Towards Next Generation Biomarkers in Natural Killer/T-Cell Lymphoma
Abstract
1. Introduction
2. Clinical Risk Stratification and Prognostication Models for NK/T-Cell Lymphoma
3. Immunohistochemical Protein Expression as Potential Biomarkers
4. Immune Microenvironment and Inflammatory Responses
5. Molecular Biomarkers for Diagnosis and Prognostication
6. Current Treatment Strategies for NKTCL and Potential Predictive Biomarkers
7. Developing Next Generation Biomarkers for NKTCL
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chan, J.Y.; Lim, S.T. Novel findings from the Asian Lymphoma Study Group: Focus on T and NK-cell lymphomas. Int. J. Hematol. 2018, 107, 413–419. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kwong, Y.-L.; Kim, W.S.; Maeda, Y.; Hashimoto, C.; Suh, C.; Izutsu, K.; Ishida, F.; Isobe, Y.; Sueoka, E.; et al. Phase II Study of SMILE Chemotherapy for Newly Diagnosed Stage IV, Relapsed, or Refractory Extranodal Natural Killer (NK)/T-Cell Lymphoma, Nasal Type: The NK-Cell Tumor Study Group Study. J. Clin. Oncol. 2011, 29, 4410–4416. [Google Scholar] [CrossRef]
- Kwong, Y.-L.; Kim, W.S.; Lim, S.T.; Kim, S.J.; Tang, T.; Tse, E.; Leung, A.Y.H.; Chim, C. SMILE for natural killer/T-cell lymphoma: Analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood 2012, 120, 2973–2980. [Google Scholar] [CrossRef]
- Wang, J.-H.; Wang, L.; Liu, C.-C.; Xia, Z.-J.; Huang, H.-Q.; Lin, T.-Y.; Jiang, W.-Q.; Lu, Y. Efficacy of combined gemcitabine, oxaliplatin and pegaspargase (P-gemox regimen) in patients with newly diagnosed advanced-stage or relapsed/refractory extranodal NK/T-cell lymphoma. Oncotarget 2016, 7, 29092–29101. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, Y.; Sun, Z.; Zhang, L.; Li, L.; Wang, X.; Wu, J.; Fu, X.; Ma, W.; Zhang, X.; et al. DDGP versus SMILE in Newly Diagnosed Advanced Natural Killer/T-Cell Lymphoma: A Randomized Controlled, Multicenter, Open-label Study in China. Clin. Cancer Res. 2016, 22, 5223–5228. [Google Scholar] [CrossRef] [PubMed]
- Jaccard, A.; Gachard, N.; Marin, B.; Rogez, S.; Audrain, M.; Suarez, F.; Tilly, H.; Morschhauser, F.; Thieblemont, C.; Ysebaert, L.; et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 2011, 117, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fu, B.B.; Gale, R.P.; Liang, Y. NK-/T-cell lymphomas. Leukemia 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xia, Y.; Feng, L.N.; Chen, J.R.; Li, H.M.; Cui, J.; Cai, Q.Q.; Sim, K.S.; Nairismägi, M.L.; Laurensia, Y.; et al. Genetic risk of extranodal natural killer T-cell lymphoma: A genome-wide association study. Lancet Oncol. 2016, 17, 1240–1247. [Google Scholar] [CrossRef]
- Lin, G.W.; Xu, C.; Chen, K.; Huang, H.Q.; Chen, J.; Song, B.; Chan, J.K.C.; Li, W.; Liu, W.; Shih, L.Y.; et al. Genetic risk of extranodal natural killer T-cell lymphoma: A genome-wide association study in multiple populations. Lancet Oncol. 2019, 21, 306–316. [Google Scholar] [CrossRef]
- Chan, J.Y.; Ng, A.Y.J.; Cheng, C.L.; Nairismägi, M.L.; Venkatesh, B.; Cheah, D.M.Z.; Li, S.T.; Chan, S.H.; Ngeow, J.; Laurensia, Y.; et al. Whole exome sequencing identifies recessive germline mutations in FAM160A1 in familial NK/T cell lymphoma. Blood Cancer J. 2018, 8, 111. [Google Scholar] [CrossRef]
- Jiang, L.; Gu, Z.H.; Yan, Z.X.; Zhao, X.; Xie, Y.Y.; Zhang, Z.G.; Pan, C.M.; Hu, Y.; Cai, C.P.; Dong, Y.; et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat. Genet. 2015, 47, 1061–1066. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Ma, G.; Zhao, G.; Liu, H. KMT2D and TP53 mutation status improve the prognostic value of the International Prognostic Index (IPI) stratification in ENKTL patients. Neoplasma 2020, 67, 636–643. [Google Scholar] [CrossRef]
- Xiong, J.; Cui, B.W.; Wang, N.; Dai, Y.T.; Zhang, H.; Wang, C.F.; Zhong, H.J.; Cheng, S.; Ou-Yang, B.S.; Hu, Y.; et al. Genomic and Transcriptomic Characterization of Natural Killer T Cell Lymphoma. Cancer Cell 2020, 37, 403–419.e6. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.P.; Ma, S.Y.; Young, K.H.; Ong, C.K.; Liu, Y.; Li, Z.; Zhai, Q.; Huang, H.Q.; Lin, T.; Li, Z.; et al. A composite single-nucleotide polymorphism prediction signature for extranodal natural killer/T-cell lymphoma. Blood 2021, 138, 452–463. [Google Scholar] [CrossRef]
- Cho, J.; Kim, S.J.; Park, W.-Y.; Kim, J.; Woo, J.; Kim, G.; Yoon, S.E.; Ko, Y.H.; Kim, W.S. Immune subtyping of extranodal NK/T-cell lymphoma: A new biomarker and an immune shift during disease progression. Mod. Pathol. 2019, 33, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Q.; Huang, D.; Tang, T.; Tan, D.; Laurensia, Y.; Peng, R.J.; Wong, E.K.Y.; Cheah, D.M.Z.; Chia, B.K.H.; Iqbal, J.; et al. Whole-genome sequencing identifies responders to Pembrolizumab in relapse/refractory natural-killer/T cell lymphoma. Leukemia 2020, 34, 3413–3419. [Google Scholar] [CrossRef] [PubMed]
- Kwong, Y.; Kim, S.; Tse, E.; Oh, S.; Kwak, J.; Eom, H.; Do, Y.; Mun, Y.; Lee, S.; Shin, H.; et al. Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T-cell lymphoma. Ann. Oncol. 2017, 29, 256–263. [Google Scholar] [CrossRef]
- Au, W.-Y.; Weisenburger, D.D.; Intragumtornchai, T.; Nakamura, S.; Kim, W.-S.; Sng, I.; Vose, J.; Armitage, J.O.; Liang, R. International Peripheral T-Cell Lymphoma Project Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: A study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 2009, 113, 3931–3937. [Google Scholar] [CrossRef]
- Kim, S.J.; Jung, H.A.; Chuang, S.-S.; Hong, H.; Guo, C.-C.; Cao, J.; Hong, X.-N.; Suzuki, R.; Kang, H.J.; Won, J.H.; et al. Extranodal natural killer/T-cell lymphoma involving the gastrointestinal tract: Analysis of clinical features and outcomes from the Asia Lymphoma study group. J. Hematol. Oncol. 2013, 6, 86. [Google Scholar] [CrossRef]
- Takata, K.; Hong, M.-E.; Sitthinamsuwan, P.; Loong, F.; Tan, S.-Y.; Liau, J.-Y.; Hsieh, P.-P.; Ng, S.-B.; Yang, S.-F.; Pongpruttipan, T.; et al. Primary Cutaneous NK/T-cell Lymphoma, Nasal Type and CD56-positive Peripheral T-cell Lymphoma. Am. J. Surg. Pathol. 2015, 39, 1–12. [Google Scholar] [CrossRef]
- Lee, J.; Suh, C.; Park, Y.H.; Ko, Y.H.; Bang, S.M.; Lee, J.H.; Lee, D.H.; Huh, J.; Oh, S.Y.; Kwon, H.-C.; et al. Extranodal Natural Killer T-Cell Lymphoma, Nasal-Type: A Prognostic Model From a Retrospective Multicenter Study. J. Clin. Oncol. 2006, 24, 612–618. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.-J.; Zhu, Y.; Cao, J.-Z.; Yuan, Z.-Y.; Xu, L.-M.; Wu, J.-X.; Wang, W.; Wu, T.; Lu, B.; et al. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T-cell lymphoma, nasal-type: A multicenter study. Leukemia 2015, 29, 1571–1577. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Yang, Y.; Qi, S.-N.; Wang, Y.; Hu, C.; He, X.; Zhang, L.-L.; Wu, G.; Qu, B.-L.; Qian, L.-T.; et al. Validation of nomogram-revised risk index and comparison with other models for extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: Indication for prognostication and clinical decision-making. Leukemia 2021, 35, 130–142. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, D.H.; Jaccard, A.; Chng, W.J.; Lim, S.T.; Hong, H.; Park, Y.; Chang, K.M.; Maeda, Y.; Ishida, F.; et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: A multicentre, retrospective analysis. Lancet Oncol. 2016, 17, 389–400. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, H.; Yamaguchi, M.; Sohn, I.; Yoon, S.E.; Byeon, S.; Hur, J.Y.; Koh, Y.; Yoon, S.-S.; Kim, S.J.; et al. Prediction and prevention of central nervous system relapse in patients with extranodal natural killer/T-cell lymphoma. Blood 2020, 136, 2548–2556. [Google Scholar] [CrossRef]
- Tan, K.M.; Chia, B.; Lim, J.Q.; Khoo, L.P.; Cheng, C.L.; Tan, L.; Poon, E.; Somasundaram, N.; Farid, M.; Tang, T.P.L.; et al. A clinicohaematological prognostic model for nasal-type natural killer/T-cell lymphoma: A multicenter study. Sci. Rep. 2019, 9, 14961. [Google Scholar] [CrossRef]
- Hong, H.; Huang, H.; Fang, X.; Wang, Z.; Ye, S.; Zhang, H.; Huang, Y.; Guo, H.; Chen, X.; Liang, C.; et al. A prognostic index for nasal-type early-stage extranodal natural killer/T-cell lymphoma: A multicenter study. Am. J. Hematol. 2019, 94, E122–E124. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, G.; Jiang, C.; Li, L.; Cui, F.; Tian, R. Prognostic value of baseline, interim and end-of-treatment 18F-FDG PET/CT parameters in extranodal natural killer/T-cell lymphoma: A meta-analysis. PLoS ONE 2018, 13, e0194435. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Ngam, P.I.; Tan, K.M.; Ng, D.C.E.; Lim, S.T.; Chan, J.Y. The exact Deauville score, NABS score and high SUVmax predicts outcome in extranodal natural killer/T-cell lymphoma. Ann. Nucl. Med. 2021, 35, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.H.; Yoon, S.E.; Kim, S.J.; Cho, J.; Ko, Y.H.; Lee, K.-H.; Kim, W.S. Metabolic activity of extranodal NK/T cell lymphoma on 18F-FDG PET/CT according to immune subtyping. Sci. Rep. 2021, 11, 5879. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xia, Z.-J.; Lu, Y.; Wang, Q.-X.; Niu, S.-Q.; Huang, H.-Q.; Zhang, Y.-J. A modified international prognostic index including pretreatment hemoglobin level for early stage extranodal natural killer/T cell lymphoma. Leuk. Lymphoma 2015, 56, 3038–3044. [Google Scholar] [CrossRef]
- Cao, J.; Lan, S.; Shen, L.; Si, H.; Xiao, H.; Yuan, Q.; Li, X.; Li, H.; Guo, R. Hemoglobin level, a prognostic factor for nasal extranodal natural killer/T-cell lymphoma patients from stage I to IV: A validated prognostic nomogram. Sci. Rep. 2017, 7, 10982. [Google Scholar] [CrossRef]
- Luo, H.; Quan, X.; Song, X.-Y.; Zhang, L.; Yin, Y.; He, Q.; Cai, S.; Li, S.; Zeng, J.; Zhang, Q.; et al. Red blood cell distribution width as a predictor of survival in nasal-type, extranodal natural killer/T-cell lymphoma. Oncotarget 2017, 8, 92522–92535. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhang, R.; Zhu, H.Y.; Wang, L.; Wu, Y.J.; Liang, J.H.; Shi, W.Y.; Liu, H.; Xu, W.; Li, J.Y. Circulating Low Absolute CD4+ T Cell Counts May Predict Poor Prognosis in Extranodal NK/T-Cell Lymphoma Patients Treating with Pegaspargase-Based Chemotherapy. Cancer Res. Treat. 2019, 51, 368–377. [Google Scholar] [CrossRef]
- Watanabe, T.; Kinoshita, T.; Itoh, K.; Yoshimura, K.; Ogura, M.; Kagami, Y.; Yamaguchi, M.; Kurosawa, M.; Tsukasaki, K.; Kasai, M.; et al. Pretreatment total serum protein is a significant prognostic factor for the outcome of patients with peripheral T/natural killer-cell lymphomas. Leuk. Lymphoma 2010, 51, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Jiang, W.Q.; Huang, J.J.; Xia, Z.J.; Huang, H.Q.; Li, Z.M. The Glasgow Prognostic Score (GPS) as a novel and significant predictor of extranodal natural killer/T-cell lymphoma, nasal type. Am. J. Hematol. 2013, 88, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Luo, X.; Liang, Y.; Rao, H.; Fang, X.; Jiang, W.; Lin, T.; Huang, H. Fasting blood glucose is a novel prognostic indicator for extranodal natural killer/T-cell lymphoma, nasal type. Br. J. Cancer 2013, 108, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Luo, X.; Zhang, G.; Huang, H.; Huang, H.; Lin, T.; Jiang, W.; Xia, Z.; Young, K.H. New prognostic model for extranodal natural killer/T cell lymphoma, nasal type. Ann. Hematol. 2014, 93, 1541–1549. [Google Scholar] [CrossRef]
- Chen, K.L.; Liu, Y.H.; Li, W.Y.; Chen, J.; Gu, Y.K.; Geng, Q.R.; Jiang, W.Q.; Huang, H.Q.; Lin, T.Y.; Xia, Z.J.; et al. The prognostic nutritional index predicts survival for patients with extranodal natural killer/T cell lymphoma, nasal type. Ann. Hematol. 2015, 94, 1389–1400. [Google Scholar] [CrossRef]
- Mao, J.; Yin, H.; Wang, L.; Wu, J.Z.; Xia, Y.; Zhu, H.Y.; Fan, L.; Li, J.Y.; Liang, J.H.; Xu, W. Prognostic value of 25-hydroxy vitamin D in extranodal NK/T cell lymphoma. Ann. Hematol. 2020, 100, 445–453. [Google Scholar] [CrossRef]
- Hong, J.; Park, S.; Baek, H.L.; Jung, J.H.; Kang, I.G.; Sym, S.J.; Park, J.; Ahn, J.Y.; Cho, E.K.; Kim, S.T.; et al. Tumor cell nuclear diameter and CD30 expression as potential prognostic parameter in patients with extranodal NK/T-cell lymphoma, nasal type. Int. J. Clin. Exp. Pathol. 2012, 5, 939–947. [Google Scholar]
- Li, P.; Jiang, L.; Zhang, X.; Liu, J.; Wang, H. CD30 expression is a novel prognostic indicator in extranodal natural killer/T-cell lymphoma, nasal type. BMC Cancer 2014, 14, 890. [Google Scholar] [CrossRef]
- Mraz-Gernhard, S.; Natkunam, Y.; Hoppe, R.T.; LeBoit, P.; Kohler, S.; Kim, Y.H. Natural Killer/Natural Killer-Like T-Cell Lymphoma, CD56+, Presenting in the Skin: An Increasingly Recognized Entity with an Aggressive Course. J. Clin. Oncol. 2001, 19, 2179–2188. [Google Scholar] [CrossRef]
- Kim, W.Y.; Nam, S.J.; Kim, S.; Kim, T.M.; Heo, D.S.; Kim, C.-W.; Jeon, Y.K. Prognostic implications of CD30 expression in extranodal natural killer/T-cell lymphoma according to treatment modalities. Leuk. Lymphoma 2014, 56, 1778–1786. [Google Scholar] [CrossRef]
- Lee, W.J.; Moon, I.J.; Shin, H.J.; Won, C.H.; Chang, S.E.; Choi, J.H.; Lee, M.W. CD30-positive cutaneous extranodal natural killer/T-cell lymphoma: Clinicopathological features and survival outcomes. Int. J. Dermatol. 2018, 58, 688–696. [Google Scholar] [CrossRef]
- Gaal, K.; Sun, N.C.J.; Hernandez, A.M.; Arber, D.A. Sinonasal NK/T-cell Lymphomas in the United States. Am. J. Surg. Pathol. 2000, 24, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.T.; Shih, L.Y.; Tsang, N.M. Nasal NK/T cell lymphoma in Taiwan: A clinicopathologic study of 22 cases, with analysis of histologic subtypes, Epstein-Barr virus LMP-1 gene association, and treatment modalities. Int. J. Surg. Pathol. 2004, 12, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Li, P.-F.; Lu, Y.; Xia, Z.-J.; Huang, H.-Q.; Zhang, Y.-J. CD38 expression predicts poor prognosis and might be a potential therapy target in extranodal NK/T cell lymphoma, nasal type. Ann. Hematol. 2015, 94, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, P.; Wang, H.; Liu, J.; Zhang, X.; Qiu, H.; Zhang, B. Prognostic significance of Ki-67 antigen expression in extranodal natural killer/T-cell lymphoma, nasal type. Med. Oncol. 2014, 31, 218. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, B.S.; Choi, C.W.; Choi, J.; Kim, I.; Lee, Y.H.; Kim, J.S. Ki-67 expression is predictive of prognosis in patients with stage I/II extranodal NK/T-cell lymphoma, nasal type. Ann. Oncol. 2007, 18, 1382–1387. [Google Scholar] [CrossRef]
- Ye, Q.; Chen, H.; Wen, Z.; Guo, W.; Huang, Y.; Mo, X. Abnormal expression of p-ATM/CHK2 in nasal extranodal NK/T cell lymphoma, nasal type, is correlated with poor prognosis. J. Clin. Pathol. 2020, 74, 223–227. [Google Scholar] [CrossRef]
- Chen, R.; Lu, M.; Wang, J.; Zhang, D.; Lin, H.; Zhu, H.; Zhang, W.; Xiong, L.; Ma, J.; Mao, Y.; et al. Increased expression of Trop2 correlates with poor survival in extranodal NK/T cell lymphoma, nasal type. Virchows Arch. 2013, 463, 713–719. [Google Scholar] [CrossRef]
- Liu, J.; Liang, L.; Huang, S.; Nong, L.; Li, D.; Zhang, B.; Li, T. Aberrant differential expression of EZH2 and H3K27me3 in extranodal NK/T-cell lymphoma, nasal type, is associated with disease progression and prognosis. Hum. Pathol. 2018, 83, 166–176. [Google Scholar] [CrossRef]
- Huang, D.; Song, T.L.; Nairismägi, M.L.; Laurensia, Y.; Pang, W.L.; Zhe, D.C.M.; Wong, E.K.Y.; Wijaya, G.G.; Tan, J.; Tan, S.H.; et al. Evaluation of the PIK3 pathway in peripheral T-cell lymphoma and NK/T-cell lymphoma. Br. J. Haematol. 2020, 189, 731–744. [Google Scholar] [CrossRef]
- Cao, W.; Liu, Y.; Zhang, H.; Wang, S.; Zhang, L.; Zhang, L.; Sun, B. Expression of LMP-1 and Cyclin D1 protein is correlated with an unfavorable prognosis in nasal type NK/T cell lymphoma. Mol. Med. Rep. 2008, 1, 363–368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mao, Y.; Zhang, D.W.; Zhu, H.; Lin, H.; Xiong, L.; Cao, Q.; Liu, Y.; Li, Q.D.; Xu, J.R.; Xu, L.F.; et al. LMP1 and LMP2A are potential prognostic markers of extranodal NK/T-cell lymphoma, nasal type (ENKTL). Diagn. Pathol. 2012, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, N.; Isobe, Y.; Masuda, A.; Momose, S.; Higashi, M.; Tamaru, J.; Sugimoto, K.; Komatsu, N. Expression of Epstein-Barr virus-encoded proteins in extranodal NK/T-cell Lymphoma, nasal type (ENKL): Differences in biologic and clinical behaviors of LMP1-positive and -negative ENKL. Clin. Cancer Res. 2012, 18, 2164–2172. [Google Scholar] [CrossRef][Green Version]
- Yamaguchi, M.; Takata, K.; Yoshino, T.; Ishizuka, N.; Oguchi, M.; Kobayashi, Y.; Isobe, Y.; Ishizawa, K.; Kubota, N.; Itoh, K.; et al. Prognostic biomarkers in patients with localized natural killer/T-cell lymphoma treated with concurrent chemoradiotherapy. Cancer Sci. 2014, 105, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, B.; Ma, X.; Guo, Y. NF-kappaB activation through the alternative pathway correlates with chemoresistance and poor survival in extranodal NK/T-cell lymphoma, nasal type. Jpn. J. Clin. Oncol. 2009, 39, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liao, Y.; Li, J.; Wu, J.; Zhang, Y.; Feng, G.; Tan, B.; Reng, S.; Zhang, Z.; Feng, X.; et al. Association between higher expression of YB-1 and poor prognosis in early-stage extranodal nasal-type natural killer/T-cell lymphoma. Biomarkers Med. 2014, 8, 581–588. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, L.; Wang, Y.; Li, P.; Zhu, J.; Wang, L.; Zhang, W.; Zhang, Y.; Huang, G. Tumor-associated macrophages promote tumor cell proliferation in nasopharyngeal NK/T-cell lymphoma. Int. J. Clin. Exp. Pathol. 2014, 7, 5429–5435. [Google Scholar] [PubMed]
- Lin, Z.X.; Bai, B.; Cai, Q.C.; Cai, Q.Q.; Wang, X.X.; Wu, X.Y.; Huang, H.Q. High numbers of tumor-associated macrophages correlate with poor prognosis in patients with mature T- and natural killer cell lymphomas. Med. Oncol. 2012, 29, 3522–3528. [Google Scholar] [CrossRef]
- Wang, H.; Li, P.; Wang, L.; Xia, Z.; Huang, H.; Lu, Y.; Li, Z. High numbers of CD68+ tumor-associated macrophages correlate with poor prognosis in extranodal NK/T-cell lymphoma, nasal type. Ann. Hematol. 2015, 94, 1535–1544. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jeon, Y.K.; Kim, T.M.; Kim, J.E.; Kim, Y.A.; Lee, S.H.; Kim, D.W.; Heo, D.S.; Kim, C.W. Increased quantity of tumor-infiltrating FOXP3-positive regulatory T cells is an independent predictor for improved clinical outcome in extranodal NK/T-cell lymphoma. Ann. Oncol. 2009, 20, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Zeng, G.P.; Li, J.; Fu, J.H.; Long, Y.Y.; Pan, J.Y.; Wei, L.Z.; Guan, J.Y.; Lin, Y.X.; You, H.; et al. High infiltration of CD20(+) B lymphocytes in extranodal natural killer/T-cell lymphoma is associated with better prognosis. Br. J. Haematol. 2020, 191, e116–e120. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Li, Z.M.; Xia, Y.; Huang, J.J.; Huang, H.Q.; Xia, Z.J.; Lin, T.Y.; Li, S.; Cai, X.Y.; Wu-Xiao, Z.J.; et al. Serum C-reactive protein (CRP) as a simple and independent prognostic factor in extranodal natural killer/T-cell lymphoma, nasal type. PLoS ONE 2013, 8, e64158. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.; Tomita, N.; Koyama, S.; Ogusa, E.; Ishii, Y.; Takahashi, H.; Miyashita, K.; Matsuura, S.; Tachibana, T.; Takasaki, H.; et al. Serum ferritin level is prognostic of patient outcome in extranodal NK/T cell lymphoma, nasal type. Med. Oncol. 2014, 31, 149. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Z.; Sun, Z.; Zhang, X.; Lu, L.; Wang, Y.; Zhang, M. S100A9 and ORM1 serve as predictors of therapeutic response and prognostic factors in advanced extranodal NK/T cell lymphoma patients treated with pegaspargase/gemcitabine. Sci. Rep. 2016, 6, 23695. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, Z.; Li, Z.; Lu, L.; Li, L.; Li, X.; Wang, X.; Zhang, M. Pretreatment 14-3-3 epsilon level is predictive for advanced extranodal NK/T cell lymphoma therapeutic response to asparaginase-based chemotherapy. Proteom. Clin. Appl. 2017, 11, 1600111. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.; Gao, Y.; Cai, Q.; Wang, X.; Bai, B.; Ping, L.; He, H.; Rao, H.; Zhang, Y.; Li, Z.; et al. High IL-6 expression in the tumor microenvironment is associated with poor prognosis of patients with extranodal natural/killer T-cell lymphoma (ENKTL). Expert Rev. Anticancer. Ther. 2021, 21, 121–127. [Google Scholar] [CrossRef]
- Bao, C.; Zhou, D.; Zhu, L.; Qian, W.; Ye, X. Increased serum level of interleukin-6 correlates with negative prognostic factors in extranodal NK/T-cell lymphoma. Transl. Cancer Res. 2020, 9, 2378–2389. [Google Scholar] [CrossRef]
- Wang, L.; Liao, D.Z.; Zhang, J.; Xia, Z.J.; Peng, X.W.; Lu, Y. Clinical significance of serum soluble interleukin-2 receptor-α in extranodal natural killer/T-cell lymphoma (ENKTL): A predictive biomarker for treatment efficacy and valuable prognostic factor. Med. Oncol. 2013, 30, 723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, W.-D.; Geng, Q.-R.; Wang, L.; Chen, X.-Q.; Liu, C.-C.; Lv, Y. Serum Levels of Interleukin-9 Correlate with Negative Prognostic Factors in Extranodal NK/T-Cell Lymphoma. PLoS ONE 2014, 9, e94637. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, J.Y.; Liu, C.C.; Zhu, M.Y.; Wang, J.H.; Geng, Q.R.; Lu, Y. Increased serum levels of interleukin-15 correlate with negative prognostic factors in extranodal NK/T cell lymphoma. Med. Oncol. 2014, 32, 370. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Wuxiao, Z.; Huang, H.; Jiang, W.; Li, Z.; Lu, Y.; Xia, Z. Increased serum levels of interleukin-10 predict poor prognosis in extranodal natural killer/T-cell lymphoma patients receiving asparaginase-based chemotherapy. OncoTargets Ther. 2015, 8, 2589–2599. [Google Scholar] [CrossRef]
- Kim, H.S.; Ryu, K.J.; Ko, Y.H.; Kim, H.J.; Kim, S.H.; Kim, W.S.; Kim, S.J. Macrophage inflammatory protein 1 alpha (MIP-1alpha) may be associated with poor outcome in patients with extranodal NK/T-cell lymphoma. Hematol. Oncol. 2016, 35, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Ryu, K.J.; Lee, H.; Ko, Y.H.; Kim, W.S.; Kim, S.J. Serum IL18 is associated with hemophagocytosis and poor survival in extranodal natural killer/T-cell lymphoma. Leuk. Lymphoma 2018, 60, 317–325. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jung, H.Y.; Nam, S.J.; Kim, T.M.; Heo, D.S.; Kim, C.W.; Jeon, Y.K. Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch. 2016, 469, 581–590. [Google Scholar] [CrossRef]
- Bi, X.W.; Wang, H.; Zhang, W.W.; Wang, J.H.; Liu, W.J.; Xia, Z.J.; Huang, H.Q.; Jiang, W.Q.; Zhang, Y.J.; Wang, L. PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J. Hematol. Oncol. 2016, 9, 109. [Google Scholar] [CrossRef]
- Nagato, T.; Ohkuri, T.; Ohara, K.; Hirata, Y.; Kishibe, K.; Komabayashi, Y.; Ueda, S.; Takahara, M.; Kumai, T.; Ishibashi, K.; et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: A potential rationale for immunotherapy. Cancer Immunol. Immunother. 2017, 66, 877–890. [Google Scholar] [CrossRef]
- Zeng, L.; Huang, W.; Cao, Z.; Zheng, B.; Liu, X.; Guo, L.; Feng, X. The correlation of clinicopathological features and prognosis in extranodal natural killer/T cell lymphoma: A report of 42 cases in the early stage. Ann. Hematol. 2019, 98, 1467–1476. [Google Scholar] [CrossRef]
- Muhamad, H.; Suksawai, N.; Assanasen, T.; Polprasert, C.; Bunworasate, U.; Wudhikarn, K. Programmed Cell Death 1 and Programmed Cell Death Ligands in Extranodal Natural Killer/T Cell Lymphoma: Expression Pattern and Potential Prognostic Relevance. Acta Haematol. 2019, 143, 78–88. [Google Scholar] [CrossRef]
- Jo, J.C.; Kim, M.; Choi, Y.; Kim, H.J.; Kim, J.E.; Chae, S.W.; Kim, H.; Cha, H.J. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann. Hematol. 2016, 96, 25–31. [Google Scholar] [CrossRef]
- Chan, J.Y.; Lim, J.Q.; Ong, C.K. Checkpoint immunotherapy for NK/T cell lymphoma—Time for a showdown? Precis. Clin. Med. 2021, 4, 70–72. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Liu, W.J.; Xia, Z.J.; Huang, H.Q.; Jiang, W.Q.; Li, Z.M.; Lu, Y. High post-treatment serum levels of soluble programmed cell death ligand 1 predict early relapse and poor prognosis in extranodal NK/T cell lymphoma patients. Oncotarget 2016, 7, 33035–33045. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Jing, C.; Yu, X.; Cao, X.; Xu, C. Predicting treatment response of patients with extranodal natural killer/T-cell lymphoma based on levels of PD-L1 mRNA and soluble PD-L1. Hematol. Oncol. 2020, 38, 467–477. [Google Scholar] [CrossRef]
- Li, J.W.; Wei, P.; Guo, Y.; Shi, D.; Yu, B.H.; Su, Y.F.; Li, X.Q.; Zhou, X.Y. Clinical significance of circulating exosomal PD-L1 and soluble PD-L1 in extranodal NK/T-cell lymphoma, nasal-type. Am. J. Cancer Res. 2020, 10, 4498–4512. [Google Scholar] [PubMed]
- Lei, K.I.; Chan, L.Y.; Chan, W.Y.; Johnson, P.J.; Lo, Y.M. Diagnostic and prognostic implications of circulating cell-free Epstein-Barr virus DNA in natural killer/T-cell lymphoma. Clin. Cancer Res. 2002, 8, 29–34. [Google Scholar] [PubMed]
- Au, W.Y.; Pang, A.; Choy, C.; Chim, C.S.; Kwong, Y.L. Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood 2004, 104, 243–249. [Google Scholar] [CrossRef]
- Ishii, H.; Ogino, T.; Berger, C.; Köchli-Schmitz, N.; Nagato, T.; Takahara, M.; Nadal, D.; Harabuchi, Y. Clinical usefulness of serum EBV DNA levels of BamHI W and LMP1 for Nasal NK/T-cell lymphoma. J. Med. Virol. 2007, 79, 562–572. [Google Scholar] [CrossRef]
- Suzuki, R.; Yamaguchi, M.; Izutsu, K.; Yamamoto, G.; Takada, K.; Harabuchi, Y.; Isobe, Y.; Gomyo, H.; Koike, T.; Okamoto, M.; et al. Prospective measurement of Epstein-Barr virus–DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T-cell lymphoma, nasal type. Blood 2011, 118, 6018–6022. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.H.; Kim, K.H.; Chang, M.H.; Ji, S.H.; Lim, D.H.; Kim, K.; Kim, S.J.; Ko, Y.; Ki, C.-S.; et al. Whole blood Epstein-Barr virus DNA load as a diagnostic and prognostic surrogate: Extranodal natural killer/T-cell lymphoma. Leuk. Lymphoma 2009, 50, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Liu, Q.F.; Wang, H.; Jin, J.; Wang, W.H.; Wang, S.L.; Song, Y.W.; Liu, Y.P.; Fang, H.; Ren, H.; et al. Clinical implications of plasma Epstein-Barr virus DNA in early-stage extranodal nasal-type NK/T-cell lymphoma patients receiving primary radiotherapy. Blood 2012, 120, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Kwong, Y.L.; Pang, A.W.; Leung, A.Y.; Chim, C.S.; Tse, E. Quantification of circulating Epstein-Barr virus DNA in NK/T-cell lymphoma treated with the SMILE protocol: Diagnostic and prognostic significance. Leukemia 2013, 28, 865–870. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Wang, J.H.; Xia, Z.J.; Lu, Y.; Huang, H.Q.; Jiang, W.Q.; Zhang, Y.J. Post-treatment plasma EBV-DNA positivity predicts early relapse and poor prognosis for patients with extranodal NK/T cell lymphoma in the era of asparaginase. Oncotarget 2015, 6, 30317–30326. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Choi, J.Y.; Hyun, S.H.; Ki, C.S.; Oh, D.; Ahn, Y.C.; Ko, Y.H.; Choi, S.; Jung, S.H.; Khong, P.L.; et al. Risk stratification on the basis of Deauville score on PET-CT and the presence of Epstein-Barr virus DNA after completion of primary treatment for extranodal natural killer/T-cell lymphoma, nasal type: A multicentre, retrospective analysis. Lancet Haematol. 2015, 2, e66–e74. [Google Scholar] [CrossRef]
- Cho, J.; Kim, S.J.; Park, S.; Yoo, K.H.; Ki, C.S.; Ko, Y.; Kim, W.S. Significance of circulating Epstein-Barr virus DNA monitoring after remission in patients with extranodal natural killer T cell lymphoma. Ann. Hematol. 2018, 97, 1427–1436. [Google Scholar] [CrossRef]
- Li, P.F.; Mao, Y.Z.; Bai, B.; Gao, Y.; Zhang, Y.J.; Li, Z.M.; Jiang, W.Q.; Huang, H.Q. Persistent peripheral blood EBV-DNA positive with high expression of PD-L1 and upregulation of CD4 + CD25 + T cell ratio in early stage NK/T cell lymphoma patients may predict worse outcome. Ann. Hematol. 2018, 97, 2381–2389. [Google Scholar] [CrossRef]
- Kanno, H.; Kojya, S.; Li, T.; Ohsawa, M.; Nakatsuka, S.; Miyaguchi, M.; Harabuchi, Y.; Aozasa, K. Low frequency of HLA-A*0201 allele in patients with Epstein-Barr virus-positive nasal lymphomas with polymorphic reticulosis morphology. Int. J. Cancer 2000, 87, 195–199. [Google Scholar] [CrossRef]
- Koo, G.C.; Tan, S.Y.; Tang, T.; Poon, S.L.; Allen, G.E.; Tan, L.; Chong, S.C.; Ong, W.S.; Tay, K.; Tao, M.; et al. Janus Kinase 3–Activating Mutations Identified in Natural Killer/T-cell Lymphoma. Cancer Discov. 2012, 2, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, H.Y.; Kang, S.Y.; Kim, S.J.; Hwang, J.; Lee, S.; Kwak, S.H.; Park, K.S.; Yoo, H.Y.; Kim, W.S.; et al. Genetic alterations of JAK/STAT cascade and histone modification in extranodal NK/T-cell lymphoma nasal type. Oncotarget 2015, 6, 17764–17776. [Google Scholar] [CrossRef]
- Song, T.L.; Nairismägi, M.L.; Laurensia, Y.; Lim, J.Q.; Tan, J.; Li, Z.M.; Pang, W.L.; Kizhakeyil, A.; Wijaya, G.C.; Huang, D.C.; et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood 2018, 132, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Miyoshi, H.; Sakata, S.; Dobashi, A.; Couronné, L.; Kogure, Y.; Sato, Y.; Nishida, K.; Gion, Y.; Shiraishi, Y.; et al. Frequent structural variations involving programmed death ligands in Epstein-Barr virus-associated lymphomas. Leukemia 2019, 33, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Oki, Y. Novel Immunotherapy Options for Extranodal NK/T-Cell Lymphoma. Front. Oncol. 2018, 8, 139. [Google Scholar] [CrossRef]
- Kwong, Y.L.; Chan, T.S.Y.; Tan, D.; Kim, S.J.; Poon, L.M.; Mow, B.; Khong, P.L.; Loong, F.; Au-Yeung, R.; Iqbal, J.; et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017, 129, 2437–2442. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, M.; Yan, J.; Li, L.; Fu, X.; Zhang, X.; Chang, Y.; Sun, Z.; Yu, H.; et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J. Hematol. Oncol. 2018, 11, 15. [Google Scholar] [CrossRef]
- Kim, S.J.; Lim, J.Q.; Laurensia, Y.; Cho, J.; Yoon, S.E.; Lee, J.Y.; Ryu, K.J.; Ko, Y.H.; Koh, Y.; Cho, D.; et al. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: An open-label phase 2 study. Blood 2020, 136, 2754–2763. [Google Scholar] [CrossRef]
- Li, J.; Tao, R.; Fan, L.; Song, Y.; Hu, Y.; Zhang, W.; Wang, Y.; Xu, L.; Sun, X.; Zhou, H. Sintilimab for relapsed/refractory (r/r) extranodal NK/T cell lymphoma (ENKTL): Extended follow-up on the multicenter, single-arm phase II trail (ORIENT-4). J. Clin. Oncol. 2020, 38, 8050. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.H.; Bang, H.; Cho, J.; Ko, Y.H.; Kim, S.J.; Kim, W.S. EGR1 as a potential marker of prognosis in extranodal NK/T-cell lymphoma. Sci. Rep. 2021, 11, 10342. [Google Scholar] [CrossRef]
- Rossi, D.; Spina, V.; Bruscaggin, A.; Gaidano, G. Liquid biopsy in lymphoma. Haematologica 2019, 104, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, W.; Li, J.; Xiong, J.; Liu, J.; Chen, T.; Wen, Q.; Zeng, Y.; Gao, L.; Zhang, C.; et al. Plasma circulating tumor DNA assessment reveals KMT2D as a potential poor prognostic factor in extranodal NK/T-cell lymphoma. Biomark. Res. 2020, 8, 27. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Tagawa, H.; Takahashi, N.; Watanabe, A.; Guo, Y.-M.; Iwamoto, K.; Yamashita, J.; Saitoh, H.; Kameoka, Y.; Shimizu, N.; et al. Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer–cell lymphoma/leukemia. Blood 2009, 114, 3265–3275. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.B.; Yan, J.; Huang, G.; Selvarajan, V.; Tay, J.L.; Lin, B.; Bi, C.; Tan, J.; Kwong, Y.L.; Shimizu, N.; et al. Dysregulated microRNAs affect pathways and targets of biologic relevance in nasal-type natural killer/T-cell lymphoma. Blood 2011, 118, 4919–4929. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.H.; Jang, J.Y.; Jeon, Y.K.; Kim, W.Y.; Kim, T.M.; Heo, D.S.; Kim, C.W. MicroRNA-146a downregulates NFkappaB activity via targeting TRAF6 and functions as a tumor suppressor having strong prognostic implications in NK/T cell lymphoma. Clin. Cancer Res. 2011, 17, 4761–4771. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, Y.; Kishibe, K.; Nagato, T.; Ueda, S.; Takahara, M.; Harabuchi, Y. Downregulation of miR-15a due to LMP1 promotes cell proliferation and predicts poor prognosis in nasal NK/T-cell lymphoma. Am. J. Hematol. 2013, 89, 25–33. [Google Scholar] [CrossRef]
- Guo, H.Q.; Huang, G.L.; Guo, C.C.; Pu, X.X.; Lin, T.Y. Diagnostic and prognostic value of circulating miR-221 for extranodal natural killer/T-cell lymphoma. Dis. Markers 2010, 29, 251–258. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, W.; Huang, R.; Li, L.; Wang, X.; Li, L.; Fu, X.; Sun, Z.; Li, Z.; Chen, Q.; et al. MicroRNA-155 is a potential molecular marker of natural killer/T-cell lymphoma. Oncotarget 2016, 7, 53808–53819. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.J.; Lee, J.Y.; Choi, M.E.; Yoon, S.E.; Cho, J.; Ko, Y.H.; Shim, J.H.; Kim, W.S.; Park, C.; Kim, S.J. Serum-Derived Exosomal MicroRNA Profiles Can Predict Poor Survival Outcomes in Patients with Extranodal Natural Killer/T-Cell Lymphoma. Cancers 2020, 12, 3548. [Google Scholar] [CrossRef]
- Komabayashi, Y.; Kishibe, K.; Nagato, T.; Ueda, S.; Takahara, M.; Harabuchi, Y. Circulating Epstein-Barr virus-encoded micro-RNAs as potential biomarkers for nasal natural killer/T-cell lymphoma. Hematol. Oncol. 2016, 35, 655–663. [Google Scholar] [CrossRef]
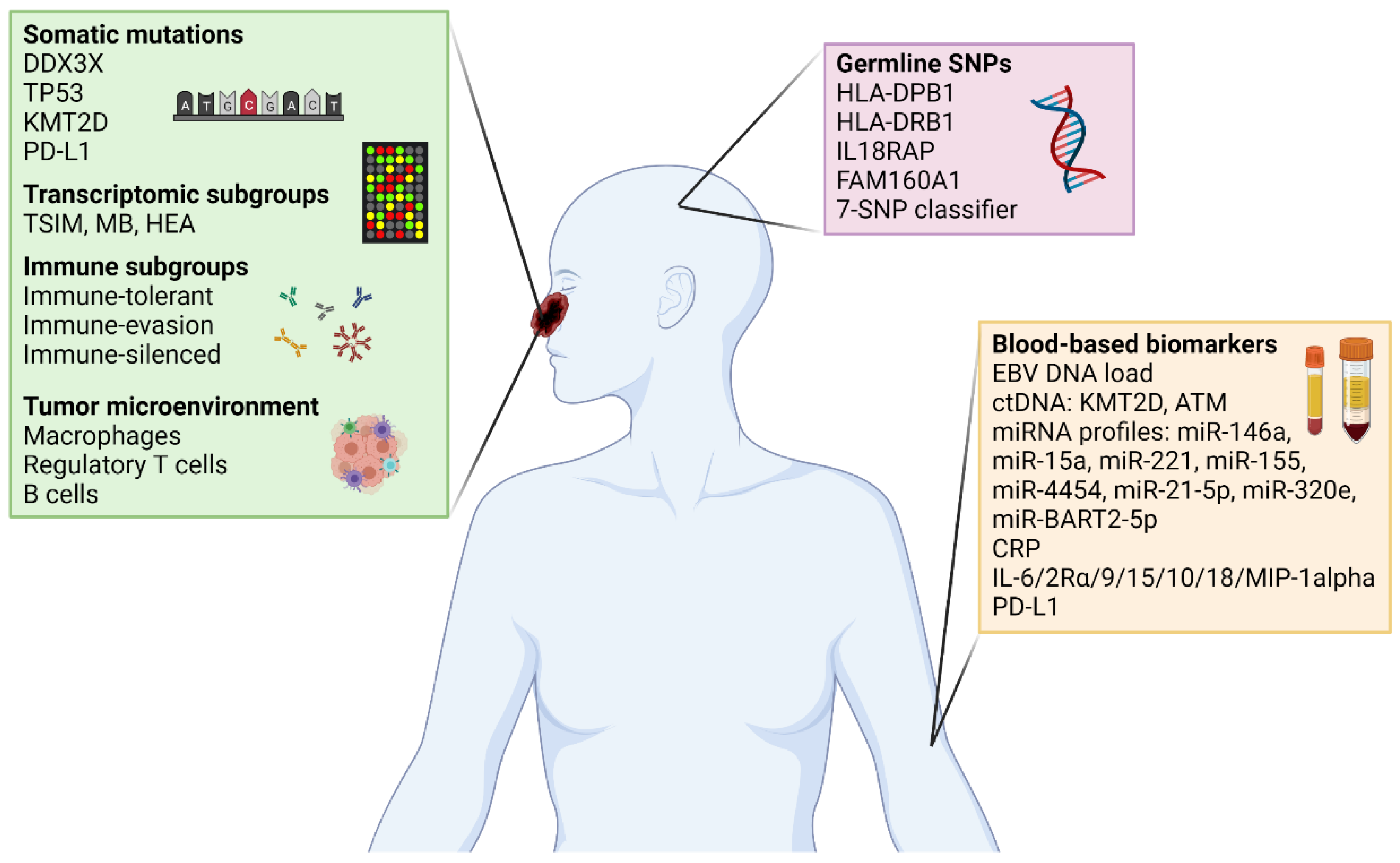
| Reference | Biomarker Type | Study Design | Main Findings |
|---|---|---|---|
| [8] | Diagnostic | GWAS | Germline SNPs at the loci of the HLA-DPB1 locus associate with increased risk of NKTCL |
| [9] | Diagnostic | GWAS | Germline SNPs at two novel loci of HLA-DRB1 and IL18RAP identified to confer risk of NKTCL |
| [10] | Diagnostic | Pedigree analysis and WES | Recessive germline SNPs in FAM160A1 reported in male siblings who were both diagnosed with NKTCL |
| [11] | Prognostic | WES and targeted sequencing | Identified recurrent mutations in DDX3X and TP53, both conferring worse OS and PFS. |
| [12] | Prognostic | Targeted sequencing | Mutations in KMT2D and TP53 associated with worse survival outcomes, and may enhance the prognostic value of the IPI model |
| [13] | Prognostic | Multi-omic profiling | Identified three molecular subtypes—Tumor-suppressor/immune-modulator (TSIM), MYC-related (MB) and Histone epigenetic altered (HEA) groups. HEA and MB subtypes were associated with the best and worst OS and PFS, respectively. |
| [14] | Prognostic | SNP genotype microarray | A 7-SNP-based classifier predicts the survival of patients with NKTCL, and improves existing risk stratification systems based on clinicopathological variables |
| [15] | Prognostic | RNA expression (NanoString) and IHC | Identified four immune subgroups—immune-tolerant (high T-reg cells and FOXP3 expression, early stage, best prognosis), immune evasion-A and immune evasion-B (high cytotoxic T-cells, high PD-L1, low T-reg), and immune-silenced (depleted immune response). The immune-silenced group represented patients with advanced disease and poor prognosis. |
| [16] | Predictive | WGS | Identified cryptic rearrangements of the PD-L1 gene disrupting the 3′-UTR to be a potential predictive biomarker of response to pembrolizumab in relapsed or refractory NKTCL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, J.Y.; Lim, J.Q.; Ong, C.K. Towards Next Generation Biomarkers in Natural Killer/T-Cell Lymphoma. Life 2021, 11, 838. https://doi.org/10.3390/life11080838
Chan JY, Lim JQ, Ong CK. Towards Next Generation Biomarkers in Natural Killer/T-Cell Lymphoma. Life. 2021; 11(8):838. https://doi.org/10.3390/life11080838
Chicago/Turabian StyleChan, Jason Yongsheng, Jing Quan Lim, and Choon Kiat Ong. 2021. "Towards Next Generation Biomarkers in Natural Killer/T-Cell Lymphoma" Life 11, no. 8: 838. https://doi.org/10.3390/life11080838
APA StyleChan, J. Y., Lim, J. Q., & Ong, C. K. (2021). Towards Next Generation Biomarkers in Natural Killer/T-Cell Lymphoma. Life, 11(8), 838. https://doi.org/10.3390/life11080838






