Declining Mortality Rate of Hospitalised Patients in the Second Wave of the COVID-19 Epidemics in Italy: Risk Factors and the Age-Specific Patterns
Abstract
:1. Introduction
2. Materials and Methods
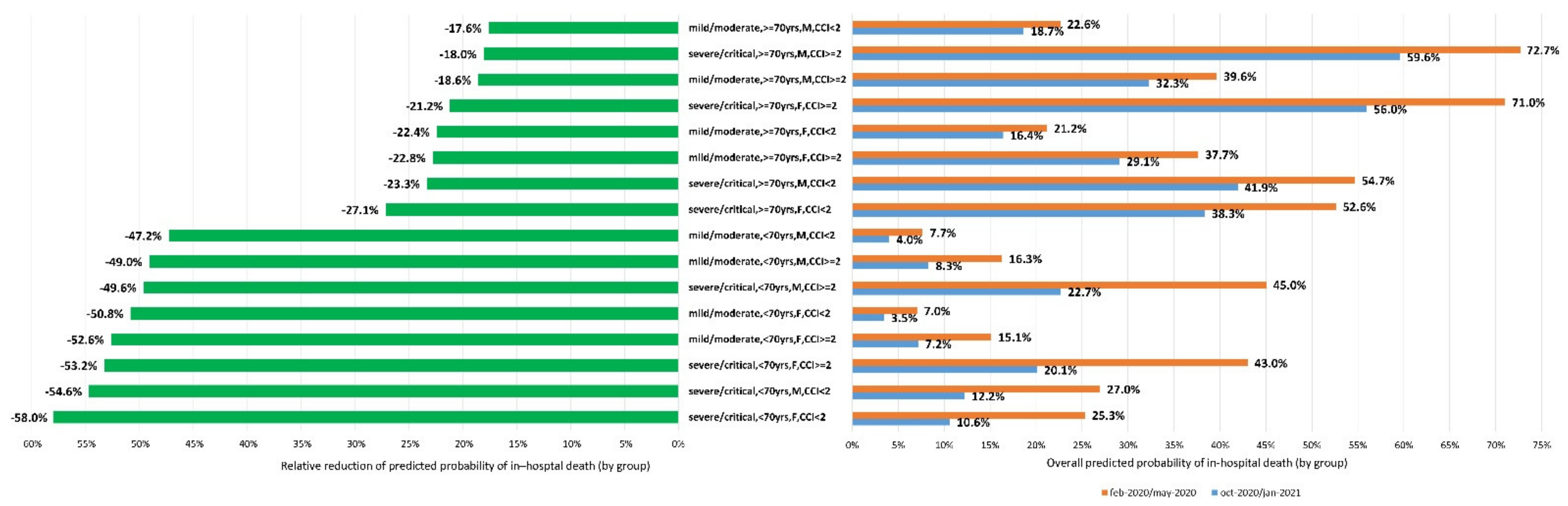
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Covid19. Available online: www.who.int (accessed on 26 July 2021).
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID 19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- ECDC. COVID-19; Communicable disease threats report; Week 36, 5–11 September 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-5-11-september-week-36 (accessed on 15 September 2021).
- Ministero Della Salute. Available online: https://www.salute.gov.it/portale/nuovocoronavirus/dettaglioNotizieNuovoCoronavirus.jsp?lingua=italiano&menu=notizie&p=dalministero&id=4648 (accessed on 26 July 2021).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chron. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of Covid-19- Preliminary report. N. Engl. J. Med. 2020, 383, 1813–1836. [Google Scholar] [CrossRef] [PubMed]
- The Recovery Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, F.; Castagna, A.; Rovere-Querini, P.; de Cobelli, F.; Ruggeri, A.; Galli, L.; Conte, C.; de Lorenzo, R.; Poli, A.; Ambrosio, A.; et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin. Immunol. 2020, 217, 108509. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Wolf, E. Older age and country-specific case fatality rates of COVID-19 in Europe, USA and Canada. Infection 2021, 49, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Noroozi, R.; Vafaee, R.; Branicki, W.; Poṡpiech, E.; Pyrc, K.; Łabaj, P.P.; Omrani, M.D.; Taheri, M.; Sanak, M. Effects of host genetic variations on response to, susceptibility and severity of respiratory infections. Biomed. Pharmacother. 2020, 128, 110296. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, C.; Li, X.; Song, Y.; Yao, X.; Wu, X.; Duan, Y.; Zhang, H.; Wang, Y.; Qian, Z.; et al. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020, 7, 1012–1023. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, S.S.; Kaur, U.; Banerjee, A.; Ganguly, U.; Banerjee, T.; Saha, S.; Parashar, G.; Prasad, S.; Chakrabarti, S.; Mittal, A.; et al. COVID-19 in India: Are biological and environmental factors helping to stem the incidence and severity? Aging Dis. 2020, 11, 480–488. [Google Scholar] [CrossRef] [PubMed]
- D’Arminio Monforte, A.; Tavelli, A.; Bai, F.; Tomasoni, D.; Falcinella, C.; Castoldi, R.; Barbanotti, D.; Mulè, G.; Allegrini, M.; Tesoro, D.; et al. The importance of patients’ case-mix for the correct interpretation of the hospital fatality rate in COVID-19 disease. Int. J. Infect. Dis. 2020, 100, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mussini, C.; Cozzi-Lepri, A.; Menozzi, M.; Meschiari, M.; Franceschini, E.; Rogati, C.; Cuomo, G.; Bedini, A.; Iadisernia, V.; Volpi, S.; et al. Better prognosis in females with severe COVID-19 pneumonia: Possible role of inflammation as potential mediator. Clin. Microbiol. Infect. 2021, 27, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Volgman, A.S.; Michos, E.D. Sex differences in mortality from COVID-19 pandemic: Are men vulnerable and women protected? JACC Case Rep. 2020, 2, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Reardon, S. How the Delta variant achieves its ultrafast spread. Nature, 21 July 2021. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High prevalence of obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
| February 2020–June 2020 | October 2020–January 2021 | Total | p | ||||
|---|---|---|---|---|---|---|---|
| N = 556 (35.6) | N = 1005 (64.4) | N = 1561 (100.0) | |||||
| Age, years, median (IQR) | 66 | 55–8 | 72 | 57–81 | 70 | 56–81 | 0.001 |
| Age, >70 years, n (%) | 249 | 44.8 | 547 | 54.0 | 796 | 51.0 | <0.001 |
| Sex, Male | 356 | 64.0 | 635 | 63.2 | 991 | 63.5 | 0.740 |
| Italian | 442 | 79.5 | 808 | 81.2 | 1250 | 80.6 | 0.414 |
| Ethnicity | 0.044 | ||||||
| Caucasian | 460 | 82.7 | 836 | 83.9 | 1296 | 83.5 | |
| Latin/Hispanic | 45 | 8.1 | 60 | 6 | 105 | 6.8 | |
| Black | 8 | 1.44 | 4 | 0.4 | 12 | 0.8 | |
| Asian | 16 | 2.9 | 45 | 4.5 | 61 | 3.9 | |
| Other | 27 | 4.9 | 52 | 5.2 | 79 | 5.1 | |
| Epidemiology, n (%) | <0.001 | ||||||
| Close contact | 84 | 15.1 | 50 | 5 | 134 | 8.6 | |
| Healthcare workers | 43 | 7.7 | 10 | 1 | 53 | 3.4 | |
| Hospitalisation | 31 | 5.6 | 171 | 17 | 202 | 12.9 | |
| Long-term care facility | 64 | 11.5 | 48 | 4.8 | 112 | 7.2 | |
| Other/Unknown | 334 | 60.1 | 726 | 72.2 | 1060 | 67.9 | |
| Smoking, n (%) | <0.001 | ||||||
| Never smoker | 54 | 9.7 | 33 | 3.3 | 87 | 5.6 | |
| Former smoker | 57 | 10.3 | 72 | 7.1 | 57 | 72 | |
| Actual smoker | 13 | 2.3 | 47 | 4.7 | 60 | 3.8 | |
| Unknown | 432 | 77.7 | 853 | 84.9 | 1285 | 82.3 | |
| Obesity, n (%) | <0.001 | ||||||
| No | 206 | 37.1 | 139 | 13.8 | 345 | 22.1 | |
| Yes | 89 | 16 | 107 | 10.7 | 196 | 12.6 | |
| Unknown | 261 | 46.9 | 759 | 75.5 | 1020 | 65.3 | |
| BMI, kg/m2, median (IQR) | 27.4 | 23.9–31.5 | 26.2 | 23.5–30.7 | 26.7 | 23.7–31.2 | 0.194 |
| Hypertension, n (%) | 260 | 46.8 | 514 | 51.1 | 774 | 49.6 | 0.097 |
| Stroke, n (%) | 49 | 8.8 | 99 | 9.9 | 148 | 9.5 | 0.503 |
| CPD, n (%) | 48 | 8.6 | 97 | 9.7 | 145 | 9.3 | 0.507 |
| IMA, n (%) | 72 | 13 | 175 | 17.4 | 247 | 15.8 | 0.021 |
| Diabetes, n (%) | 100 | 18 | 224 | 22.3 | 324 | 20.8 | 0.045 |
| Cerebrovascular diseases, n (%) | 49 | 8.8 | 99 | 9.9 | 148 | 9.5 | 0.503 |
| Cardiovascular diseases n (%) | 154 | 27.7 | 341 | 33.9 | 495 | 31.7 | 0.011 |
| COPD/Asthma, n (%) | 79 | 14.2 | 137 | 13.6 | 216 | 13.8 | 0.752 |
| Cancer (last 5 years), n (%) | 39 | 7 | 88 | 8.8 | 127 | 8.1 | 0.228 |
| CKD, n (%) | 44 | 7.9 | 84 | 8.4 | 128 | 8.2 | 0.759 |
| Rheumatological Diseases, n (%) | 15 | 2.7 | 12 | 1.2 | 27 | 1.7 | 0.029 |
| Peripheric vascular diseases, n (%) | 53 | 9.5 | 63 | 6.3 | 116 | 7.4 | 0.019 |
| HIV, n (%) | 4 | 0.7 | 11 | 1.1 | 15 | 1 | 0.467 |
| Chronic liver disease, n (%) | 20 | 3.6 | 44 | 4.9 | 64 | 4.1 | 0.456 |
| Age Unadjusted Charlson score, median (IQR) | 0 | 0–2 | 1 | 0–2 | 1 | 0–2 | 0.003 |
| Age Adjusted Charlson score, median (IQR) | 3 | 1–5 | 4 | 2–5 | 3 | 1–5 | <0.001 |
| Signs and symptoms at admission | |||||||
| Fever | 475 | 85.4 | 698 | 69.5 | 1173 | 75.1 | <0.001 |
| Dyspnoea | 308 | 55.4 | 492 | 49 | 800 | 51.3 | 0.015 |
| Cough | 276 | 50 | 332 | 33 | 608 | 39 | <0.001 |
| Fatigue | 89 | 16 | 150 | 14.9 | 239 | 15.3 | 0.570 |
| GI Symptoms | 80 | 14.4 | 115 | 11.4 | 195 | 12.5 | <0.001 |
| Erythromelalgia | 29 | 5.2 | 45 | 4.5 | 74 | 4.7 | 0.511 |
| Chest pain | 26 | 4.7 | 36 | 3.6 | 62 | 4 | 0.289 |
| Anosmia/dysgeusia | 18 | 3.2 | 57 | 5.67 | 75 | 4.8 | 0.031 |
| Syncope/Pre-syncope | 10 | 1.8 | 48 | 4.8 | 58 | 3.72 | 0.003 |
| COVID Severity at admission, n (%) | <0.001 | ||||||
| No pneumonia | 31 | 5.6 | 117 | 11.6 | 148 | 9.5 | |
| Mild | 254 | 45.7 | 394 | 39.2 | 648 | 41.5 | |
| Severe | 253 | 45.5 | 468 | 46.6 | 721 | 46.2 | |
| Critical | 18 | 3.2 | 26 | 2.6 | 44 | 2.8 | |
| Respiratory rate at admission, breaths/min, median (IQR) | 24 | 20–29 | 20 | 18–24 | 22 | 18–26 | <0.001 |
| X-ray or CT scan signs of pneumonia | 514 | 92.4 | 804 | 80 | 1318 | 84.4 | <0.001 |
| Highest grade of O2 therapy/ventilation during hospitalisation | <0.001 | ||||||
| Invasive mechanical ventilation (IMV) | 69 | 12.4 | 32 | 3.2 | 101 | 6.5 | |
| Non-invasive mechanical Ventilation (NIV) | 52 | 9.4 | 63 | 6.3 | 115 | 7.4 | |
| Continuous positive airway pressure (CPAP) | 157 | 28.2 | 273 | 27.2 | 430 | 27.6 | |
| O2 low/high flows | 207 | 37.2 | 479 | 47.7 | 686 | 44 | |
| No O2 therapy | 71 | 12.8 | 158 | 15.7 | 229 | 14.7 | |
| ICU admission, n (%) | 72 | 13 | 38 | 3.8 | 110 | 7.1 | <0.001 |
| Laboratory parameters | |||||||
| Haemoglobin/dL, median (IQR) | 13.5 | 12.1–14.8 | 13.3 | 11.7–14.5 | 13.3 | 11.8–14.6 | 0.027 |
| CRP, mg/L, median (IQR) | 60 | 26.8–102.2 | 53.4 | 22.3–92.9 | 54.9 | 24–95.6 | <0.001 |
| LDH, U/L, median (IQR) | 296 | 229–393 | 290 | 231–385 | 292 | 230–389 | 0.614 |
| Leukocytes count, 103/uL, median (IQR) | 6.58 | 4.93–9.18 | 7.31 | 5.26–10.11 | 7 | 5.13–9.69 | 0.002 |
| Lymphocyte count, 103/uL, median (IQR) | 1.02 | 0.68–1.37 | 0.98 | 0.69–1.4 | 0.99 | 0.68–1.38 | 0.719 |
| Neutrophil count, 103/uL, median (IQR) | 4.76 | 3.35–7.44 | 5.34 | 3.64–8.12 | 3.15 | 3.53–7.85 | 0.002 |
| Monocyte count, 103/uL, median (IQR) | 0.46 | 0.31–0.65 | 0.5 | 0.34–0.73 | 0.49 | 0.33–0.71 | 0.004 |
| Platlets,103/uL, median (IQR) | 204 | 158–263 | 208 | 161–266 | 206 | 160–265 | 0.443 |
| Creatine phosphokinase, U/L, median (IQR) | 94 | 53–185 | 82 | 51–159 | 86 | 52–166 | 0.052 |
| D-Dimer,ng/mL, median (IQR) | 413 | 247–865 | 350 | 218–660 | 364 | 226–724 | 0.002 |
| ALT, U/L, median (IQR) | 29 | 20–49 | 26 | 18–44 | 27 | 19–45 | 0.009 |
| AST, U/L, median (IQR) | 41 | 30.5–60 | 39 | 29–56 | 40 | 30–57 | 0.074 |
| Creatinine, mg/dL, median (IQR) | 0.9 | 0.7–1.2 | 0.9 | 0.7–1.2 | 0.9 | 0.7–1.2 | |
| Procalcitonin, ng/mL, median (IQR) | 0.18 | 0.07–0.85 | 0.15 | 0.05–0.45 | 0.16 | 0.05–0.56 | 0.031 |
| Ferritin, ng/mL, median (IQR) | 447 | 219–858 | 392 | 164–905 | 419 | 180–872 | 0.119 |
| Days from symptoms onset and hospitalisation, median (IQR) | 7 | 3–10 | 5 | 2–7 | 5 | 3–8 | <0.001 |
| Pharmacological treatments | |||||||
| Azithromycin, n (%) | 168 | 30.2 | 25 | 2.5 | 193 | 12.4 | <0.001 |
| Lopinavir/r or Darunavir/c, n (%) | 133 | 23.9 | 0 | 0 | 133 | 8.5 | <0.001 |
| Hydroxychloroquine, n (%) | 434 | 78.1 | 1 | 0.1 | 435 | 27.9 | <0.001 |
| Remdesivir, n (%) | 9 | 1.6 | 206 | 20.5 | 215 | 13.8 | <0.001 |
| Heparin prophylaxis, n (%) | 360 | 64.8 | 784 | 78 | 1144 | 73.3 | <0.001 |
| Corticosteroid treatment, n (%) | 127 | 22.8 | 719 | 71.5 | 846 | 54.2 | <0.001 |
| Biological (Tocilizumab, Sarilumab), n (%) | 44 | 7.9 | 29 | 2.9 | 73 | 4.7 | <0.001 |
| Days of Hospitalisation, median (IQR) | 10 | 6–21 | 10 | 6–19 | 10 | 6–20 | 0.304 |
| In-hospital death, n (%) | 178 | 32 | 245 | 24.4 | 423 | 27.1 | <0.001 |
| Unadjusted | Model1 (Without Drugs) | Model2 (With Drugs) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SHR | 95% CI | p | aSHR | 95% CI | p | aSHR | 95% CI | p | ||||
| Age, per 10 years older | 1.73 | 1.61 | 1.85 | <0.001 | 1.66 | 1.52 | 1.82 | <0.001 | 1.67 | 1.52 | 1.83 | <0.001 |
| Sex, male (vs. female) | 1.08 | 0.89 | 1.32 | 0.438 | 1.24 | 1.00 | 1.54 | 0.045 | 1.26 | 1.01 | 1.56 | 0.040 |
| Obesity (BMI > 30 kg/m2) | ||||||||||||
| No | 1.00 | 1.00 | 1.00 | |||||||||
| Yes | 1.39 | 0.98 | 1.96 | 0.064 | 1.50 | 1.04 | 2.15 | 0.029 | 1.47 | 1.02 | 2.11 | 0.038 |
| Unknown | 1.44 | 1.12 | 1.86 | 0.005 | 1.33 | 1.01 | 1.74 | 0.040 | 1.30 | 0.99 | 1.70 | 0.060 |
| Charlson age unadjusted index | ||||||||||||
| 0 | 1.00 | 1.00 | 1.00 | |||||||||
| 1 | 2.61 | 2.00 | 3.42 | <0.001 | 1.83 | 1.39 | 2.42 | <0.001 | 1.85 | 1.40 | 2.44 | <0.001 |
| 2 | 2.79 | 2.05 | 3.80 | <0.001 | 1.79 | 1.28 | 2.48 | 0.001 | 1.79 | 1.29 | 2.49 | 0.001 |
| ≥3 | 4.50 | 3.48 | 5.81 | <0.001 | 2.35 | 1.76 | 3.12 | <0.001 | 2.30 | 1.72 | 3.07 | <0.001 |
| LDH >300 U/L (vs ≤ 300) | 1.99 | 1.62 | 2.45 | <0.001 | 1.52 | 1.21 | 1.91 | <0.001 | 1.51 | 1.20 | 1.90 | <0.001 |
| Lymphocyte < 1.00 103/uL (vs ≥ 1.000) | 1.95 | 1.60 | 2.39 | <0.001 | 1.27 | 1.03 | 1.66 | 0.027 | 1.29 | 1.03 | 1.61 | 0.024 |
| CRP > 60 mg/L (vs ≤ 60) | 2.80 | 2.29 | 3.43 | <0.001 | 1.80 | 1.44 | 2.25 | <0.001 | 1.80 | 1.43 | 2.25 | <0.001 |
| D-dimer > 1.000 ng/mL (vs.≤1.000) | 2.27 | 1.80 | 2.85 | <0.001 | 1.28 | 1.00 | 1.65 | 0.049 | 1.28 | 1.00 | 1.64 | 0.053 |
| Severity | ||||||||||||
| mild/moderate | 1.00 | 1.00 | 1.00 | |||||||||
| severe | 2.75 | 2.23 | 3.39 | <0.001 | 1.96 | 1.56 | 2.46 | <0.001 | 1.98 | 1.57 | 2.50 | <0.001 |
| critical | 9.09 | 6.07 | 13.60 | <0.001 | 5.19 | 3.43 | 7.86 | <0.001 | 5.18 | 3.45 | 7.77 | <0.001 |
| anti-COVID-19 regimen | ||||||||||||
| None | 1.00 | 1.00 | ||||||||||
| Immunomodulators only | 1.0481 | 0.53 | 2.07 | 0.892 | 0.66 | 0.30 | 1.49 | 0.320 | ||||
| Immunomodulators + Corticosteroids + Remdesivir | 0.5717 | 0.16 | 2.04 | 0.388 | 0.88 | 0.24 | 3.18 | 0.841 | ||||
| Immunomodulators + Corticosteroids | 1.3293 | 0.75 | 2.35 | 0.327 | 1.24 | 0.66 | 2.32 | 0.505 | ||||
| Remdesivir | 0.4228 | 0.14 | 1.28 | 0.127 | 0.47 | 0.15 | 1.48 | 0.197 | ||||
| Corticosteroids | 1.0591 | 0.86 | 1.30 | 0.586 | 0.98 | 0.76 | 1.28 | 0.902 | ||||
| Corticosteroids + Remdesivir | 0.6977 | 0.50 | 0.98 | 0.036 | 0.78 | 0.53 | 1.14 | 0.195 | ||||
| Waves | ||||||||||||
| March 2020–May 2020 | 1.00 | 1.00 | 1.00 | |||||||||
| October 2020–January 2021 | 0.71 | 0.58 | 0.86 | <0.001 | 0.59 | 0.48 | 0.74 | <0.001 | 0.61 | 0.47 | 0.78 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Arminio Monforte, A.; Tavelli, A.; Bai, F.; Tomasoni, D.; Falcinella, C.; Castoldi, R.; Barbanotti, D.; Mulè, G.; Allegrini, M.; Suardi, E.; et al. Declining Mortality Rate of Hospitalised Patients in the Second Wave of the COVID-19 Epidemics in Italy: Risk Factors and the Age-Specific Patterns. Life 2021, 11, 979. https://doi.org/10.3390/life11090979
D’Arminio Monforte A, Tavelli A, Bai F, Tomasoni D, Falcinella C, Castoldi R, Barbanotti D, Mulè G, Allegrini M, Suardi E, et al. Declining Mortality Rate of Hospitalised Patients in the Second Wave of the COVID-19 Epidemics in Italy: Risk Factors and the Age-Specific Patterns. Life. 2021; 11(9):979. https://doi.org/10.3390/life11090979
Chicago/Turabian StyleD’Arminio Monforte, Antonella, Alessandro Tavelli, Francesca Bai, Daniele Tomasoni, Camilla Falcinella, Roberto Castoldi, Diletta Barbanotti, Giovanni Mulè, Marina Allegrini, Elisa Suardi, and et al. 2021. "Declining Mortality Rate of Hospitalised Patients in the Second Wave of the COVID-19 Epidemics in Italy: Risk Factors and the Age-Specific Patterns" Life 11, no. 9: 979. https://doi.org/10.3390/life11090979
APA StyleD’Arminio Monforte, A., Tavelli, A., Bai, F., Tomasoni, D., Falcinella, C., Castoldi, R., Barbanotti, D., Mulè, G., Allegrini, M., Suardi, E., Tesoro, D., Tagliaferri, G., Mondatore, D., Augello, M., Cona, A., Beringheli, T., Gemignani, N., Sala, M., Varisco, B., ... Marchetti, G. (2021). Declining Mortality Rate of Hospitalised Patients in the Second Wave of the COVID-19 Epidemics in Italy: Risk Factors and the Age-Specific Patterns. Life, 11(9), 979. https://doi.org/10.3390/life11090979








