagnulo A, Cerciello A, Di Maio U, Petrella A. The Procoagulant Activity of Emoxilane®: A New Appealing Therapeutic Use in Epistaxis of the Combination of Sodium Hyaluronate, Silver Salt, α-tocopherol and D-panthenol. Life. 2021; 11(9):992. https://doi.org/10.3390/life11090992
Chicago/Turabian StyleBelvedere, Raffaella, Nunzia Novizio, Daniela Eletto, Amalia Porta, Antonino Bagnulo, Andrea Cerciello, Umberto Di Maio, and Antonello Petrella. 2021. "The Procoagulant Activity of Emoxilane®: A New Appealing Therapeutic Use in Epistaxis of the Combination of Sodium Hyaluronate, Silver Salt, α-tocopherol and D-panthenol" Life 11, no. 9: 992. https://doi.org/10.3390/life11090992
APA StyleBelvedere, R., Novizio, N., Eletto, D., Porta, A., Bagnulo, A., Cerciello, A., Di Maio, U., & Petrella, A. (2021). The Procoagulant Activity of Emoxilane®: A New Appealing Therapeutic Use in Epistaxis of the Combination of Sodium Hyaluronate, Silver Salt, α-tocopherol and D-panthenol. Life, 11(9), 992. https://doi.org/10.3390/life11090992








