3D Printing—A Cutting Edge Technology for Treating Post-Infarction Patients
Abstract
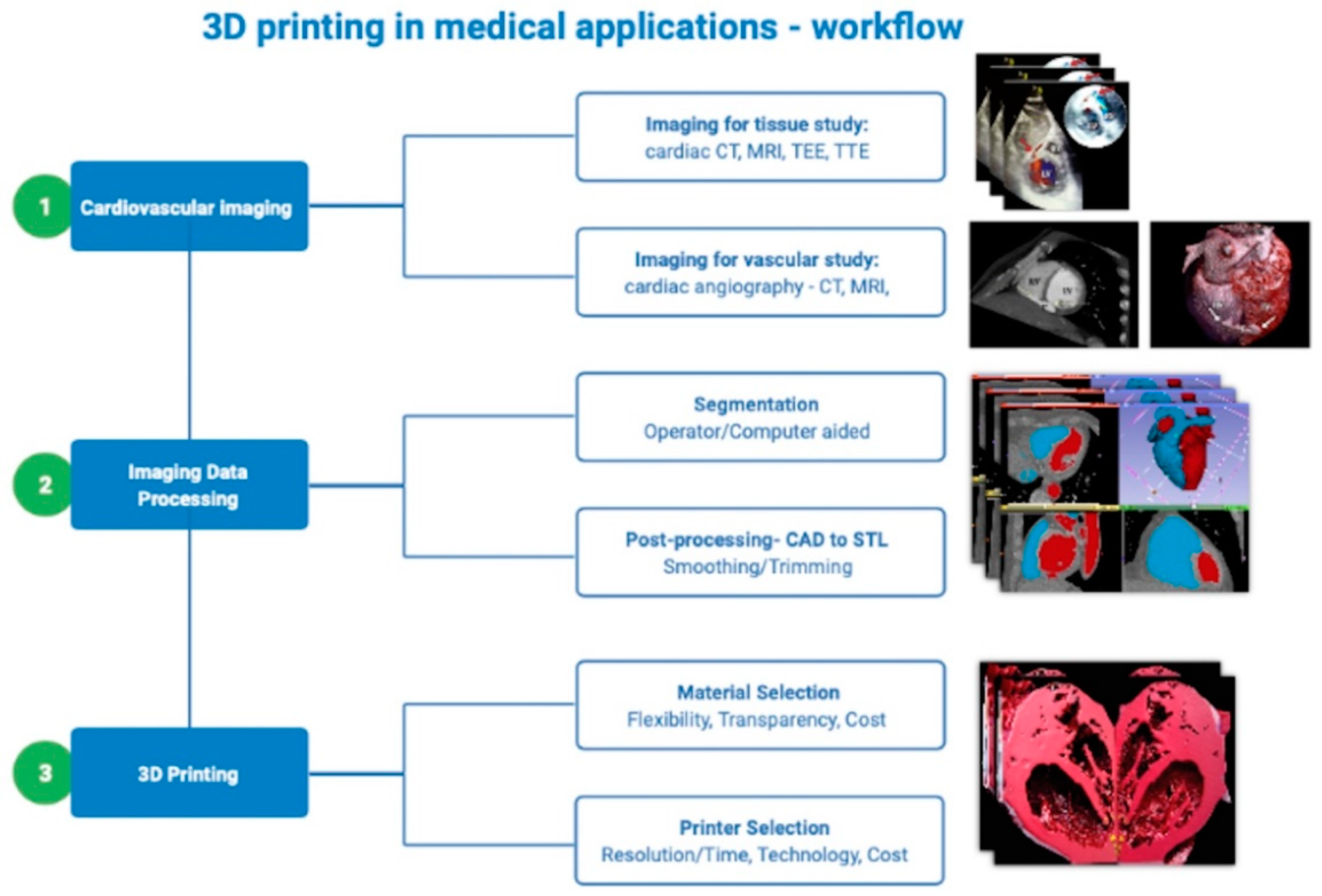
1. 3D Printing in Cardiology—Introduction
2. 3D Printing in Medical Applications—Basic Concepts and Technology
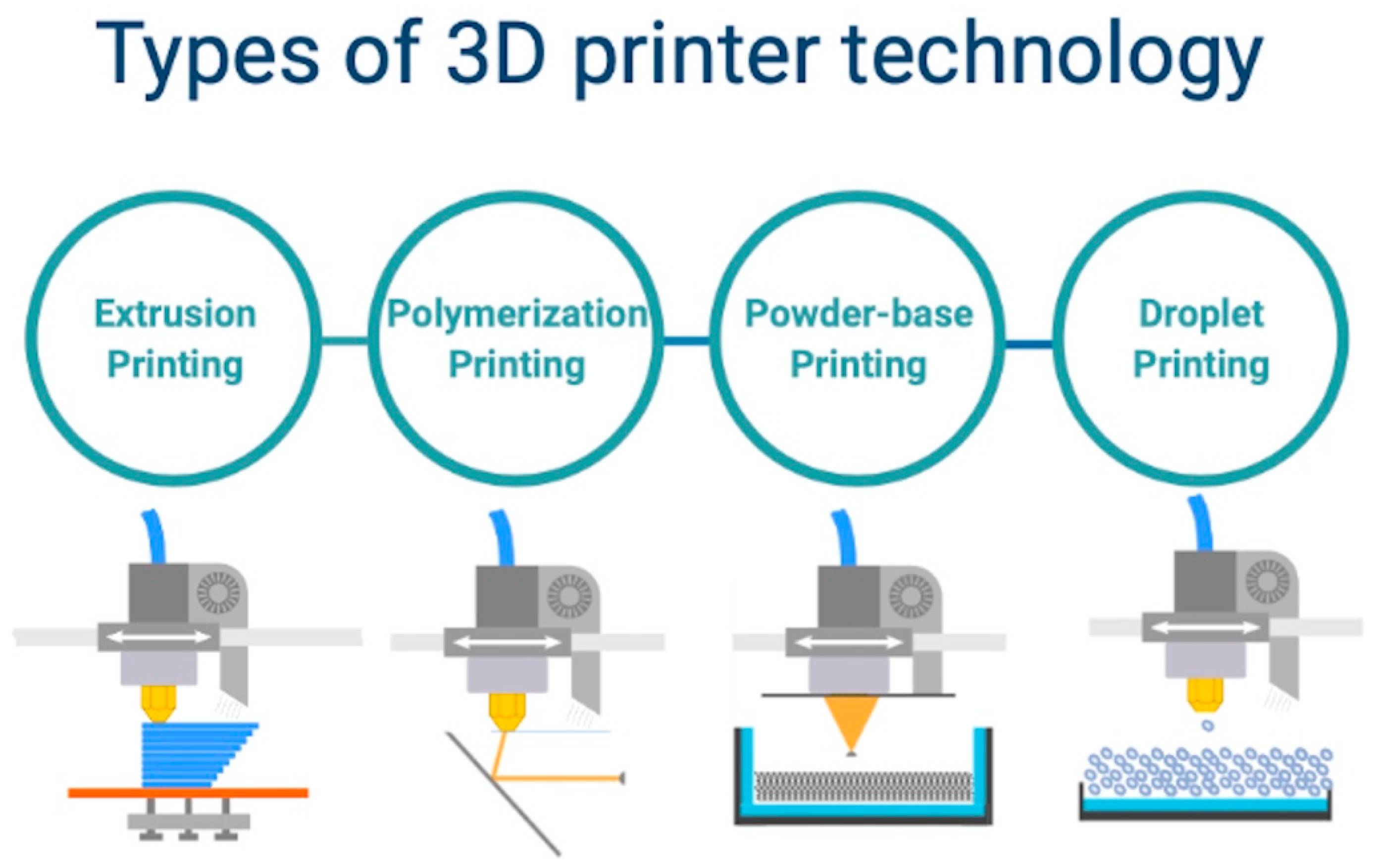
- Printing by using the polymerization method which includes stereolithography and digital light processing; it consists of a laser beam directed to a selected resin material to cure layer by layer [21]; phantoms printed with this technique generally have predictable mechanical properties (isotropic); this method can be used to mimic soft and hard tissues or to fabricate complex anatomical parts, including cardiac valves; the major advantage of this technique consists in the possibility to print complex structures with refined surfaces; however, this method requires higher cost for the used materials and printing machines [22,23].
- Powder-based printing includes selective laser sintering or melting and consist of focal heating beam to fuse the selected powdered materials [24]; materials compatible with this method range from plastic powder to ceramics and metal alloys, which gives it a major advantage; nevertheless, it is considered the most expensive method due to increased costs related to the mechanical parts of the printing system and print materials [25].
- Printing by using the droplet method requires multi-jet modeling with wax or trough laser beam induced forward transfer, which is commonly used to deploy photo-curable resins into layers [26]; the technique usually uses photo-polymer bio-resins to fabricate scaffolds for tissue development; the advantage consists of an augmented printing precision regardless of the model complexity and dimensions; however, it requires an extended time frame for model prototyping [27];
- The extrusion printing method includes a fused deposition and direct ink writing modeling, by using a thermo-sensitive plastic or ink materials, which are delivered through a special printhead, layer by layer on a dedicated support area [28]; the use of thermoplastic ink or hydrogels facilitates the fabrication of anisotropic models with increased density, that are able to resist high loads and increased strain; despite having a lower precision, this method uses the lowest costs in terms of materials and printing machines [29].
3. Selection of Materials for 3D Printing
3.1. Rigid Materials
3.2. Flexible Materials
3.3. Printing with Multiple Materials
4. Clinical Applications of 3D Printing in Cardiology
5. 3D Printing to Understand Complex Anatomy and Procedural Planning
6. 3D Printing for Procedural Simulation
7. 3D Printing for Modeling of Patient-Specific Anatomy
8. 3D Printing to Assist Interventional Procedures
9. 3D Printing for Computational Flow Dynamics Simulation
10. 3D Printing for Bioprinting
| First Author | Clinical Application | Supportive Material | Printing Method | Year | Country | Objective | Results |
|---|---|---|---|---|---|---|---|
| Gozde B. et al. [88] | Conductive composite in treatment of myocardial infarction | Titanium carbide mxene | Aerosol Jet Printing | 2020 | USA | To investigate cardiac patches as conductive myocardial tissue | Increase of MYH7, SERCA2, TNNT2 expression and conductive velocity |
| Izadifar M. et al. [89] | Intracoronary hybrid cardiac patch in ischemic heart disease | Methacrylated collagen | UV-integrated 3D-bioprinting-hydrogel | 2017 | Canada | To investigate hydrogel carboxyl functionalized carbon nanotubes | 3D-bioprinted nanotubes presented significant cellular proliferation, migration and differentiation incubation time |
| Yeung E. et al. [91] | Regenerative patch in heart failure | Biomaterial-free | Monolayer bioprint on needle array | 2019 | USA | To investigate bioprinted patch in vivo scaffold free | Increased the average vessel counts patch group; the scar area in the cardiac patch group was significantly smaller |
| Gaetani R. et al. [92] | Role of epicardial tissue in myocardial infarction | Gelatin and hyaluronic acid matrix (hystem matrix) | Bioscaffolder-ink jet | 2015 | Netherlands | To evaluate the role of a 3D-printed patch composed of human cardiac-derived progenitor cells in a hyaluronic acid/gelatin matrix | Reduction in remodeling and preservation of cardiac performance and increase in cardiac and vascular differentiation at four weeks follow up |
| Jang J. et al. [93] | Treatment of ischemic heart disease | Decellularized extracellular matrix bioinks | Bio-ink printing | 2016 | Korea | To evaluate 3D pre-vascularized stem cell patch of cardiac remodeling and fibrosis, and promotional effects of cardiomyogenesis and neovascularization | Reduction of cardiac remodeling and fibrosis by modulating inflammation, apoptosis, and cardiac metabolism; increasing of neovascularization |
| Gao L. et al. [94] | Treatment of ischemic heart disease | Photoactive gelatin polymer | Multiphoton-excited photochemistry printing | 2018 | USA | To evaluate bioprinted scaffold with cardiomyocytes, smooth muscle cells, and endothelial cells in a murine model of myocardial infarction | The scaffold promoted cell viability and electromechanical coupling in vitro, high levels of cell engraftment, as well as significant improvements in cardiac function, infarct size, apoptosis, vascularity, and cell proliferation in a murine MI model |
| Wang Z. et al. [95] | Heart failure treatment | Fibrin-based composite hydrogel | Bioink hydrogel | 2018 | USA | Develop a functional cardiac tissue in heart failure | Bioprinted cardiac tissue is not structural and functional like native tissue after printing, instead the tissues are matured after three to four weeks in culture |
| Park S. et al. [96] | Treating coronary thrombosis | Polycaprolactone mixed with poly-lactide-co-glycolide and polyethylene glycol | Rotational 3D printing | 2014 | Korea | To evaluate feasibility of 3D-printed biocompatible and biodegradable stent | It showed a reduction of neointimal hyperplasia, the inflammatory cells and fibrin infiltrated around the struts |
11. Future Directions
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farooqi, K.; Cooper, C. 3D Printing and Heart Failure: The Present and the Future. JACC Heart Fail. 2019, 7, 132–142. [Google Scholar] [CrossRef]
- Giannopoulos, A.A. Cardiothoracic applications of 3-dimensional printing. J. Thorac. Imaging 2016, 31, 253–272. [Google Scholar] [CrossRef]
- Kim, M.S.; Hansgen, A.R.; Carroll, J. Rapid prototyping: A new tool in understanding and treating structural heart disease. Circulation 2008, 117, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.R. A novel approach to neonatal management of tetralogy of Fallot, with pulmonary atresia, and multiple aortopulmonary collaterals. JACC Cardiovasc. Imaging 2015, 8, 103–104. [Google Scholar] [CrossRef]
- Schmauss, D.; Haeberle, S. Three-dimensional printing in cardiac surgery and interventional cardiology: A single-centre experience. Eur. J. Cardiothorac. Surg. 2015, 47, 1044–1052. [Google Scholar] [CrossRef]
- Pellegrino, P.L.; Fassini, G.; Di Biase, M. Left atrial appendage closure guided by 3D-printed cardiac reconstruction: Emerging directions and future trends. J. Cardiovasc. Electrophysiol. 2016, 27, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H. Myocardial 3-dimensional printing for septal myectomy guidance in a patient with obstructive hypertrophic cardiomyopathy. Circulation 2015, 132, 300–301. [Google Scholar] [CrossRef]
- Mashari, A. Hemodynamic testing of patient-specific mitral valves using a pulse duplicator: A clinical application of three-dimensional printing. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1278–1285. [Google Scholar] [CrossRef]
- Sulkin, M.S. Three-dimensional printing physiology laboratory technology. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1569–H1573. [Google Scholar] [CrossRef]
- Biglino, G. 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: Feasibility and acceptability. BMJ Open 2015, 5, e007165. [Google Scholar] [CrossRef]
- Costello, J. Incorporating three-dimensional printing into a simulation-based congenital heart disease and critical care training curriculum for resident physicians. Congenit. Heart Dis. 2015, 10, 185–190. [Google Scholar] [CrossRef]
- Wurm, G.; Tomancok, B. Cerebrovascular stereolithographic biomodeling for aneurysm surgery. J. Neurosurg. 2004, 100, 139–145. [Google Scholar] [CrossRef]
- Farooqi, K.M.; Nielsen, J.C. Use of 3-dimensional printing to demonstrate complex intracardiac relationships in double-outlet right ventricle for surgical planning. Circ. Cardiovasc. Imaging 2015, 8, e003043. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.; Domingos, M.A. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Parimi, M.; Buelter, J. Feasibility and validity of printing 3d heart models from rotational angiography. Pediatr. Cardiol. 2018, 39, 653–658. [Google Scholar] [CrossRef]
- Mitsouras, D. Medical 3D printing for the radiologist. Radiographics 2015, 35, 1965–1988. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Gheewala, N. Three-Dimensional Printing for Planning of Structural Heart Interventions. Interv. Cardiol. Clin. 2018, 7, 415–423. [Google Scholar] [CrossRef]
- Wang, K.; Wu, C. Dual-material 3D-printed metamaterials with tunable mechanical properties for patient-specific tissue-mimicking phantoms. Addit. Manuf. 2016, 12, 31–37. [Google Scholar] [CrossRef]
- Kim, G.B. Three-dimensional printing: Basic principles and applications in medicine and radiology. Korean J. Radiol. 2016, 17, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Vandenhaute, M. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Wong, K.V.; Hernandez, A. A review of additive manufacturing. ISRN Mech. Eng. 2012, 1–10. [Google Scholar] [CrossRef]
- Castilho, M.D.; Malda, J.; Levato, R. Bio-resin for high resolution lithography-based biofabrication of complex cell-laden constructs. Biofabrication 2018, 10, 034101. [Google Scholar]
- Carve, M.; Wlodkowic, D. 3D-Printed Chips: Compatibility of Additive Manufacturing Photopolymeric Substrata with Biological Applications. Micromachines 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.Y. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Rahman, Z.; Barakh Ali, S.F.; Ozkan, T.; Charoo, N.A.; Reddy, I.K.; Khan, M.A. Additive Manufacturing with 3D Printing: Progress from Bench to Bedside. AAPS J. 2018, 20, 101. [Google Scholar] [CrossRef]
- Do, A.V.; Khorsand, B. 3D printing of scaffolds for tissue regeneration applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Tao, X.; Damon, B.J.; Manley, B.; Kesari, P.; Jalota, S.; Bhaduri, S. Drop-on-demand printing of cells and materials for designer tissue constructs. Mater. Sci. Eng. C 2006, 27, 372–376. [Google Scholar] [CrossRef]
- Bohandy, J.; Kim, B.F. Metal deposition from a supported metal !lm using an excimer laser. J. Appl. Phys. 1986, 60, 1538–1539. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Garcia, J.; Yang, Z.; Mongrain, R.; Leask, R.L.; Lachapelle, K. 3D printing materials and their use in medical education: A review of current technology and trends for the future. BMJ Simul. Technol. Enhanc. Learn. 2018, 4, 27–40. [Google Scholar] [CrossRef]
- Chan, H.H.; Siewerdsen, J.H.; Vescan, A. 3D rapid prototyping for otolaryngology-head and neck surgery: Applications in image-guidance, surgical simulation and patient-specific modeling. PLoS ONE 2015, 10, e0136370. [Google Scholar] [CrossRef] [PubMed]
- Ripley, B.; Kelil, T.; Cheezum, M.K. 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2016, 10, 28–36. [Google Scholar] [CrossRef]
- Emmott, A.; Garcia, J.; Chung, J. Biomechanics of the ascending thoracic aorta: A clinical perspective on engineering data. Can. J. Cardiol. 2016, 32, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A. Direct ink writing of 3D functional materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Bramlet, M.; Olivieri, L.; Farooqi, K. Impact of three-dimensional printing on the study and treatment of congenital heart disease. Circ. Res. 2017, 120, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Ngoa, T.D.; Kashania, A.; Imbalzanoa, G.; Nguyena, K.T.Q.; Huib, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Tack, P.; Victor, J. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef]
- Sun, Z.; Lau, I.; Wong, Y.H.; Yeong, C.H. Personalized Three-Dimensional Printed Models in Congenital Heart Disease. J. Clin. Med. 2019, 8, 522. [Google Scholar] [CrossRef]
- Batteux, C. 3D-Printed Models for Surgical Planning in Complex Congenital Heart Diseases: A Systematic Review. Front. Pediatrics 2019, 7, 23. [Google Scholar] [CrossRef]
- Padalino, M.A.; Basso, C. Surgically treated primary cardiac tumors in early infancy and childhood. J. Thorac. Cardiovasc. Surg. 2005, 129, 1358–1363. [Google Scholar] [CrossRef]
- Schmauss, D.; Schmitz, C. Three-dimensional printing of models for preoperative planning and simulation of transcatheter valve replacement. Ann. Thorac. Surg. 2012, 93, e31–e33. [Google Scholar] [CrossRef] [PubMed]
- Sodian, R.; Weber, S. Pediatriccardiac transplantation: Three-dimensional printing of anatomic models for surgical planning of heart transplantation in patients with univentricular heart. J. Thorac. Cardiovasc. Surg. 2008, 136, 1098–1099. [Google Scholar] [CrossRef]
- Bateman, M.; William, L. Cardiac patient–specific three-dimensional models as surgical planning tools. Surgery 2020, 167, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Meier, L.M.; Meineri, M. Structural and congenital heart disease interventions: The role of three-dimensional printing. Neth. Heart J. 2017, 25, e65–e75. [Google Scholar] [CrossRef] [PubMed]
- Hazeveld, A.; Slater, H.J.J.R.; Ren, Y. Accuracy and reproducibility of dental replica models reconstructed by different rapid prototyping techniques. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, I.; Yamagishi, M. Simulative operation on congenital heart disease using rubber- like urethane stereolithographic biomodels based on 3D datasets of multislice computed tomography. Eur. J. Cardiothorac. Surg. 2010, 37, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J.; Van Arsdell, G.S. 3D printing in surgical management of double outlet right ventricle. Front. Pediatr. 2018, 5, 289. [Google Scholar] [CrossRef]
- Motwani, M.; Burley, O.; Luckie, M.; Cunnington, C.; Pisaniello, A.D.; Hasan, R.; Malik, I.; Fraser, D.G. 3D-printing assisted closure of paravalvular leak. J. Cardiovasc. Comput. Tomogr. 2020, 14, e66–e68. [Google Scholar] [CrossRef]
- Engelhardt, S.; Sauerzapf, S.; Preim, B.; Karck, M.; Wolf, I.; De Simone, R. Flexible and comprehensive patient-specific mitral valve silicone models with chordae tendineae made from 3D-printable molds. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Zopf, D.A.; Flanagan, C.L.; Wheeler, M.; Hollister, S.J.; Green, G.E. Treatment of severe porcine tracheomalacia with a 3-dimensionally printed, bioresorbable, external airway splint. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 66–71. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, F.; Cheung, G.S.; Chan, A.K.; Wang, D.D.; Lam, Y.Y.; Chow, M.C.; Leong, M.C.; Kam, K.K.; So, K.C.; et al. Device Sizing Guided by Echocardiography-Based Three-Dimensional Printing Is Associated with Superior Outcome after Percutaneous Left Atrial Appendage Occlusion. J. Am. Soc. Echocardiogr. 2019, 32, 708–719.e1. [Google Scholar] [CrossRef]
- Lazkani, M.; Faran, B. Postinfarct VSD management using 3D computer printing assisted percutaneous closure. Indian Heart J. 2015, 67, 581–585. [Google Scholar] [CrossRef][Green Version]
- Kim, M.S.; Hansgen, A.R.; Carroll, J.D. Use of rapid prototyping in the care of patients with structural heart disease. Trends Cardiovasc. Med. 2008, 18, 210–216. [Google Scholar] [CrossRef]
- Riesenkampff, E.; Rietdorf, U. The practical clinical value of three-dimensional models of complex congenitally mal- formed hearts. J. Thorac. Cardiovasc. Surg. 2009, 138, 571–580. [Google Scholar] [CrossRef]
- Olivieri, L.J.; Krieger, A. Three-dimensional printing of intracardiac defects from three-dimensional echocardiographic images: Feasibility and relative accuracy. J. Am. Soc. Echocardiogr. 2015, 28, 392–397. [Google Scholar] [CrossRef]
- Valverde, I.; Gomez, G. Three-dimensional patient-specific cardiac model for surgical planning in Nikaidoh procedure. Cardiol. Young. 2015, 25, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Penela, D.; Doni, L.; Marazzi, R.; Napoli, V.; Napoli, L. Development of simulation combining a physical heart model and three-dimensional system for electrophysiology training. Pacing Clin. Electrophysiol. 2018, 41, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Valverde, I.; Gomez, G. 3D-printed models for planning endovascular stenting in transverse aortic arch hypo-plasia. Catheter. Cardiovasc. Interv. 2015, 85, 1006–1012. [Google Scholar] [CrossRef]
- Yeazel, T.R.; Becker, M.L. Advancing Towards 3D Printing of Bioresorbable Shape Memory Polymer Stents. Biomacromolecules 2020, 21, 3957–3965. [Google Scholar] [CrossRef]
- Modi, B.N.; Ryan, M.; Chattersingh, A. Optimal Application of Fractional Flow Reserve to Assess Serial Coronary Artery Disease: A 3D-Printed Experimental Study with Clinical Validation. J. Am. Heart Assoc. 2018, 7, e010279. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Santos, M.; Oliveira-Santos, E.; Marinho, A.V.; Leite, L.; Guardado, J.; Matos, V.; Pego, G.M.; Marques, J.S. Patient-specific 3D printing simulation to guide complex coronary intervention. Rev. Port. Cardiol. 2018, 37, 541.e1–541.e4. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Zheng, X. Three-dimensional virtual surgery models for percutaneous coronary intervention (PCI) optimization strategies. Sci. Rep. 2015, 5, 10945. [Google Scholar] [CrossRef] [PubMed]
- Velasco Forte, M.N.; Byrne, N.; Valverde Perez, I.; Bell, A.; Gómez-Ciriza, G.; Krasemann, T.; Sievert, H.; Simpson, J.; Pushparajah, K.; Razavi, R.; et al. 3D-printed models in patients with coronary artery fistulae: Anatomical assessment and interventional planning. Eurointervention 2017, 13, e1080–e1083. [Google Scholar] [CrossRef]
- Sedaghat, A.; Wolpers, A.C. Percutaneous treatment of a saccular coronary artery aneurysm using multimodal imaging and rapid prototyping. Eur. Heart J. 2018, 39, 4125. [Google Scholar] [CrossRef]
- Mohamed, E.; Telila, T.; Osaki, S.; Jacobson, K. Percutaneous closure of left ventricle pseudoaneurysm using 3D-printed heart model for procedure planning: A novel approach. Catheter. Cardiovasc. Interv. 2019, 94, 874–877. [Google Scholar] [CrossRef]
- Bompotis, G.; Meletidou, M.; Karakanas, A.; Sotiriou, S.; Sachpekidis, V.; Konstantinidou, M.; Lazaridis, I. Transcatheter Aortic Valve Implantation using 3-D printing modeling assistance. A single center experience. Hell. J. Cardiol. 2020, 61, 131–132. [Google Scholar] [CrossRef]
- Baribeau, Y.; Sharkey, A.; Mahmood, E.; Feng, R.; Chaudhary, O.; Baribeau, V.; Khabbaz, K. Three-Dimensional Printing and Transesophageal Echocardiographic Imaging of Patient-Specific Mitral Valve Models in a Pulsatile Phantom Model. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Iriart, X.; Ciobotaru, V.; Martin, C.; Cochet, H.; Jalal, Z.; Thambo, J.-B.; Quessard, A. Role of cardiac imaging and three-dimensional printing in percutaneous appendage closure. Arch. Cardiovasc. Dis. 2018, 111, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Lodziński, P.; Balsam, P.; Peller, M. Three-dimensional print facilitated ventricular tachycardia ablation in patient with corrected congenital heart disease. Cardiol. J. 2017, 24, 584–585. [Google Scholar] [CrossRef]
- Randles, A.; Frakes, D.H.; Leopold, J.A. Computational Fluid Dynamics and Additive Manufacturing to Diagnose and Treat. Cardiovascular Disease. Trends Biotechnol. 2017, 35, 1049–1061. [Google Scholar] [CrossRef]
- De Zélicourt, D.; Pekkan, K.; Kitajima, H.; Frakes, D.; Yoganathan, A.P. Single-step stereolithography of complex anatomical models for optical flow measurements. J. Biomech. Eng. 2005, 127, 204–207. [Google Scholar] [CrossRef]
- Taylor, C.A.; Fonte, T.A. Computational Fluid Dynamics Applied to Cardiac Computed Tomography for Noninvasive Quantification of Fractional Flow Reserve. J. Am. Coll. Cardiol. 2013, 61, 2233–2241. [Google Scholar] [CrossRef]
- Esses, S.J.; Berman, P. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am. J. Roentgenol. 2011, 196, W683–W688. [Google Scholar] [CrossRef]
- Maragiannis, D.; Jackson, M.S. Replicating Patient-Specific Severe Aortic Valve Stenosis With Functional 3D Modeling. Circ. Cardiovasc. Imaging 2015, 8, e003626. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, K. Quantitative Prediction of Paravalvular Leak in Transcatheter Aortic Valve Replacement Based on Tissue-Mimicking 3D Printing. JACC Cardiovasc. Imaging 2017, 10, 719–731. [Google Scholar] [CrossRef]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Yu, Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Mahrholdt, H.; Wagner, A. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur. Heart J. 2005, 26, 1461–1474. [Google Scholar] [CrossRef]
- Liao, J.; Huang, W.; Liu, G. Animal models of coronary heart disease. J. Biomed. Res. 2016, 30, 3–10. [Google Scholar] [CrossRef]
- Tanimoto, A.; Kawaguchi, H. Microminipig, a non-rodent experimental animal optimized for life science research: Novel atherosclerosis model induced by high fat and cholesterol diet. J. Pharmacol. Sci. 2011, 115, 115–121. [Google Scholar]
- Beg, S.; Almalki, W.H. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Gear, J.; Craig, S. Radioactive 3D printing for the production of molecular imaging phantoms. Phys. Med. Biol. 2020, 65, 17. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Boland, T. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat. Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Gorenek, B.; Blomström, C. Cardiac arrhythmias in acute coronary syndromes: Position paper from the joint EHRA, ACCA, and EAPCI task force. EP Eur. 2014, 16, 1655–1673. [Google Scholar] [CrossRef]
- Henkel, D.M.; Witt, B.J. Ventricular arrhythmias after acute myocardial infarction: A 20-year community study. Am. Heart J. 2006, 151, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Jung, S.M. Carbon-Nanotube-Embedded Hydrogel Sheets for Engineering Cardiac Constructs and Bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef]
- Basara, G.; Saeidi-Javash, M. Electrically conductive 3D-printed Ti3C2Tx MXene-PEG composite constructs for cardiac tissue engineering. Acta Biomater. 2020, S1742–S7061, 30747–30749. [Google Scholar] [CrossRef]
- Izadifar, M.; Chapman, D. UV-Assisted 3D Bioprinting of Nanoreinforced Hybrid Cardiac Patch for Myocardial Tissue Engineering. Tissue Eng. Part C Methods 2018, 24, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, N.; Haddow, G.; Seymour, T.; Faulkner-Jones, A.; Shu, W. 3D bioprint me: A socioethical view of bioprinting human organs and tissues. J. Med. Ethics. 2017, 43, 618–624. [Google Scholar] [CrossRef]
- Yeung, E.; Fukunishi, T. Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo. J. Tissue Eng. Regen. Med. 2019, 13, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, R.; Feyen, D.A. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339–348. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.J. 3D-printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Gao, L.; Kupfer, M.E. Myocardial Tissue Engineering with Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circ. Res. 2017, 120, 1318–1325. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, S.J. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Sang, J.L. In vivo evaluation and characterization of a bio-absorbable drug-coated stent fabricated using a 3D-printing system. Mater. Lett. 2015, 141, 355–358. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. PT Peer-Rev. J. Formul. Manag. 2014, 39, 704–711. [Google Scholar]
- Hoy, M.B. 3D printing: Making things at the library. Med. Ref. Serv. Q 2013, 32, 94–99. [Google Scholar] [CrossRef]

| First Author | Clinical Application | Imaging Modality | 3D-Printed Material | Printing Method | Year | Country | Objective | Results |
|---|---|---|---|---|---|---|---|---|
| Lazkani M. et al. [52] | Postinfarct VSD treatment | Computed tomography Angiography | Gypsum-cyanoacrylate | Stereolithography | 2015 | USA | To evaluate the role of 3D-printed occluder in guided percutaneous treatment of complex PIVSD | 3D printing model guide size and choice of septal occluder with no residual shunt |
| Modi B. et al. [60] | Assess Coronary Artery Disease | Coronary angiogram | Photopolymer | Ink Jet | 2018 | United Kingdom | Three-dimensional-printing to characterize serial stenosis interplay in vitro | Generated and tested a mathematical equation to improve estimation of the true physiological impact of each stenosis, using measurements from a routine pressure-wire study |
| Oliveira-Santos M. et al. [61] | Simulation percutaneous coronary intervention | Coronary angiogram | Hybrid flexible filled with fluid | Stereolithography | 2018 | Portugal | Patient-specific simulation of percutaneous coronary intervention | Patient-specific simulation is feasible to guide the treatment strategy of complex coronary artery disease (e.g., treating a critical ostial Cx stenosis) |
| Wang H. et al. [62] | Optimized stent implantation strategy | Coronary angiogram | Polydimethylsiloxane | Wax Jet | 2015 | China | Optimized stent position in microfluidic settings through 3D printing technology | Microfluidic model has demonstrated to be a feasible and novel approach for hemodynamic study of coronary disease |
| Velasco M. et al. [63] | Diagnosis and interventional planning of coronary artery fistulae | Computed tomography and cardiac magnetic resonance | Polyjet photopolymer | Polyjet | 2017 | United Kingdom | To evaluate the 3D-printed models contribution to the diagnosis and interventional planning of coronary artery fistulae | Improvement in selection of the correct equipment and in reduction of procedure times |
| Sedaghat A. et al. [64] | Percutaneous treatment of coronary artery aneurysm | Computed tomography Angiography | Silicone | Stereolithography | 2018 | Germany | To investigate a multimodal preprocedural planning in percutaneous coronary aneurysm treatment | Confirm the benefit of ex vivo 3D-printed model in interventional treatment of an extensive coronary aneurysm |
| Mohamed E. et al. [65] | Percutaneous treatment of left ventricle pseudoaneurysm | Computed tomography Angiography | Clear resin | Stereolithography | 2019 | USA | To investigate the role of 3D model in complications of myocardial infarction | Improve the accuracy to cannulate the aneurysm, select the appropriate sheath/catheter shape and determine the most suitable occluder device type and size |
| Bompotis G. et al. [66] | Transcatheter aortic valve implantation | Computed tomography aortography | Clear resin | Stereolithography | 2019 | Greece | To investigate the role of 3D model in transcatheter valve implantation | It was demonstrated to be a significant tool for the optimal sizing and positioning of the transcatheter implantation of the bioprosthetic valves and the reduction of the para- valvular leak |
| Baribeau Y. et al. [67] | Complex procedures on patient-specific models | Transesophageal Echocardiographic | Clear resin | Stereolithography | 2019 | USA | Procedural simulation that use a pulsatile left-sided heart printed model | Provided a better understanding of the anatomy and assessing the effect of interventions |
| Iriarta X. et al. [68] | Percutaneous appendage closure | Computed tomography angiography | Rubber-like material | Stereolithography | 2018 | France | To study the role of cardiac three-dimensional printing in percutaneous appendage closure | 3D model allow testing of several designs of prosthesis to better choose the optimal shape and size of the patient-specific prothesis to prevent procedural complications |
| Lodziński P. et al. [69] | Ventricular tachycardia ablation | Computed tomography angiography | Gypsum-cyanoacrylate | Stereolithography | 2017 | Poland | To investigate the role of 3D model in complex tachycardia treatment | Improve spatial orientation in patients with complex anatomy and provide a novel technology of mapping in ablation procedures |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cernica, D.; Benedek, I.; Polexa, S.; Tolescu, C.; Benedek, T. 3D Printing—A Cutting Edge Technology for Treating Post-Infarction Patients. Life 2021, 11, 910. https://doi.org/10.3390/life11090910
Cernica D, Benedek I, Polexa S, Tolescu C, Benedek T. 3D Printing—A Cutting Edge Technology for Treating Post-Infarction Patients. Life. 2021; 11(9):910. https://doi.org/10.3390/life11090910
Chicago/Turabian StyleCernica, Daniel, Imre Benedek, Stefania Polexa, Cosmin Tolescu, and Theodora Benedek. 2021. "3D Printing—A Cutting Edge Technology for Treating Post-Infarction Patients" Life 11, no. 9: 910. https://doi.org/10.3390/life11090910
APA StyleCernica, D., Benedek, I., Polexa, S., Tolescu, C., & Benedek, T. (2021). 3D Printing—A Cutting Edge Technology for Treating Post-Infarction Patients. Life, 11(9), 910. https://doi.org/10.3390/life11090910







