The Ideal Time for Iron Administration in Anemia Secondary to Blood Loss—An Experimental Animal Model
Abstract
:1. Introduction
2. Materials and Methods
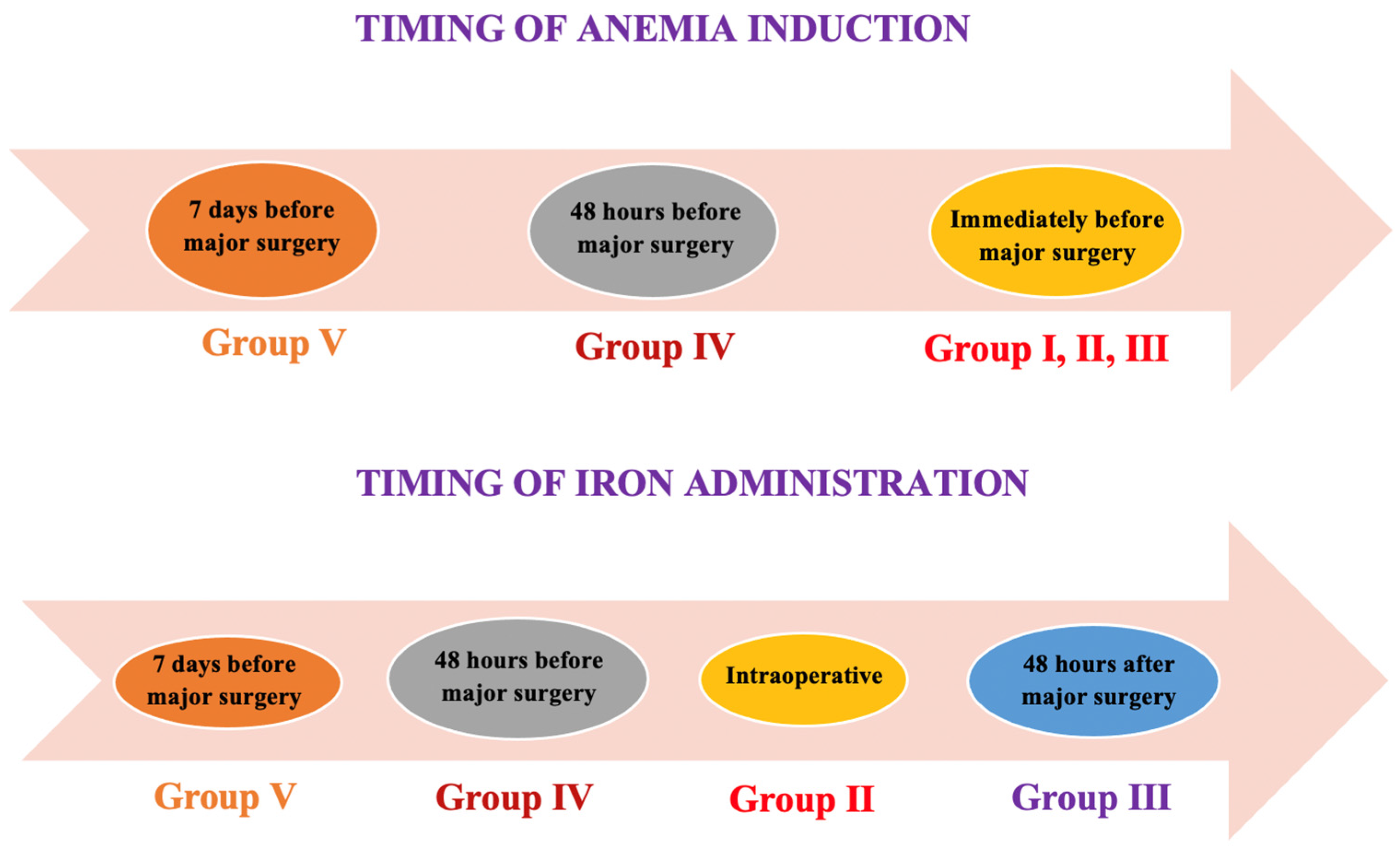
2.1. Animal Care and Group Allocation
2.2. Anemia Induction, Therapeutic Intervention, and Anesthetic and Surgical Procedures
2.3. Clinical Evaluation
2.4. Blood Sampling and Biomarkers Determination
2.5. Histology
2.6. Statistical Analysis
3. Results
3.1. Clinical Findings
3.2. Routine Blood Tests
3.3. Iron Profile
3.4. Morphopathological Findings
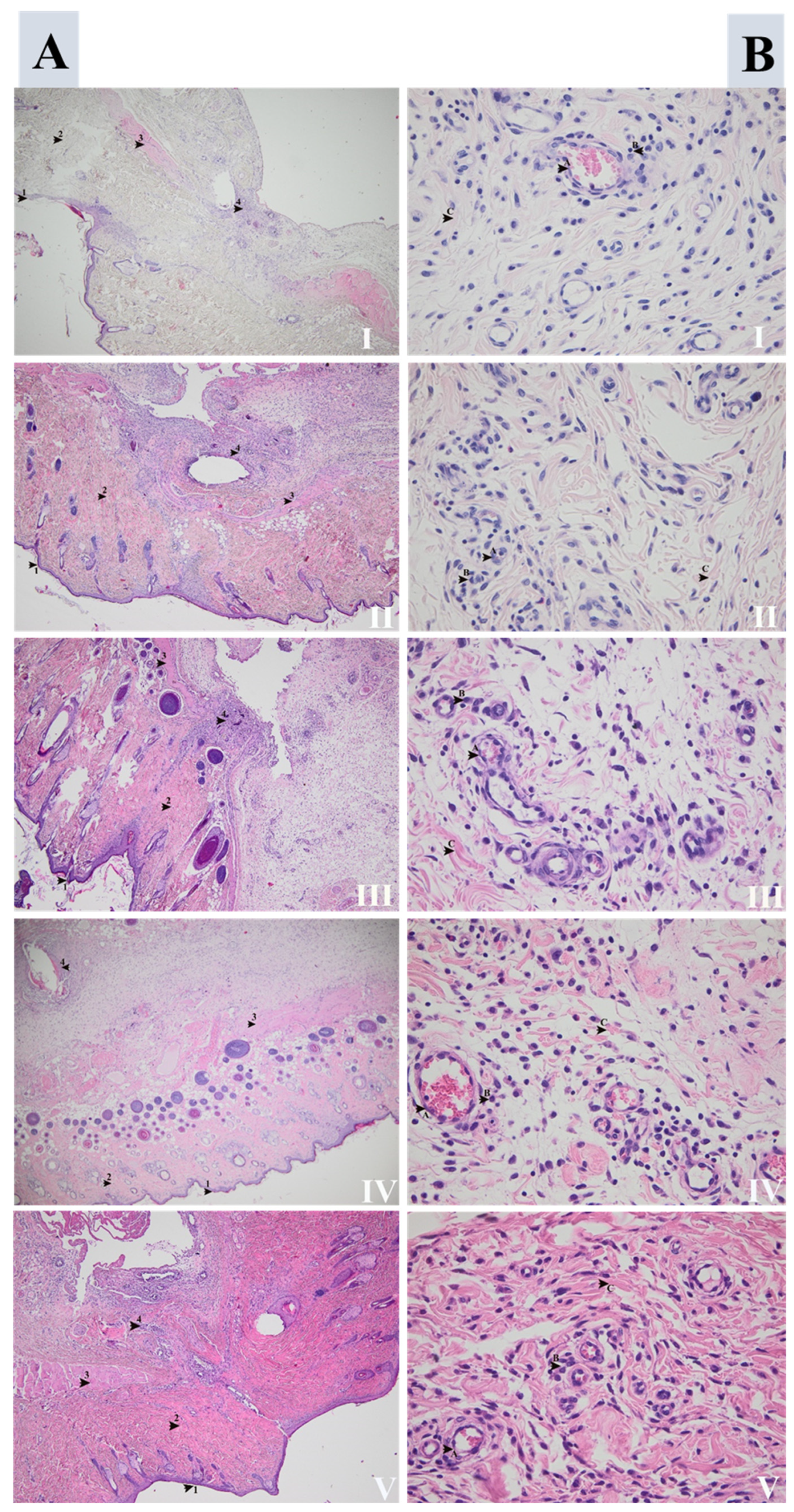
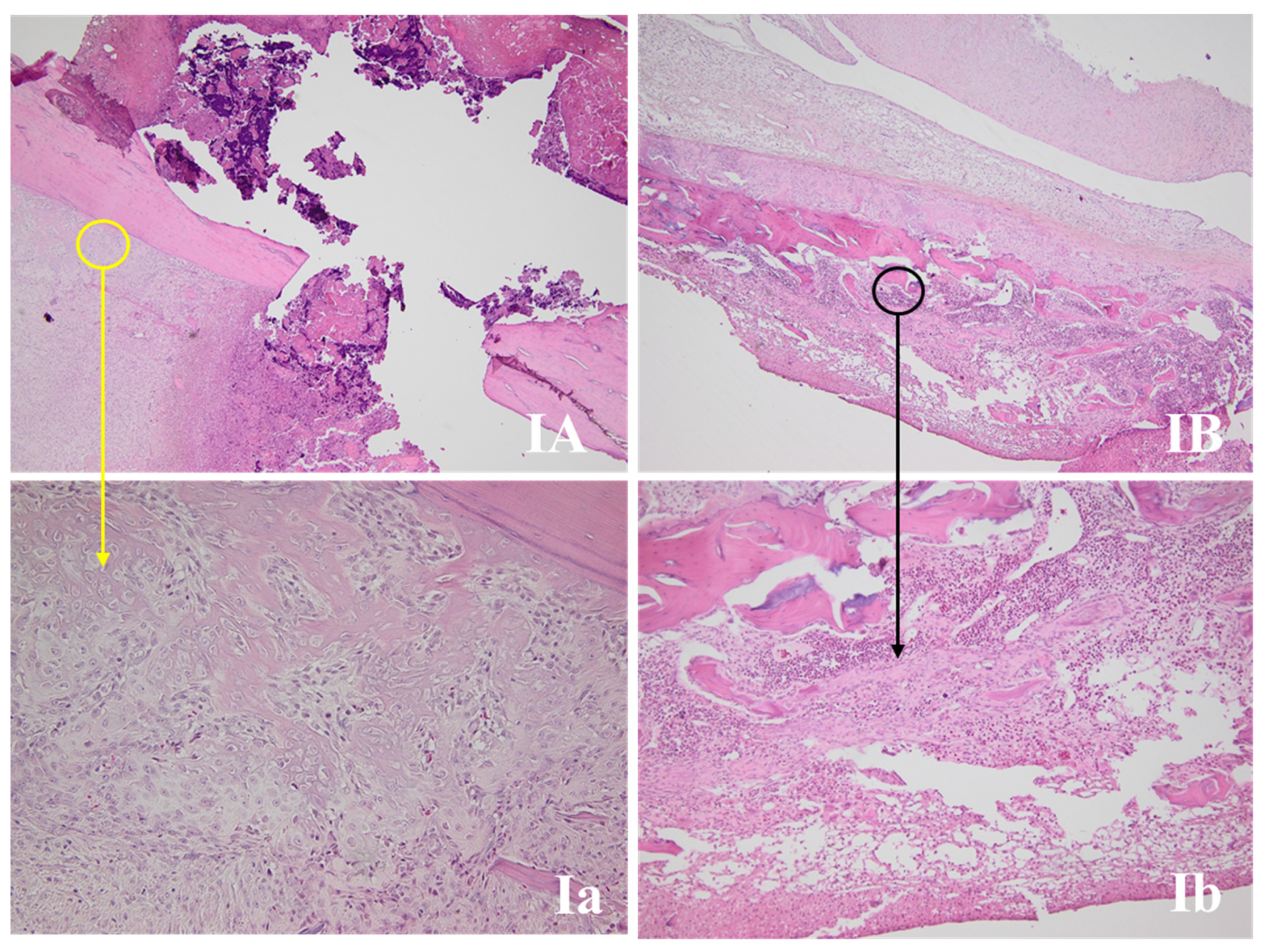
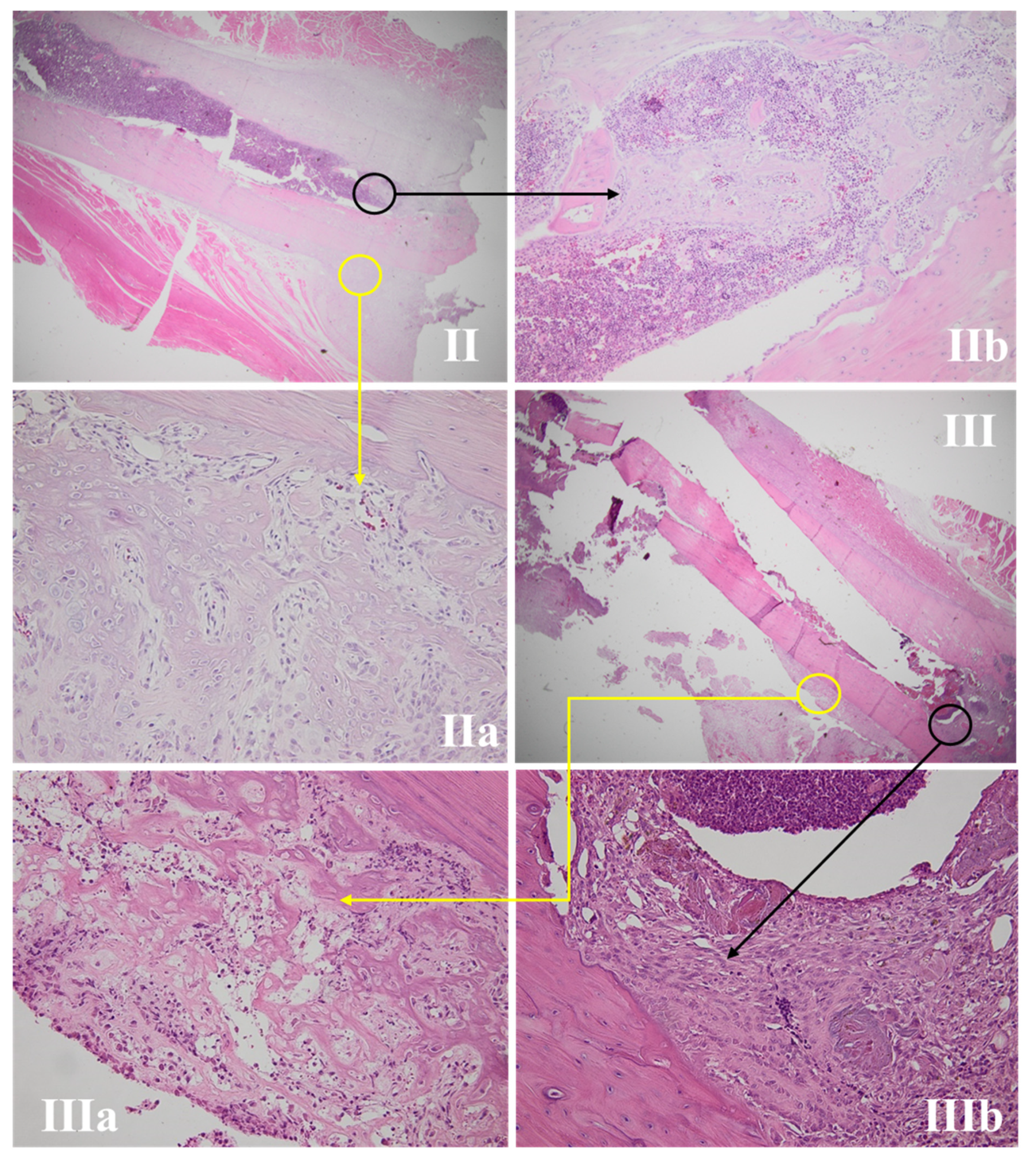
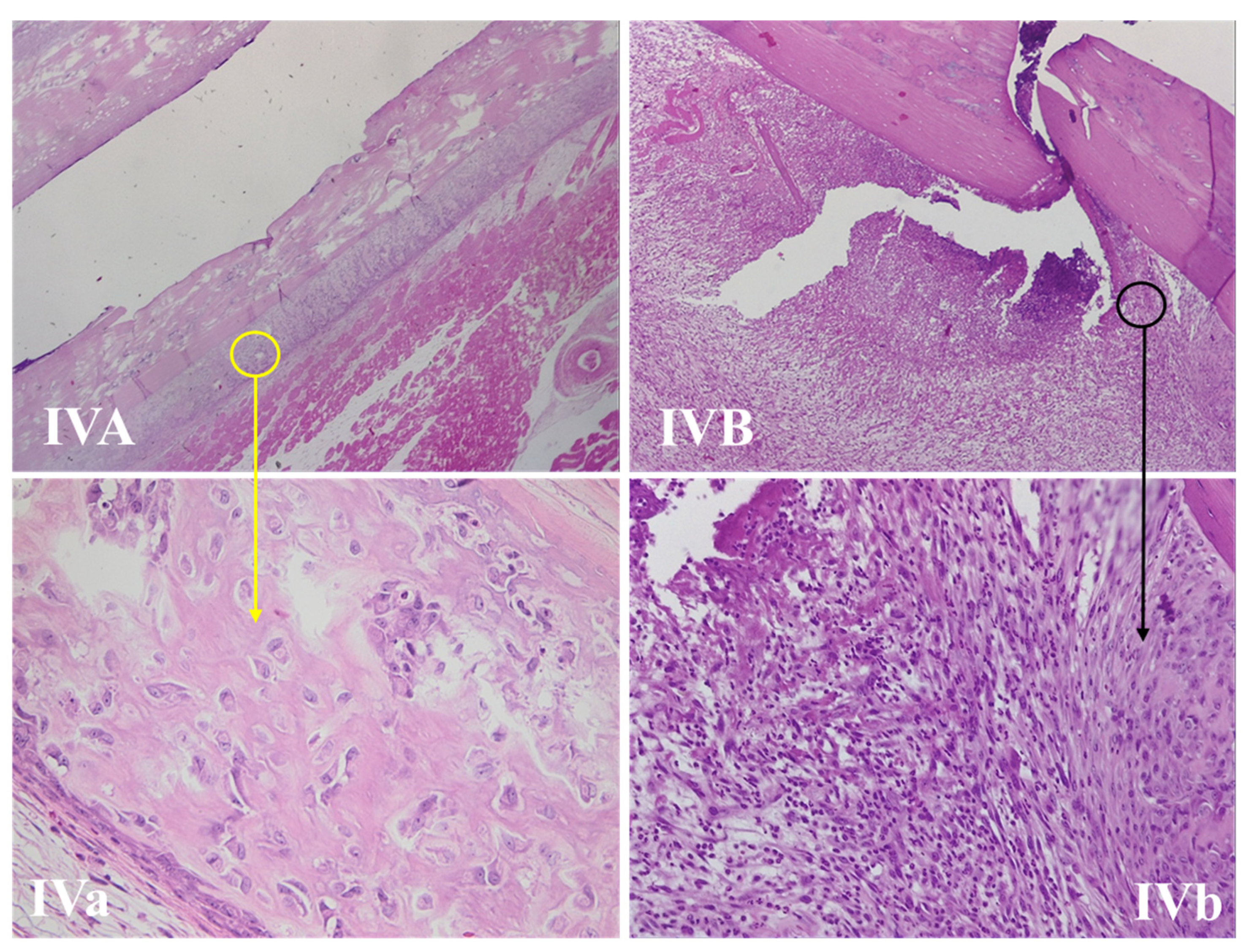
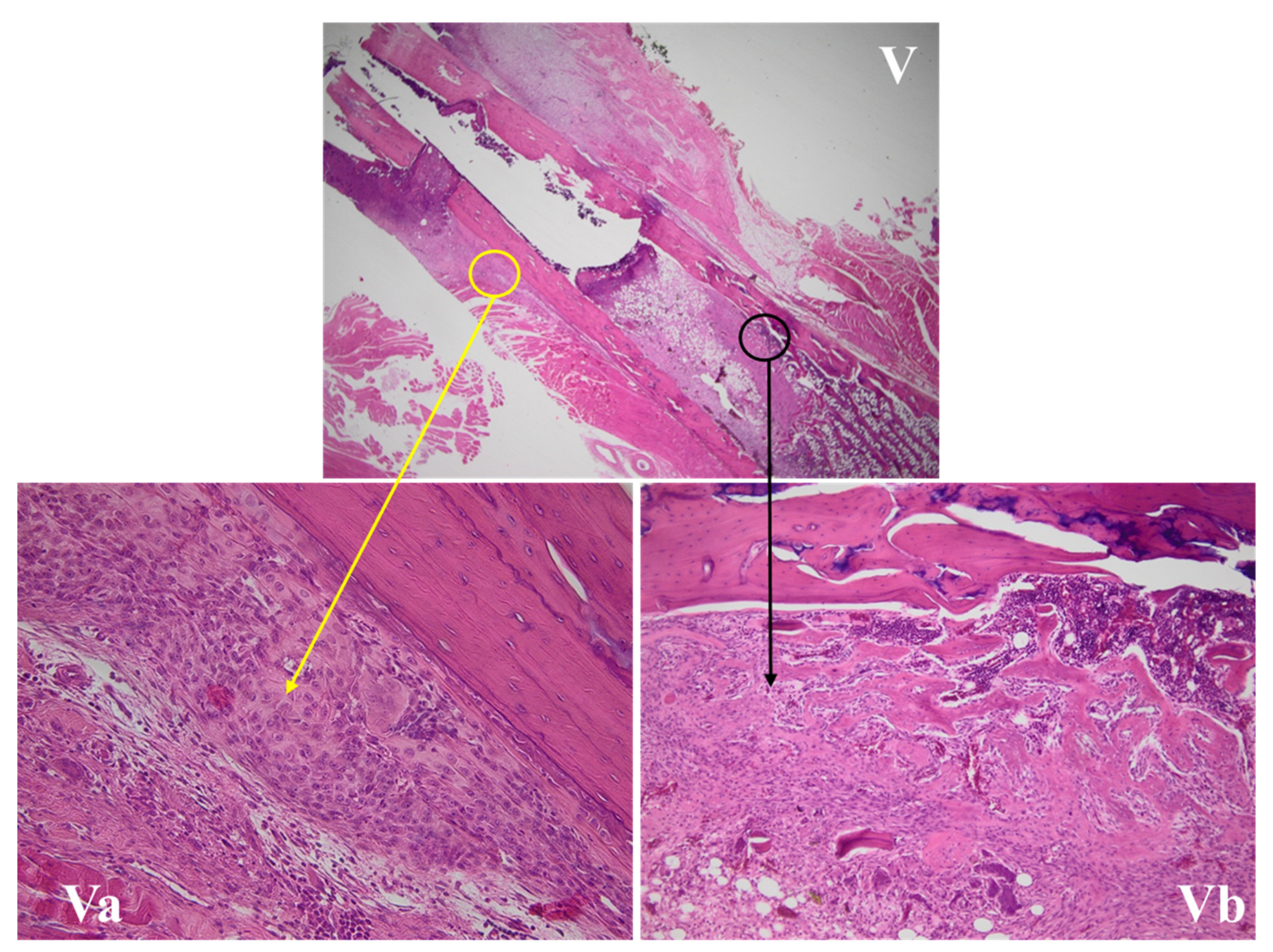
3.4.1. Wound Assessment
3.4.2. Fracture Site Assessment
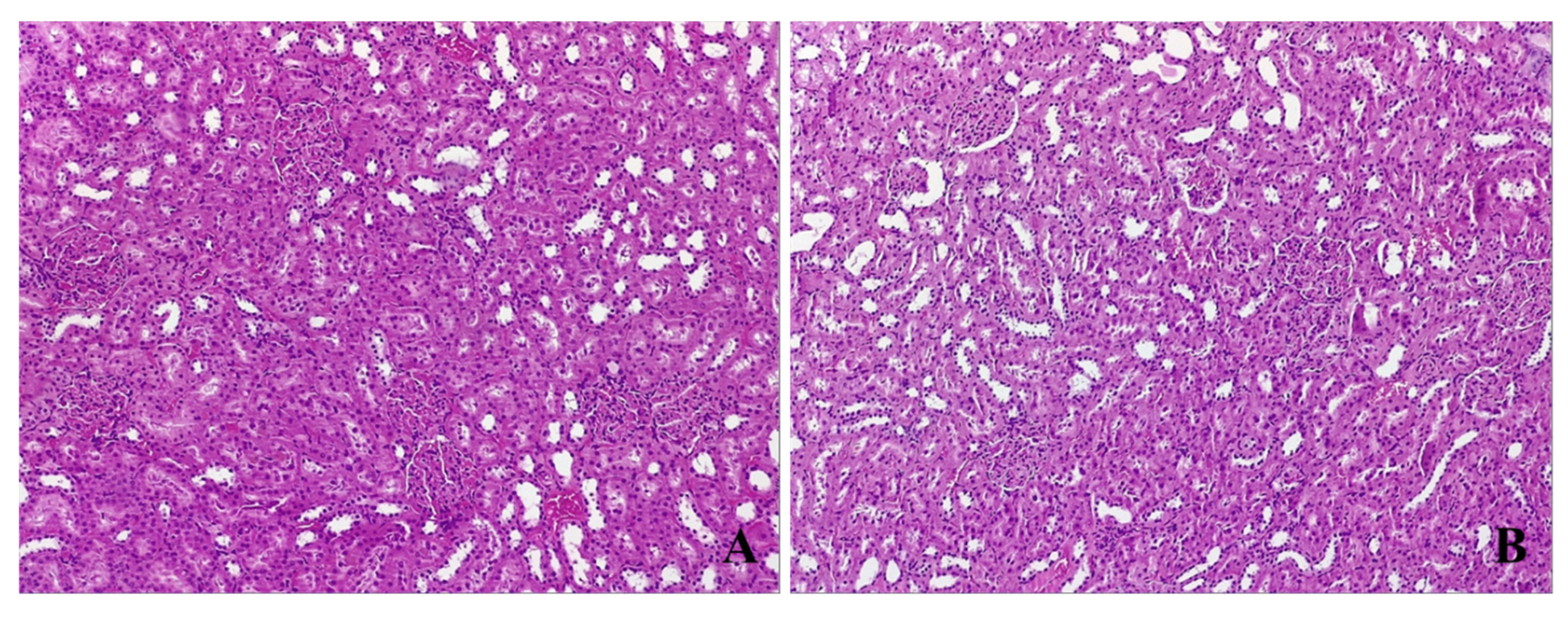
3.4.3. Kidney Histological Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gómez-Ramírez, S.; Khalafallah, A.A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girelli, D.; Ugolini, S.; Busti, F.; Marchi, G.; Castagna, A. Modern iron replacement therapy: Clinical and pathophysiological insights. Int. J. Hematol. 2018, 107, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Bacuzzi, A.; Dionigi, G.; Piffaretti, G.; Tozzi, M.; Del Romano, M.; Guzzetti, L.; Paracchini, F.; Villa, F.; Cuffari, S. Preoperative Methods to Improve Erythropoiesis. Transplant. Proc. 2011, 43, 324–326. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Flecknoe-Brown, S.C.; Allen, K.; Gibson, P.R.; McMahon, L.P.; Olynyk, J.K.; Roger, S.D.; Savoia, H.F.; Tampi, R.; Thomson, A.R.; et al. Diagnosis and management of iron deficiency anaemia: A clinical update. Med. J. Aust. 2010, 193, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Deloughery, T.G. Safety of Oral and Intravenous Iron. Acta Haematol. 2019, 142, 8–12. [Google Scholar] [CrossRef]
- Litton, E.; Xiao, J.; Allen, C.T.; Ho, K.M. Iron-Restricted Erythropoiesis and Risk of Red Blood Cell Transfusion in the Intensive Care Unit: A Prospective Observational Study. Anaesth. Intensive Care 2015, 43, 612–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elstrott, B.; Khan, L.; Olson, S.; Raghunathan, V.; Deloughery, T.; Shatzel, J.J. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur. J. Haematol. 2020, 104, 153–161. [Google Scholar] [CrossRef]
- Yoon, B.-H.; Lee, B.S.; Won, H.; Kim, H.-K.; Lee, Y.-K.; Koo, K.-H. Preoperative Iron Supplementation and Restrictive Transfusion Strategy in Hip Fracture Surgery. Clin. Orthop. Surg. 2019, 11, 265–269. [Google Scholar] [CrossRef]
- Kam, P.M.-H.; Chu, C.W.-H.; Chan, E.M.-Y.; Liu, O.-L.; Kwok, K.-H. Use of intravenous iron therapy in colorectal cancer patient with iron deficiency anemia: A propensity-score matched study. Int. J. Colorectal Dis. 2020, 35, 521–527. [Google Scholar] [CrossRef]
- Elhenawy, A.M.; Meyer, S.R.; Bagshaw, S.M.; MacArthur, R.G.; Carroll, L.J. Role of preoperative intravenous iron therapy to correct anemia before major surgery: A systematic review and meta-analysis. Syst. Rev. 2021, 10, 36. [Google Scholar] [CrossRef]
- Peters, F.; Ellermann, I.; Steinbicker, A.U. Intravenous Iron for Treatment of Anemia in the 3 Perisurgical Phases: A Review and Analysis of the Current Literature. Anesth. Analg. 2018, 126, 1268–1282. [Google Scholar] [CrossRef]
- Jeong, J.H.; Chang, M.J.; Kang, S.-B.; Park, H.J.; Lee, K.H.; Chang, C.B. Postoperative Intravenous Iron Supplementation Does Not Improve Hemoglobin Level and Transfusion Rate Following Staged Bilateral Total Knee Arthroplasty. J. Arthroplast. 2020, 35, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Takayama, Y.; Aoki, R. Roles of lactoferrin on skin wound healing. Biochem. Cell Biol. 2011, 90, 497–503. [Google Scholar] [CrossRef]
- Sandberg, N.; Zederfeldt, B. Influence of acute hemorrhage on wound healing in the rabbit. Acta Chir. Scand. 1960, 118, 367–371. [Google Scholar]
- Katsumata, S.-I.; Katsumata-Tsuboi, R.; Uehara, M.; Suzuki, K. Severe Iron Deficiency Decreases Both Bone Formation and Bone Resorption in Rats. J. Nutr. 2009, 139, 238–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.-Y.; Zhao, L.-P.; He, Y.-F.; Li, G.-F.; Gao, C.; Li, K.; Xu, Y.-J. A Comparison of the Biological Activities of Human Osteoblast hFOB1.19 Between Iron Excess and Iron Deficiency. Biol. Trace Elem. Res. 2012, 150, 487–495. [Google Scholar] [CrossRef]
- Wright, I.; Blanco-Rojo, R.; Fernández, M.C.; Toxqui, L.; Moreno, G.; Pérez-Granados, A.M.; De La Piedra, C.; Remacha, Á.F.; Vaquero, M.P. Bone remodelling is reduced by recovery from iron-deficiency anaemia in premenopausal women. J. Physiol. Biochem. 2013, 69, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, V.J.; Agarwal, A. Targeting iron homeostasis in acute kidney injury. In Seminars in Nephrology; WB Saunders: Philadelphia, PA, USA, 2016; Volume 36, pp. 62–70. [Google Scholar]
- Total Iron Binding Capacity (TIBC), Transferrin and Transferrin Saturation. Available online: https://labpedia.net/total-iron-binding-capacity-tibc-transferrin-and-transferrin-saturation/ (accessed on 12 February 2021).
- Ma, J.; Wen, X.; Mo, F.; Wang, X.; Shen, Z.; Li, M. Effects of Different Doses and Duration of Iron Supplementation on Curing Iron Deficiency Anemia: An Experimental Study. Biol. Trace Elem. Res. 2014, 162, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Sample Size Calculations (IACUC). Available online: http://www.bu.edu/researchsupport/compliance/animal-care/working-with-animals/research/sample-size-calculations-iacuc/ (accessed on 24 January 2021).
- O’Connell, K.E.; Mikkola, A.M.; Stepanek, A.M.; Vernet, A.; Hall, C.D.; Sun, C.C.; Yildirim, E.; Staropoli, J.F.; Lee, J.T.; Brown, D.E. Practical Murine Hematopathology: A Comparative Review and Implications for Research. Comp. Med. 2015, 65, 96–113. [Google Scholar] [PubMed]
- Knoblaugh, S.E.; Hohl, T.M.; La Perle, K.M.D. Pathology Principles and Practices for Analysis of Animal Models. ILAR J. 2018, 59, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-P.; Liu, Z.-Y.; Liu, J.-P.; Kang, Y.; Mao, C.-S.; Shang, J. Diagnostic Value of Common Inflammatory Markers on Fever of Unknown Origin. Jpn. J. Infect. Dis. 2016, 69, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Hassan, T.H.; Badr, M.A.; Karam, N.A.; Zkaria, M.; El Saadany, H.F.; Abdel Rahman, D.M.; Shahbah, D.A.; Al Morshedy, S.M.; Fathy, M.; Esh, A.M.H.; et al. Impact of iron deficiency anemia on the function of the immune system in children. Medicine 2016, 95, e5395. [Google Scholar] [CrossRef]
- Wang, F.-R.; Xie, Z.-G.; Ye, X.-Q.; Deng, S.-G.; Hu, Y.Q.; Guo, X.; Chen, S.G. Effectiveness of treatment of iron deficiency anemia in rats with squid ink melanin-Fe. Food Funct. 2014, 5, 123–128. [Google Scholar] [CrossRef]
- He, H.; An, F.; Huang, Q.; Kong, Y.; He, D.; Chen, L.; Song, H. Metabolic effect of AOS-iron in rats with iron deficiency anemia using LC-MS/MS based metabolomics. Food Res. Int. 2020, 130, 108913. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.L.; Hurrie, D.; Graham, J.; Perija, B.; Rimmer, E.; Rabbani, R.; Bernstein, C.N.; Turgeon, A.F.; Fergusson, D.A.; Houston, D.S.; et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: A systematic review of randomised controlled trials. BMJ Open 2018, 8, e019240. [Google Scholar] [CrossRef] [Green Version]
- Wenger, M.J.; Dellavalle, D.M.; Murray-Kolb, L.E.; Haas, J.D. Effect of iron deficiency on simultaneous measures of behavior, brain activity, and energy expenditure in the performance of a cognitive task. Nutr. Neurosci. 2019, 22, 196–206. [Google Scholar] [CrossRef]
- Murphy, J.F. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System; WHO/NMH/NHD/MNM/11.1; World Health Organization: Geneva, Switzerland, 2011; Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 2 May 2021).
- Anderson, F.A., Jr.; Spencer, F.A. Risk Factors for Venous Thromboembolism. Circulation 2003, 107, I9–I16. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, K.; Jensen, J.A.; Goodson, W.H., 3rd; Scheuenstuhl, H.; West, J.; Hopf, H.W.; Hunt, T.K. Tissue Oxygenation, Anemia, and Perfusion in Relation to Wound Healing in Surgical Patients. Ann. Surg. 1991, 214, 605–613. [Google Scholar] [CrossRef]
- Pavlidis, T.E.; Galatianos, I.N.; Papaziogas, B.T.; Lazaridis, C.N.; Atmatzidis, K.S.; Makris, J.G.; Papaziogas, T.B. Complete Dehiscence of the Abdominal Wound and Incriminating Factors. Eur. J. Surg. 2001, 167, 351–354. [Google Scholar] [CrossRef]
- Oliveira Sampaio, S.C.P.; de C. Monteiro, J.S.; Cangussú, M.C.T.; Pires Santos, G.M.; dos Santos, M.A.; dos Santos, J.N.; Pinheiro, A.L. Effect of laser and LED phototherapies on the healing of cutaneous wound on healthy and iron-deficient Wistar rats and their impact on fibroblastic activity during wound healing. Lasers Med. Sci. 2013, 28, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.N. Collagen deposition in the subcutaneous tissue during wound healing in humans: A model evaluation. APMIS Suppl. 2003, 115, 1–56. [Google Scholar]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Ann. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pikuleva, I.A.; Waterman, M.R. Cytochromes P450: Roles in Diseases. J. Biol. Chem. 2013, 288, 17091–17098. [Google Scholar] [CrossRef] [Green Version]
- D’Amelio, P.; Cristofaro, M.A.; Tamone, C.; Morra, E.; Di Bella, S.; Isaia, G.; Grimaldi, A.; Gennero, L.; Gariboldi, A.; Ponzetto, A.; et al. Role of iron metabolism and oxidative damage in postmenopausal bone loss. Bone 2008, 43, 1010–1015. [Google Scholar] [CrossRef]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The Early Fracture Hematoma and Its Potential Role in Fracture Healing. Tissue Eng. Part B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef]
- Shapiro, F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cells Mater. 2008, 15, 53–76. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Cavdar, C.; Temiz, A.; Yeniçerioğlu, Y.; Çalişkan, S.; Çelik, A.; Sifil, A.; Önvural, B.; Camsari, T. The Effects of Intravenous Iron Treatment on Oxidant Stress and Erythrocyte Deformability in Hemodialysis Patients. Scand. J. Urol. Nephrol. 2003, 37, 77–82. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Formula | Standard Measurement Unit |
|---|---|---|
| TSAT | = 100 × serum iron/serum transferrin × 1.25 | % |
| TIBC | = serum transferrin (mg/dL) × 1.25 | μg/dL |
| Weight | Day 1 (G-I–GIV) (Day 7 for G-V) after Anemia and Surgery, Reference Values for G-0 | Weight Day 3 after Surgery | Weight Day 5 after Surgery | Weight Day 7 after Surgery | |||||
|---|---|---|---|---|---|---|---|---|---|
| Group | N | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| G-I | 5 | 363 | 28 | 354 | 30 | 346 | 24 | 349 | 29 |
| G-II | 6 | 350 | 29 | 340 | 31 | 336 | 33 | 343 | 28 |
| G-III | 6 | 349 | 43 | 340 | 46 | 338 | 47 | 341 | 44 |
| G-IV | 5 | 355 | 34 | 348 | 33 | 347 | 33 | 349 | 36 |
| G-V | 5 | 364 | 33 | 358 | 32 | 357 | 31 | 359 | 32 |
| G-0 | 5 | 340 | 44 | - | - | - | - | - | - |
| F-ratio * (p) | 0.334 (0.888) | 0.276 (0.890) | 0.306 (0.871) | 0.208 (0.931) | |||||
| Group | Hb (g/dL) | Ht (%) | PLT (109/L) | RBC (1012/L) | MCH (pg) | MCV (fL) |
|---|---|---|---|---|---|---|
| G-0 (n = 5) | 14.32 ± 2.01 | 36.88 ± 4.81 | 227.20 ± 27.87 | 7.64 ± 0.94 | 18.70 ± 0.39 | 48.28 ± 0.98 |
| G-I (n = 5) | 10.4 ± 0.54 | 23.62 ± 1.23 | 518.80 ± 56.53 | 5.56 ± 0.11 | 18.44 ± 0.54 | 41.98 ± 1.51 |
| G-II (n = 6) | 13.15 ± 0.61 | 34.58 ± 1.15 | 300 ± 23.41 | 7.01 ± 0.32 | 18.78 ± 0.50 | 49.25 ± 2.38 |
| G-III (n = 6) | 12.2 ± 0.33 | 32.67 ± 1.63 | 277.33 ± 43.54 | 6.35 ± 0.23 | 19.32 ± 0.67 | 51.75 ± 2.87 |
| G-IV (n = 5) | 12.72 ± 0.54 | 34.18 ± 1.50 | 276.60 ± 33.66 | 6.74 ± 0.38 | 18.94 ± 0.77 | 50.82 ± 2.48 |
| G-V (n = 5) | 14.26 ± 1.51 | 37.34 ± 3.43 | 316.20 ± 17.37 | 7.56 ± 0.78 | 18.88 ± 0.86 | 49.56 ± 3.40 |
| F-ratio * (p) | 9.368 (<0.001) | 18.768 (<0.001) | 40.447 (<0.001) | 11.031 (<0.001) | 1.146 (0.361) | 10.532 (<0.001) |
| Group 0 different from ** | I, III | I | I, II, III, IV, V | I, III, IV | - | I |
| Group | Serum Iron (μg/dL) | Ferritin (ng/mL) | Transferrin (ng/mL) | TSAT (%) | TIBC (μg/dL) |
|---|---|---|---|---|---|
| G-0 (n = 5) | 60.50 ± 2.34 | 29.86 ± 3.97 | 67.54 ± 7.14 | 72.42 ± 9.06 | 84.38 ± 8.95 |
| G-I (n = 5) | 27.04 ± 6.92 | 22.52 ± 0.53 | 88.28 ± 7.65 | 23.30 ± 6.53 | 110.36 ± 9.59 |
| G-II (n = 6) | 61.50 ± 11.43 | 28.73 ± 3.03 | 71.85 ± 9.74 | 68.93 ± 12.47 | 89.80 ± 12.18 |
| G-III (n = 6) | 55.95 ± 7.26 | 38.00 ± 4.89 | 71.05 ± 6.65 | 63.05 ± 5.99 | 88.77 ± 8.30 |
| G-IV (n = 5) | 38.84 ± 12.34 | 27.34 ± 4.01 | 77.26 ± 8.24 | 40.20 ± 13.01 | 96.52 ± 10.35 |
| G-V (n = 5) | 71.68 ± 21.68 | 28.38 ± 1.59 | 105.24 ± 12.51 | 56.62 ± 13.89 | 127.40 ± 23.41 |
| F-ratio * (p) | 9.887 (<0.001) | 12.143 (<0.001) | 13.353 (<0.001) | 16.475 (<0.001) | 8.182 (<0.001) |
| Group 0 different from ** | I, IV, V | I, III | I, V | I, IV, V | I, V |
| Group | Collagen Fiber Dimension (µm) | Different from Group nr. ** |
|---|---|---|
| G-I | 4.12 ± 0.92 | V |
| G-II | 4.45 ± 0.69 | V |
| G-III | 4.54 ± 0.95 | V |
| G-IV | 3.99 ± 0.81 | V |
| G-V | 6.15 ± 2.34 | I, II, III, IV |
| F-ratio * (p) | 4.549 (0.004) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiglis, M.; Peride, I.; Petcu, L.C.; Neagu, T.P.; Niculae, A.; Totan, A.; Zurac, S.A.; Checherita, I.A.; Grintescu, I.M. The Ideal Time for Iron Administration in Anemia Secondary to Blood Loss—An Experimental Animal Model. Life 2021, 11, 898. https://doi.org/10.3390/life11090898
Tiglis M, Peride I, Petcu LC, Neagu TP, Niculae A, Totan A, Zurac SA, Checherita IA, Grintescu IM. The Ideal Time for Iron Administration in Anemia Secondary to Blood Loss—An Experimental Animal Model. Life. 2021; 11(9):898. https://doi.org/10.3390/life11090898
Chicago/Turabian StyleTiglis, Mirela, Ileana Peride, Lucian Cristian Petcu, Tiberiu Paul Neagu, Andrei Niculae, Alexandra Totan, Sabina Andrada Zurac, Ionel Alexandru Checherita, and Ioana Marina Grintescu. 2021. "The Ideal Time for Iron Administration in Anemia Secondary to Blood Loss—An Experimental Animal Model" Life 11, no. 9: 898. https://doi.org/10.3390/life11090898
APA StyleTiglis, M., Peride, I., Petcu, L. C., Neagu, T. P., Niculae, A., Totan, A., Zurac, S. A., Checherita, I. A., & Grintescu, I. M. (2021). The Ideal Time for Iron Administration in Anemia Secondary to Blood Loss—An Experimental Animal Model. Life, 11(9), 898. https://doi.org/10.3390/life11090898






