The Detection of Stem-Like Circulating Tumor Cells Could Increase the Clinical Applicability of Liquid Biopsy in Ovarian Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Cohort
2.2. Enumeration of Circulating Tumor Cells
2.3. Statistical Processing
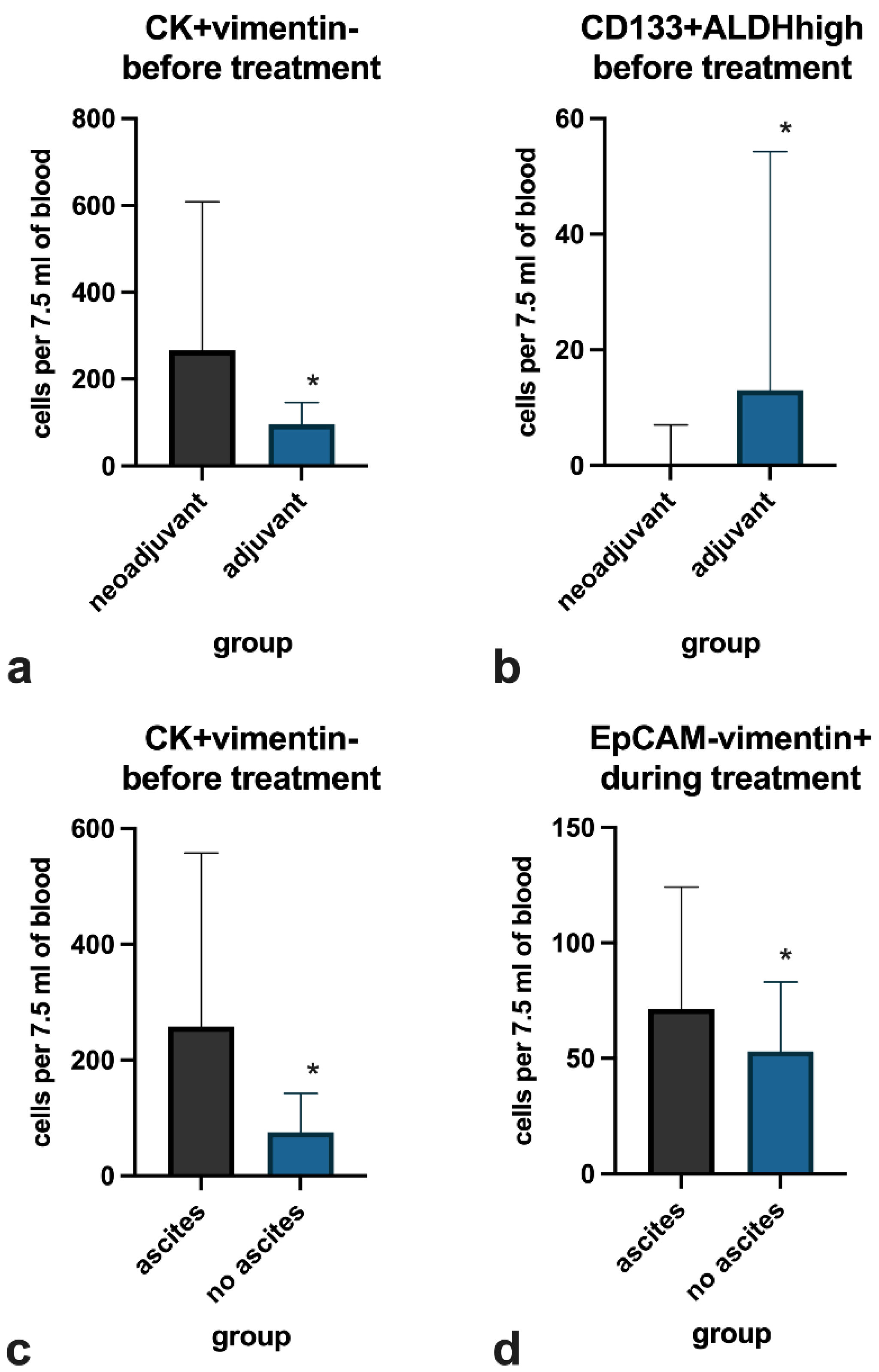
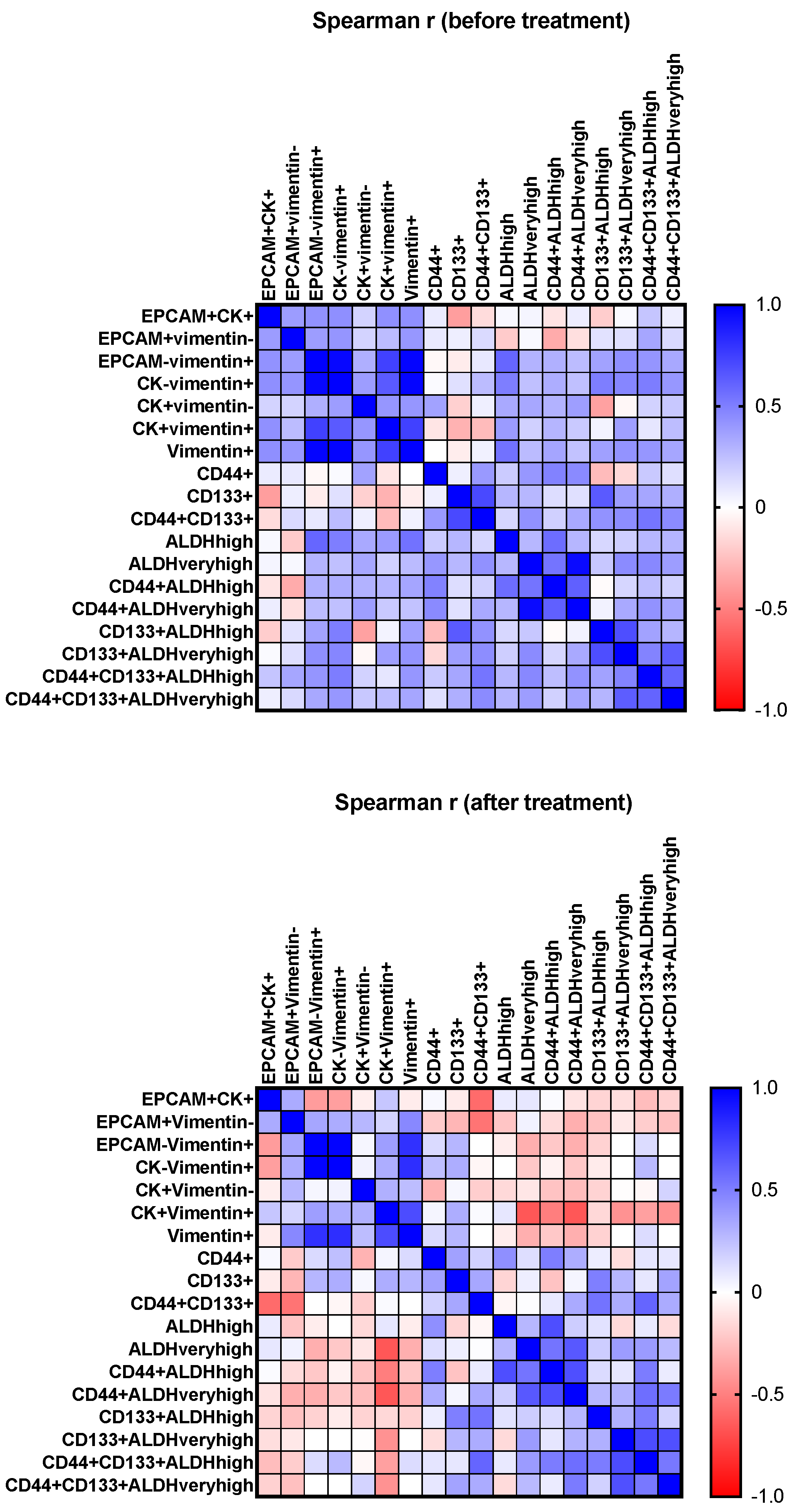
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.-L.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in Cancer Survival, Mortality, and Incidence in Seven High-Income Countries 1995–2014 (ICBP SURVMARK-2): A Population-Based Study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, R.F.; Ng, C.K.Y.; Cooke, S.L.; Newman, S.; Temple, J.; Piskorz, A.M.; Gale, D.; Sayal, K.; Murtaza, M.; Baldwin, P.J.; et al. Spatial and Temporal Heterogeneity in High-Grade Serous Ovarian Cancer: A Phylogenetic Analysis. PLoS Med. 2015, 12, e1001789. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.K.; Asher, R.; Friedlander, M.; Gebski, V.; Gonzalez-Martin, A.; Lortholary, A.; Lesoin, A.; Kurzeder, C.; Largillier, R.; Hilpert, F.; et al. Development and Validation of a Prognostic Nomogram for Overall Survival in Patients with Platinum-Resistant Ovarian Cancer Treated with Chemotherapy. Eur. J. Cancer 2019, 117, 99–106. [Google Scholar] [CrossRef]
- van de Laar, R.; IntHout, J.; Van Gorp, T.; Verdonschot, S.; van Altena, A.M.; Gerestein, C.G.; Massuger, L.F.A.G.; Zusterzeel, P.L.M.; Kruitwagen, R.F.P.M. External Validation of Three Prognostic Models for Overall Survival in Patients with Advanced-Stage Epithelial Ovarian Cancer. Br. J. Cancer 2014, 110, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Hernández, L.E.; Eslami-S, Z.; Alix-Panabières, C. Circulating Tumor Cell as the Functional Aspect of Liquid Biopsy to Understand the Metastatic Cascade in Solid Cancer. Mol. Asp. Med. 2020, 72, 100816. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ma, Q.; Zhang, Y.; Wang, X.; Xu, K.; Yan, K.; Dong, W.; Fan, Q.; Zhang, Y.; Qiu, X. Self-Seeding Circulating Tumor Cells Promote the Proliferation and Metastasis of Human Osteosarcoma by Upregulating Interleukin-8. Cell Death Dis. 2019, 10, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradeep, S.; Kim, S.W.; Wu, S.Y.; Nishimura, M.; Chaluvally-Raghavan, P.; Miyake, T.; Pecot, C.V.; Kim, S.-J.; Choi, H.J.; Bischoff, F.Z.; et al. Hematogenous Metastasis of Ovarian Cancer: Rethinking Mode of Spread. Cancer Cell 2014, 26, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Tellez-Gabriel, M.; Heymann, M.-F.; Heymann, D. Circulating Tumor Cells as a Tool for Assessing Tumor Heterogeneity. Theranostics 2019, 9, 4580–4594. [Google Scholar] [CrossRef]
- Yang, C.; Zou, K.; Zheng, L.; Xiong, B. Prognostic and Clinicopathological Significance of Circulating Tumor Cells Detected by RT-PCR in Non-Metastatic Colorectal Cancer: A Meta-Analysis and Systematic Review. BMC Cancer 2017, 17, 725. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Gong, L.; Zhang, T.; Ye, J.; Chai, L.; Ni, C.; Mao, Y. Prognostic Value of Circulating Tumor Cells in Metastatic Breast Cancer: A Systemic Review and Meta-Analysis. Clin. Transl. Oncol. 2016, 18, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Pearl, M.L.; Dong, H.; Tulley, S.; Zhao, Q.; Golightly, M.; Zucker, S.; Chen, W.-T. Treatment Monitoring of Patients with Epithelial Ovarian Cancer Using Invasive Circulating Tumor Cells (ICTCs). Gynecol. Oncol. 2015, 137, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Loh, C.-Y.; Chai, J.; Tang, T.; Wong, W.; Sethi, G.; Shanmugam, M.; Chong, P.; Looi, C. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Teeuwssen, M.; Fodde, R. Wnt Signaling in Ovarian Cancer Stemness, EMT, and Therapy Resistance. J. Clin. Med. 2019, 8, 1658. [Google Scholar] [CrossRef] [Green Version]
- Chirshev, E.; Hojo, N.; Bertucci, A.; Sanderman, L.; Nguyen, A.; Wang, H.; Suzuki, T.; Brito, E.; Martinez, S.R.; Castañón, C.; et al. Epithelial/Mesenchymal Heterogeneity of High-grade Serous Ovarian Carcinoma Samples Correlates with MiRNA Let-7 Levels and Predicts Tumor Growth and Metastasis. Mol. Oncol. 2020, 14, 2796–2813. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Jolly, M.K.; Woodward, W.A.; Levine, H.; Deem, M.W. Analysis of Hierarchical Organization in Gene Expression Networks Reveals Underlying Principles of Collective Tumor Cell Dissemination and Metastatic Aggressiveness of Inflammatory Breast Cancer. Front. Oncol. 2018, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kim, D.; Kim, D.K.; Choi, K.U.; Suh, D.S.; Kim, J.H. Therapeutic Strategies for Targeting Ovarian Cancer Stem Cells. Int. J. Mol. Sci. 2021, 22, 5059. [Google Scholar] [CrossRef]
- Lei, M.M.L.; Lee, T.K.W. Cancer Stem Cells: Emerging Key Players in Immune Evasion of Cancers. Front. Cell Dev. Biol. 2021, 9, 692940. [Google Scholar] [CrossRef]
- Liao, M.; Wang, C.; Yang, B.; Huang, D.; Zheng, Y.; Wang, S.; Wang, X.; Zhang, J.; Tang, C.; Xu, Z.; et al. Autophagy Blockade by Ai Du Qing Formula Promotes Chemosensitivity of Breast Cancer Stem Cells Via GRP78/β-Catenin/ABCG2 Axis. Front. Pharmacol. 2021, 12, 659297. [Google Scholar] [CrossRef]
- Quan, Q.; Wang, X.; Lu, C.; Ma, W.; Wang, Y.; Xia, G.; Wang, C.; Yang, G. Cancer Stem-like Cells with Hybrid Epithelial/Mesenchymal Phenotype Leading the Collective Invasion. Cancer Sci. 2020, 111, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Blassl, C.; Kuhlmann, J.D.; Webers, A.; Wimberger, P.; Fehm, T.; Neubauer, H. Gene Expression Profiling of Single Circulating Tumor Cells in Ovarian Cancer—Establishment of a Multi-Marker Gene Panel. Mol. Oncol. 2016, 10, 1030–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemba, A.; Purzycka-Olewiecka, J.K.; Wcisło, G.; Czarnecka, A.M.; Lewicki, S.; Lesyng, B.; Szczylik, C.; Kieda, C. Surface Markers of Cancer Stem-like Cells of Ovarian Cancer and Their Clinical Relevance. Współczesna Onkol. 2018, 2018, 48–55. [Google Scholar] [CrossRef]
- Heong, V.; Topp, M.; Ananda, S.; McNally, O.; Lindeman, G.J.; Haluska, P.; Wakefield, M.; Swisher, E.M.; Tan, D.S.; Ruby, H.; et al. Targeting the C5 Subclass of High-Grade Serous Ovarian Cancer Using Patient-Derived Xenografts: Microtubule Polymerisation Inhibitors. J. Clin. Oncol. 2015, 33, e22202. [Google Scholar] [CrossRef]
- Matte, I.; Legault, C.M.; Garde-Granger, P.; Laplante, C.; Bessette, P.; Rancourt, C.; Piché, A. Mesothelial Cells Interact with Tumor Cells for the Formation of Ovarian Cancer Multicellular Spheroids in Peritoneal Effusions. Clin. Exp. Metastasis 2016, 33, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.; Trimble, E.; Tinker, A.; Alberts, D.; Avall-Lundqvist, E.; Brady, M.; Harter, P.; Pignata, S.; Pujade- Lauraine, E.; Sehouli, J.; et al. Clinical Trials in Recurrent Ovarian Cancer. Int. J. Gynecol. Cancer 2011, 21, 771–775. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard Chemotherapy with or without Bevacizumab for Women with Newly Diagnosed Ovarian Cancer (ICON7): Overall Survival Results of a Phase 3 Randomised Trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Watanabe, T.; Okumura, T.; Hirano, K.; Yamaguchi, T.; Sekine, S.; Nagata, T.; Tsukada, K. Circulating Tumor Cells Expressing Cancer Stem Cell Marker CD44 as a Diagnostic Biomarker in Patients with Gastric Cancer. Oncol. Lett. 2017, 13, 281–288. [Google Scholar] [CrossRef]
- Poveda, A.; Kaye, S.B.; McCormack, R.; Wang, S.; Parekh, T.; Ricci, D.; Lebedinsky, C.A.; Tercero, J.C.; Zintl, P.; Monk, B.J. Circulating Tumor Cells Predict Progression Free Survival and Overall Survival in Patients with Relapsed/Recurrent Advanced Ovarian Cancer. Gynecol. Oncol. 2011, 122, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Behbakht, K.; Sill, M.W.; Darcy, K.M.; Rubin, S.C.; Mannel, R.S.; Waggoner, S.; Schilder, R.J.; Cai, K.Q.; Godwin, A.K.; Alpaugh, R.K. Phase II Trial of the MTOR Inhibitor, Temsirolimus and Evaluation of Circulating Tumor Cells and Tumor Biomarkers in Persistent and Recurrent Epithelial Ovarian and Primary Peritoneal Malignancies: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2011, 123, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Banys-Paluchowski, M.; Fehm, T.; Neubauer, H.; Paluchowski, P.; Krawczyk, N.; Meier-Stiegen, F.; Wallach, C.; Kaczerowsky, A.; Gebauer, G. Clinical Relevance of Circulating Tumor Cells in Ovarian, Fallopian Tube and Peritoneal Cancer. Arch. Gynecol. Obstet. 2020, 301, 1027–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obermayr, E.; Reiner, A.; Brandt, B.; Braicu, E.I.; Reinthaller, A.; Loverix, L.; Concin, N.; Woelber, L.; Mahner, S.; Sehouli, J.; et al. The Long-Term Prognostic Significance of Circulating Tumor Cells in Ovarian Cancer—A Study of the OVCAD Consortium. Cancers 2021, 13, 2613. [Google Scholar] [CrossRef]
- Po, J.W.; Roohullah, A.; Lynch, D.; DeFazio, A.; Harrison, M.; Harnett, P.R.; Kennedy, C.; de Souza, P.; Becker, T.M. Improved Ovarian Cancer EMT-CTC Isolation by Immunomagnetic Targeting of Epithelial EpCAM and Mesenchymal N-Cadherin. J. Circ. Biomark. 2018, 7, 184945441878261. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Fan, W.-H.; Song, Z.; Lou, H.; Kang, X. Comparison of Circulating Tumor Cell (CTC) Detection Rates with Epithelial Cell Adhesion Molecule (EpCAM) and Cell Surface Vimentin (CSV) Antibodies in Different Solid Tumors: A Retrospective Study. PeerJ 2021, 9, e10777. [Google Scholar] [CrossRef]
- Hatina, J.; Boesch, M.; Sopper, S.; Kripnerova, M.; Wolf, D.; Reimer, D.; Marth, C.; Zeimet, A.G. Ovarian Cancer Stem Cell Heterogeneity. In Stem Cells Heterogeneity in Cancer; Birbrair, A., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1139, pp. 201–221. [Google Scholar]
- Alvero, A.B.; Chen, R.; Fu, H.-H.; Montagna, M.; Schwartz, P.E.; Rutherford, T.; Silasi, D.-A.; Steffensen, K.D.; Waldstrom, M.; Visintin, I.; et al. Molecular Phenotyping of Human Ovarian Cancer Stem Cells Unravels the Mechanisms for Repair and Chemoresistance. Cell Cycle 2009, 8, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhang, H.; Zhang, G.; Shi, Y.; Huang, J. Co-Expression of CD44/MyD88 Is a Poor Prognostic Factor in Advanced Epithelial Ovarian Cancer. Ann. Transl. Med. 2019, 7, 91. [Google Scholar] [CrossRef]
- Loreth, D.; Schuette, M.; Zinke, J.; Mohme, M.; Piffko, A.; Schneegans, S.; Stadler, J.; Janning, M.; Loges, S.; Joosse, S.A.; et al. CD74 and CD44 Expression on CTCs in Cancer Patients with Brain Metastasis. Int. J. Mol. Sci. 2021, 22, 6993. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.; Bobbs, A.; Sattler, R.; Kurkewich, J.L.; Dausinas, P.B.; Nallathamby, P.; Cowden Dahl, K.D. CD133 Promotes Adhesion to the Ovarian Cancer Metastatic Niche. Cancer Growth Metastasis 2018, 11, 1179064418767882. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.; Chang, D.Y.; Rosen, D.G.; Mercado-Uribe, I.; Liu, J. CD133 Expression Associated with Poor Prognosis in Ovarian Cancer. Mod. Pathol. 2012, 25, 456–464. [Google Scholar] [CrossRef] [Green Version]
- Ferrandina, G.; Bonanno, G.; Pierelli, L.; Perillo, A.; Procoli, A.; Mariotti, A.; Corallo, M.; Martinelli, E.; Rutella, S.; Paglia, A.; et al. Expression of CD133-1 and CD133-2 in Ovarian Cancer. Int. J. Gynecol. Cancer 2008, 18, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Liu, S.; Roh, M.; Vatan, L.; Szeliga, W.; Wei, S.; Banerjee, M.; Mao, Y.; Kotarski, J.; Wicha, M.S.; et al. Expression of Aldehyde Dehydrogenase and CD133 Defines Ovarian Cancer Stem Cells. Int. J. Cancer 2012, 130, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Ruscito, I.; Darb-Esfahani, S.; Kulbe, H.; Bellati, F.; Zizzari, I.G.; Rahimi Koshkaki, H.; Napoletano, C.; Caserta, D.; Rughetti, A.; Kessler, M.; et al. The Prognostic Impact of Cancer Stem-like Cell Biomarker Aldehyde Dehydrogenase-1 (ALDH1) in Ovarian Cancer: A Meta-Analysis. Gynecol. Oncol. 2018, 150, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Chefetz, I.; Grimley, E.; Yang, K.; Hong, L.; Vinogradova, E.V.; Suciu, R.; Kovalenko, I.; Karnak, D.; Morgan, C.A.; Chtcherbinine, M.; et al. A Pan-ALDH1A Inhibitor Induces Necroptosis in Ovarian Cancer Stem-like Cells. Cell Rep. 2019, 26, 3061–3075. [Google Scholar] [CrossRef] [Green Version]
- Silva, I.A.; Bai, S.; McLean, K.; Yang, K.; Griffith, K.; Thomas, D.; Ginestier, C.; Johnston, C.; Kueck, A.; Reynolds, R.K.; et al. Aldehyde Dehydrogenase in Combination with CD133 Defines Angiogenic Ovarian Cancer Stem Cells That Portend Poor Patient Survival. Cancer Res. 2011, 71, 3991–4001. [Google Scholar] [CrossRef] [Green Version]
- Meng, E.; Mitra, A.; Tripathi, K.; Finan, M.A.; Scalici, J.; McClellan, S.; da Silva, L.M.; Reed, E.; Shevde, L.A.; Palle, K.; et al. ALDH1A1 Maintains Ovarian Cancer Stem Cell-Like Properties by Altered Regulation of Cell Cycle Checkpoint and DNA Repair Network Signaling. PLoS ONE 2014, 9, e107142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Brocker, C.; Koppaka, V.; Chen, Y.; Jackson, B.C.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Aldehyde Dehydrogenases in Cellular Responses to Oxidative/Electrophilicstress. Free Radic. Biol. Med. 2013, 56, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Casagrande, N.; Borghese, C.; Agostini, F.; Durante, C.; Mazzucato, M.; Colombatti, A.; Aldinucci, D. In Ovarian Cancer Multicellular Spheroids, Platelet Releasate Promotes Growth, Expansion of ALDH+ and CD133+ Cancer Stem Cells, and Protection against the Cytotoxic Effects of Cisplatin, Carboplatin and Paclitaxel. Int. J. Mol. Sci. 2021, 22, 3019. [Google Scholar] [CrossRef]


| No. | Manufacturer | Product | Usage |
|---|---|---|---|
| 1 | BioLegend, CA, USA | PE anti-human CD45 Antibody Clone 2D1 | CD45 |
| 2 | BioLegend | PE Mouse IgG1, κ Isotype Ctrl (FC) Antibody clone MOPC-21 | Isotype |
| 3 | BioLegend | APC anti-human CD44 Antibody Clone BJ18 | CD44 |
| 4 | BioLegend | APC Mouse IgG1, κ Isotype Ctrl Antibody clone MOPC-21 | Isotype |
| 5 | Miltenyi biotec, Bergisch Gladbach, Germany | CD133/1 VioBright 667 mouse IgG1 clone AC133 | CD133 |
| 6 | Miltenyi biotec | VioBright 515 mouse IgG1, Clone IS5-21F5 | Isotype |
| 7 | Stemcell, Canada | ALDEFLUOR kit #01700 | ALDH |
| 8 | Invitrogen, CA, USA | CD326 (EpCAM) Monoclonal Antibody PE-Cyanine7 clone 1B7 | EpCAM |
| 9 | Invitrogen | PE-Cyanine7 mouse/IgG1, kappa clone P3.6.2.8.1 | Isotype |
| 10 | Abcam, Cambridge, United Kingdom | Mouse Anti-Cytokeratin 8 + 18 + 19 antibody clone 2A4 (ab41825) IgG1 | Primary |
| 11 | BioLegend | Brilliant Violet 421™ anti-mouse IgG1 Antibody clone RMG1-1 | Secondary |
| 12 | BioLegend | Alexa Fluor 647 anti-Vimentin Antibody clone 091D3 Mouse IgG2a | Vimentin |
| 13 | BioLegend | Alexa Fluor 647 anti-mouse IgG2a clone RMG2a-62 | Isotype |
| Parameter | Number of Patients (%) |
|---|---|
| Age—median (IQR Q1–Q3), years—66 (53–70) | |
| Serum CA-125 before treatment—median (IQR), U/mL—863 (309–1595) | |
| FIGO stage | |
| I | 2 (5%) |
| III | 22 (58%) |
| IV | 14 (37%) |
| Ascites at diagnosis | |
| yes | 31 (82%) |
| no | 7 (18%) |
| Histological subtype | |
| serous high-grade adenocarcinoma | 16 (42%) |
| other subtypes | 4 (11%) |
| not determined due to CRS 3 | 5 (13%) |
| no histological assessment (only cytological verification) | 13 (34%) |
| Chemotherapy | |
| neoadjuvant | 31 (82%) |
| adjuvant | 7 (18%) |
| Cytoreductive surgery within first-line treatment | |
| performed | 20 (53%) |
| not performed | 18 (47%) |
| Maintenance therapy with bevacizumab after first-line CT | |
| performed | 12 (32%) |
| not performed | 26 (68%) |
| Phenotype | EpCAM+CK+ (Median (Min–Max)) | EpCAM+Vimentin- | EpCAM-Vimentin+ | CK-Vimentin+ | CK+Vimentin- | CK+Vimentin+ | Vimentin+ |
|---|---|---|---|---|---|---|---|
| Before | 0 (0–11) | 25 (9–48) | 59 (15–108) | 50 (14–89) | 218 (112–392) | 6 (2–24) | 59 (20–137) |
| During | 1 (0–15) | 28 (12–45) | 57 (40–80) | 48 (24–77) | 217 (101–310) | 5 (0–12) | 79 (50–93) |
| Phenotype | CD44+ | CD133+ | CD44+CD133+ | ALDHhigh | ALDHveryhigh | ||
| Before | 527 (121–1519) | 101 (15–291) | 1 (0–14) | 90 (50–152) | 12 (1–57) | ||
| During | 205 (120–339) | 172 (57–391) | 4 (0–23) | 110 (37–154) | 6 (0–9) | ||
| Phenotype | CD44+ALDHhigh | CD44+ALDH veryhigh | CD133+ALDHhigh | CD133+ALDHveryhigh (Median (Min–Max)) | |||
| Before | 15 (2–49) | 7 (0–26) | 0 (0–10) | 0 (0–83) | |||
| During | 16 (6–49) | 1 (0–5) | 6 (0–26) | 0 (0–300) | |||
| No. | Age | Stage | Histology | CT Regimen | Ascites | Platinum Sensitivity | CD44+CD133+ ALDHhigh Count | CD44+CD133+ ALDHveryhigh Count |
|---|---|---|---|---|---|---|---|---|
| Patients positive for the cells at baseline | ||||||||
| 1 | 61 | IV | HGS | NACT | Yes | No | 11 | 34 |
| 2 | 69 | IV | HGS | NACT | Yes | Yes (M) | 7 | 0 |
| 3 | 72 | III | NS | NACT | Yes | No | 2 | 2 |
| 4 | 70 | III | NS | NACT | Yes | No | 2 | 2 |
| 5 | 64 | III | HGS | NACT | Yes | Yes | 1 | 4 |
| 6 | 49 | IV | HGS | NACT | Yes | No | 5 | 0 |
| Patients positive for the cells during treatment only | ||||||||
| 7 | 37 | IV | NS | NACT | Yes | Yes (M) | 29 | 44 |
| 8 | 73 | IV | HGS | NACT | Yes | Yes | 1 | 1 |
| 9 | 52 | III | HGS | NACT | Yes | Yes | 4 | 7 |
| 10 | 57 | IV | Clear cell | ACT | No | Yes | 3 | 0 |
| Category | Univariable Analysis, HR (95% CI) | Multivariable Analysis, HR (95% CI) |
|---|---|---|
| FIGO stage | ||
| Stage III | ||
| Stage IV | 1.13 (0.61–2.10), p = 0.699 | |
| Ascites | ||
| No ascites | ||
| Ascites present | 2.07 (1.03–4.18), p = 0.042 | 1.65 (0.81–3.37), p = 0.169 |
| Surgery in first line | ||
| Surgery not performed | ||
| Surgery performed | 0.14 (0.03–0.67), p = 0.014 | 0.06 (0.01–0.48), p = 0.009 |
| Chemotherapy | ||
| Adjuvant regimen | ||
| Neoadjuvant regimen | 2.06 (1.00–4.25), p = 0.051 | 1.27 (0.47–2.86), p = 0.562 |
| Age | 1.01 (0.98–1.04), p = 0.458 | |
| Initial serum CA-125 | 1.00 (1.00–1.00), p = 0.619 | |
| Initial WBC count in blood | 1.19 (1.01–1.41), p = 0.040 | 1.21 (0.91–1.62), p = 0.195 |
| Maintenance after first line | 0.28 (0.11–0.68), p = 0.006 | 0.27 (0.06–1.27), p = 0.096 |
| CTC number before treatment | ||
| EpCAM+CK+ | 1.01 (0.89–1.15), p = 0.856 | |
| EpCAM+vimentin- | 1.00 (1.00–1.01), p = 0.260 | |
| EpCAM-vimentin+ | 1.00 (1.00–1.00), p = 0.456 | |
| CK-vimentin+ | 1.00 (1.00–1.00), p = 0.484 | |
| CK+vimentin- | 1.00 (1.00–1.00), p=0.711 | |
| CK+vimentin+ | 1.00 (0.98–1.01), p = 0.727 | |
| CD44+ | 1.00 (1.00–1.00), p = 0.377 | |
| CD133+ | 1.00 (1.00–1.00), p = 0.885 | |
| ALDHhigh | 1.00 (1.00–1.00), p = 0.375 | |
| ALDHveryhigh | 1.00 (1.00–1.01), p = 0.080 | |
| CD44+CD133+ | 1.01 (0.99–1.03), p = 0.292 | |
| CD44+ALDHhigh | 1.00 (0.99–1.00), p = 0.160 | |
| CD44+ALDHveryhigh | 1.01 (1.00–1.02), p = 0.175 | |
| CD133+ALDHhigh | 1.01 (0.97–1.04), p = 0.764 | |
| CD133+ALDHveryhigh | 1.03 (1.01–1.06), p = 0.014 | 1.06 (1.02–1.12), p = 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gening, S.O.; Abakumova, T.V.; Gafurbaeva, D.U.; Rizvanov, A.A.; Antoneeva, I.I.; Miftakhova, R.R.; Peskov, A.B.; Gening, T.P. The Detection of Stem-Like Circulating Tumor Cells Could Increase the Clinical Applicability of Liquid Biopsy in Ovarian Cancer. Life 2021, 11, 815. https://doi.org/10.3390/life11080815
Gening SO, Abakumova TV, Gafurbaeva DU, Rizvanov AA, Antoneeva II, Miftakhova RR, Peskov AB, Gening TP. The Detection of Stem-Like Circulating Tumor Cells Could Increase the Clinical Applicability of Liquid Biopsy in Ovarian Cancer. Life. 2021; 11(8):815. https://doi.org/10.3390/life11080815
Chicago/Turabian StyleGening, Snezhanna O., Tatyana V. Abakumova, Dina U. Gafurbaeva, Albert A. Rizvanov, Inna I. Antoneeva, Regina R. Miftakhova, Andrey B. Peskov, and Tatyana P. Gening. 2021. "The Detection of Stem-Like Circulating Tumor Cells Could Increase the Clinical Applicability of Liquid Biopsy in Ovarian Cancer" Life 11, no. 8: 815. https://doi.org/10.3390/life11080815
APA StyleGening, S. O., Abakumova, T. V., Gafurbaeva, D. U., Rizvanov, A. A., Antoneeva, I. I., Miftakhova, R. R., Peskov, A. B., & Gening, T. P. (2021). The Detection of Stem-Like Circulating Tumor Cells Could Increase the Clinical Applicability of Liquid Biopsy in Ovarian Cancer. Life, 11(8), 815. https://doi.org/10.3390/life11080815








