Shared Molecular Mechanisms of Hypertrophic Cardiomyopathy and Its Clinical Presentations: Automated Molecular Mechanisms Extraction Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Mechanisms Extraction
2.2. Networks Generation
2.3. Network Analysis
3. Results
3.1. Network Analysis
3.1.1. Networks
3.1.2. The Most Important Nodes
3.1.3. Nodes’ Centrality Scores
3.1.4. Reliability of Interactions
3.1.5. Cooperatively Working Elements
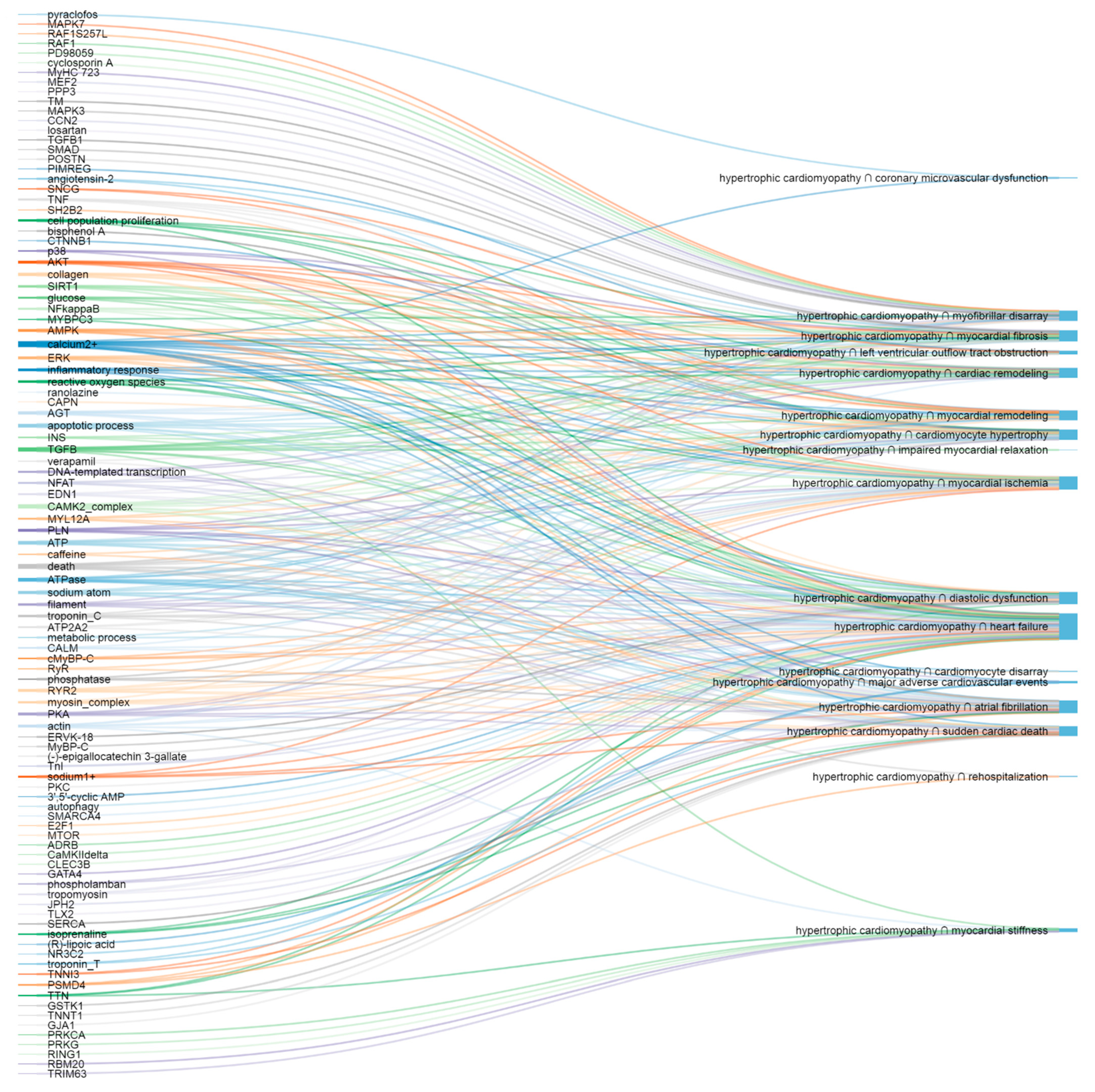
3.2. Shared Molecular Elements and Pathways
3.2.1. Hypertrophic Cardiomyopathy and Structural Changes
3.2.2. Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction
3.2.3. Hypertrophic Cardiomyopathy and Contractile Dysfunction
3.2.4. Hypertrophic Cardiomyopathy and Arrhythmia
3.2.5. Hypertrophic Cardiomyopathy and Ischemia
3.2.6. Hypertrophic Cardiomyopathy and Endpoints
3.2.7. The Most Important Shared Elements and Pathways
4. Discussion
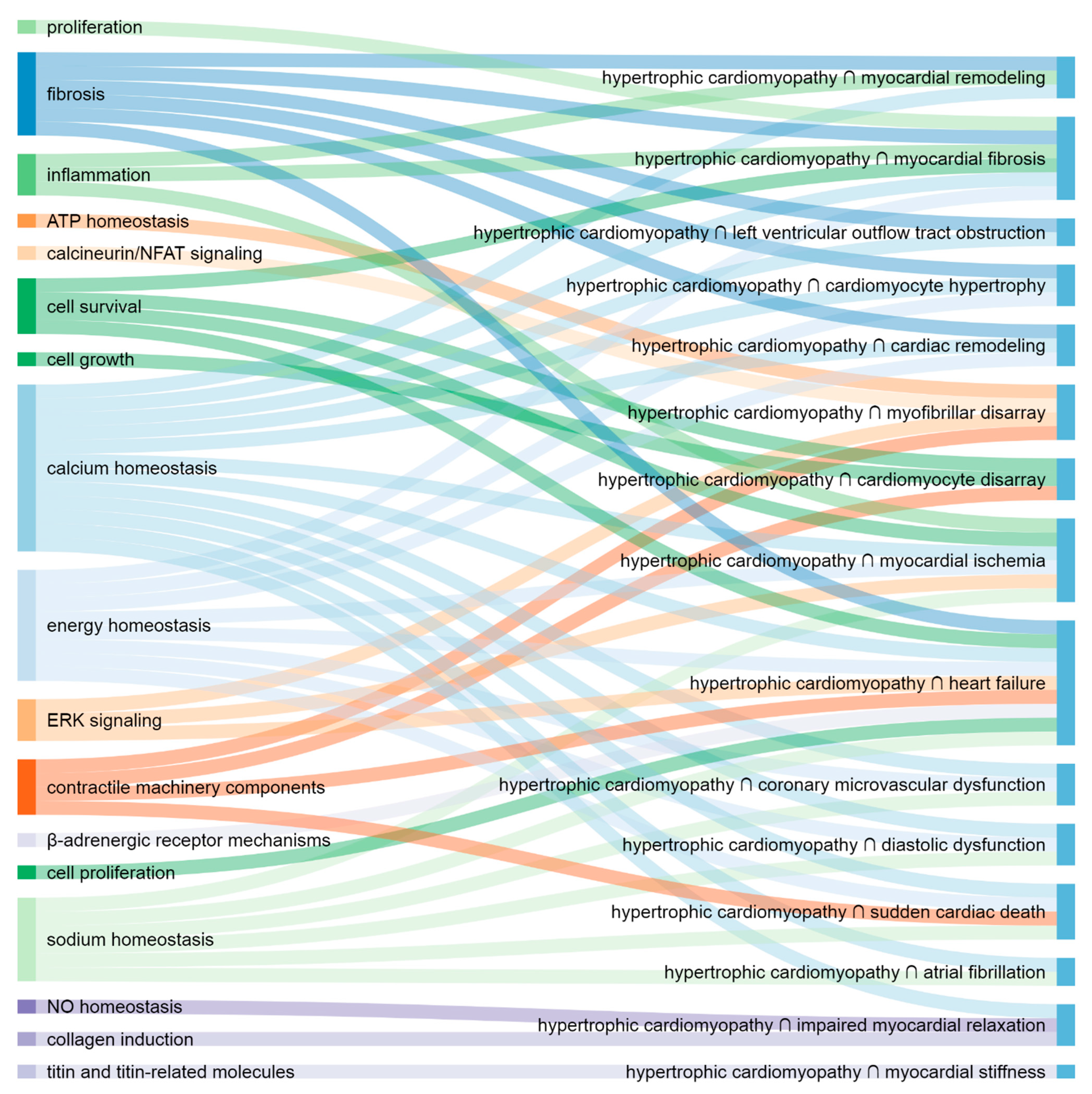
4.1. Shared Molecular Elements and Pathways
4.1.1. Hypertrophic Cardiomyopathy and Structural Changes
4.1.2. Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction
4.1.3. Hypertrophic Cardiomyopathy and Contractile Dysfunction
4.1.4. Hypertrophic Cardiomyopathy and Arrhythmia
4.1.5. Hypertrophic Cardiomyopathy and Ischemia
4.1.6. Hypertrophic Cardiomyopathy and Endpoints
4.1.7. Calcium in Hypertrophic Cardiomyopathy Presentations
4.1.8. The Most Important Shared Elements and Pathways
4.2. Non-Molecular Factors That Affect Clinical Presentations of HCM
4.3. General
4.4. Limitations
4.5. Significance and Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabater-Molina, M.; Pérez-Sánchez, I.; Hernández del Rincón, J.P.; Gimeno, J.R. Genetics of hypertrophic cardiomyopathy: A review of current state. Clin. Genet. 2018, 93, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Firth, J. Cardiology: Hypertrophic cardiomyopathy. Clin. Med. 2019, 19, 61–63. [Google Scholar]
- Geske, J.B.; Ommen, S.R.; Gersh, B.J. Hypertrophic cardiomyopathy: Clinical update. JACC Heart Fail. 2018, 6, 364–375. [Google Scholar] [CrossRef]
- Deranek, A.E.; Klass, M.M.; Tardiff, J.C. Moving beyond simple answers to complex disorders in sarcomeric cardiomyopathies: The role of integrated systems. Pflügers Arch. Eur. J. Physiol. 2019, 471, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Prondzynski, M.; Mearini, G.; Carrier, L. Gene therapy strategies in the treatment of hypertrophic cardiomyopathy. Pflügers Arch. Eur. J. Physiol. 2019, 471, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.P.; Shimada, Y.J.; Ginns, J.; Weiner, S.D.; Takayama, H. Septal myectomy for hypertrophic cardiomyopathy: Important surgical knowledge and technical tips in the era of increasing alcohol septal ablation. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 192–200. [Google Scholar] [CrossRef]
- Tuohy, C.V.; Kaul, S.; Song, H.K.; Nazer, B.; Heitner, S.B. Hypertrophic cardiomyopathy: The future of treatment. Eur. J. Heart Fail. 2020, 22, 228–240. [Google Scholar] [CrossRef]
- Price, J.; Clarke, N.; Turer, A.; Quintana, E.; Mestres, C.; Huffman, L.; Peltz, M.; Wait, M.; Ring, W.S.; Jessen, M.; et al. Hypertrophic obstructive cardiomyopathy: Review of surgical treatment. Asian Cardiovasc. Thorac. Ann. 2017, 25, 594–607. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, P.Y. Review of recent advances in the management of hypertrophic cardiomyopathy. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5207–5210. [Google Scholar]
- Gómez, J.; Reguero, J.R.; Coto, E. The ups and downs of genetic diagnosis of hypertrophic cardiomyopathy. Rev. Española Cardiol. 2016, 69, 61–68. [Google Scholar] [CrossRef]
- Tower-Rader, A.; Desai, M.Y. Phenotype–genotype correlation in hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e006066. [Google Scholar] [CrossRef]
- Velicki, L.; Jakovljevic, D.G.; Preveden, A.; Golubovic, M.; Bjelobrk, M.; Ilic, A.; Stojsic, S.; Barlocco, F.; Tafelmeier, M.; Okwose, N.; et al. Genetic determinants of clinical phenotype in hypertrophic cardiomyopathy. BMC Cardiovasc. Disord. 2020, 20, 516. [Google Scholar] [CrossRef] [PubMed]
- Smole, T.; Žunkovič, B.; Pičulin, M.; Kokalj, E.; Robnik-Šikonja, M.; Kukar, M.; Fotiadis, D.I.; Pezoulas, V.C.; Tachos, N.S.; Barlocco, F.; et al. A machine learning-based risk stratification model for ventricular tachycardia and heart failure in hypertrophic cardiomyopathy. Comput. Biol. Med. 2021, 135, 104648. [Google Scholar] [CrossRef]
- Farrell, E.T.; Grimes, A.C.; de Lange, W.J.; Armstrong, A.E.; Ralphe, J.C. Increased postnatal cardiac hyperplasia precedes cardiomyocyte hypertrophy in a model of hypertrophic cardiomyopathy. Front Physiol. 2017, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.A.; Mai Ja, K.P.M.; Lin, Y.H.; Shim, W.; Boisvert, W.A.; Hausenloy, D.J. Induced pluripotent stem cells for modelling energetic alterations in hypertrophic cardiomyopathy. Cond. Med. 2019, 2, 142–151. [Google Scholar]
- MacIver, D.H.; Clark, A.L. Contractile dysfunction in sarcomeric hypertrophic cardiomyopathy. J. Card. Fail. 2016, 22, 731–737. [Google Scholar] [CrossRef]
- Sukhacheva, T.V.; Chudinovskikh, Y.A.; Eremeeva, M.V.; Serov, R.A.; Bockeria, L.A. Proliferative potential of cardiomyocytes in hypertrophic cardiomyopathy: Correlation with myocardial remodeling. Bull. Exp. Biol. Med. 2016, 162, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Fernlund, E.; Gyllenhammar, T.; Jablonowski, R.; Carlsson, M.; Larsson, A.; Ärnlöv, J.; Liuba, P. Serum biomarkers of myocardial remodeling and coronary dysfunction in early stages of hypertrophic cardiomyopathy in the young. Pediatr. Cardiol. 2017, 38, 853–863. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Kp, M.M.J.; Chua, J.; Hernandez-Resendiz, S.; Liehn, E.A.; Gan, L.M.; Michaëlsson, E.; Jonsson, M.K.B.; Ryden-Markinhuhta, K.; Bhat, R.V.; et al. Inhibiting cardiac myeloperoxidase alleviates the relaxation defect in hypertrophic cardiomyocytes. Cardiovasc. Res. 2021, in press. [Google Scholar] [CrossRef]
- Coppini, R.; Ferrantini, C.; Mugelli, A.; Poggesi, C.; Cerbai, E. Altered Ca2+ and Na+ homeostasis in human hypertrophic cardiomyopathy: Implications for arrhythmogenesis. Front. Physiol. 2018, 9, 1391. [Google Scholar] [CrossRef]
- Argirò, A.; Zampieri, M.; Berteotti, M.; Marchi, A.; Tassetti, L.; Zocchi, C.; Iannone, L.; Bacchi, B.; Cappelli, F.; Stefàno, P.; et al. Emerging Medical Treatment for Hypertrophic Cardiomyopathy. J. Clin. Med. 2021, 10, 951. [Google Scholar] [CrossRef]
- Toepfer, C.N.; Wakimoto, H.; Garfinkel, A.C.; McDonough, B.; Liao, D.; Jiang, J.; Tai, A.C.; Gorham, J.M.; Lunde, I.G.; Lun, M.; et al. Hypertrophic cardiomyopathy mutations in MYBPC3 dysregulate myosin. Sci. Transl. Med. 2019, 11, eaat1199. [Google Scholar] [CrossRef] [PubMed]
- Cordts, K.; Seelig, D.; Lund, N.; Carrier, L.; Böger, R.H.; Avanesov, M.; Tahir, E.; Schwedhelm, E.; Patten, M. Association of asymmetric dimethylarginine and diastolic dysfunction in patients with hypertrophic cardiomyopathy. Biomolecules 2019, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Aguiar Rosa, S.; Rocha Lopes, L.; Fiarresga, A.; Ferreira, R.C.; Mota Carmo, M. Coronary microvascular dysfunction in hypertrophic cardiomyopathy: Pathophysiology, assessment, and clinical impact. Microcirculation 2021, 28, e12656. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Xu, H.Y.; Zheng, S.S.; Zhu, Y.; Xiao, J.X.; Zhou, W.; Yu, S.S.; Gong, L.G. 3.0 T magnetic resonance myocardial perfusion imaging for semi-quantitative evaluation of coronary microvascular dysfunction in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2017, 33, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Raphael, C.E.; Cooper, R.; Parker, K.H.; Collinson, J.; Vassiliou, V.; Pennell, D.J.; de Silva, R.; Hsu, L.Y.; Greve, A.M.; Nijjer, S.; et al. Mechanisms of myocardial ischemia in hypertrophic cardiomyopathy: Insights from wave intensity analysis and magnetic resonance. J. Am. Coll. Cardiol. 2016, 68, 1651–1660. [Google Scholar] [CrossRef]
- INDRA Database. Available online: https://indra-db.readthedocs.io/en/latest/ (accessed on 31 May 2021).
- Huh, S. How to add a journal to the international databases, Science Citation Index Expanded and MEDLINE. Arch. Plast. Surg. 2016, 43, 487. [Google Scholar] [CrossRef]
- Gyori, B.M.; Bachman, J.A.; Subramanian, K.; Muhlich, J.L.; Galescu, L.; Sorger, P.K. From word models to executable models of signaling networks using automated assembly. Mol. Syst. Biol. 2017, 13, 954. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Pratt, D.; Chen, J.; Welker, D.; Rivas, R.; Pillich, R.; Rynkov, V.; Ono, K.; Miello, C.; Hicks, L.; Szalma, S.; et al. NDEx, the Network Data Exchange. Cell Syst. 2015, 1, 302–305. [Google Scholar] [CrossRef]
- Pillich, R.T.; Chen, J.; Rynkov, V.; Welker, D.; Pratt, D. NDEx: A community resource for sharing and publishing of biological networks. Methods Mol. Biol. 2017, 1558, 271–301. [Google Scholar] [PubMed]
- Pratt, D.; Chen, J.; Pillich, R.; Rynkov, V.; Gary, A.; Demchak, B.; Ideker, T. NDEx 2.0: A clearinghouse for research on cancer pathways. Cancer Res. 2017, 77, e58–e61. [Google Scholar] [CrossRef] [PubMed]
- Cytoscape App Store, wk-shell-decomposition. Available online: http://apps.cytoscape.org/apps/wkshelldecomposition (accessed on 31 May 2021).
- Zaki, N.; Efimov, D.; Berengueres, J. Protein complex detection using interaction reliability assessment and weighted clustering coefficient. BMC Bioinform. 2013, 14, 163. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Tadaka, S.; Kinoshita, K. NCMine: Core-peripheral based functional module detection using near-clique mining. Bioinformatics 2016, 32, 3454–3460. [Google Scholar] [CrossRef]
- Tanaka, A.; Yuasa, S.; Mearini, G.; Egashira, T.; Seki, T.; Kodaira, M.; Kusumoto, D.; Kuroda, Y.; Okata, S.; Suzuki, T.; et al. Endothelin-1 induces myofibrillar disarray and contractile vector variability in hypertrophic cardiomyopathy-induced pluripotent stem cell-derived cardiomyocytes. J. Am. Heart Assoc. 2014, 3, e001263. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, H.; Xin, X.; Yang, J.; Hou, Y.; Fang, M.; Lu, X.; Xu, Y. An MRTF-A–Sp1–PDE5 axis mediates angiotensin-II-induced cardiomyocyte hypertrophy. Front. Cell Dev. Biol. 2020, 8, 839. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, J.; Chen, Q.; Wu, Q.; Deng, W.; Zhou, H.; Shen, D. Long non-coding RNA cytoskeleton regulator RNA (CYTOR) modulates pathological cardiac hypertrophy through miR-155-mediated IKKi signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1421–1427. [Google Scholar] [CrossRef]
- Yu, X.J.; Huang, Y.Q.; Shan, Z.X.; Zhu, J.N.; Hu, Z.Q.; Huang, L.; Feng, Y.Q.; Geng, Q.S. MicroRNA-92b-3p suppresses angiotensin II-induced cardiomyocyte hypertrophy via targeting HAND2. Life Sci. 2019, 232, 116635. [Google Scholar] [CrossRef]
- Shanmugam, P.; Valente, A.J.; Prabhu, S.D.; Venkatesan, B.; Yoshida, T.; Delafontaine, P.; Chandrasekar, B. Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J. Mol. Cell Cardiol. 2011, 50, 928–938. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frustaci, A.; De Luca, A.; Guida, V.; Biagini, T.; Mazza, T.; Gaudio, C.; Letizia, C.; Russo, M.A.; Galea, N.; Chimenti, C. Novel α-actin gene mutation p.(Ala21Val) causing familial hypertrophic cardiomyopathy, myocardial noncompaction, and transmural crypts. Clinical-pathologic correlation. J. Am. Heart. Assoc. 2018, 7, e008068. [Google Scholar] [CrossRef]
- Kraft, T.; Montag, J. Altered force generation and cell-to-cell contractile imbalance in hypertrophic cardiomyopathy. Pflügers Arch. Eur. J. Physiol. 2019, 471, 719–733. [Google Scholar] [CrossRef]
- Schramm, C.; Fine, D.M.; Edwards, M.A.; Reeb, A.N.; Krenz, M. The PTPN11 loss-of-function mutation Q510E-Shp2 causes hypertrophic cardiomyopathy by dysregulating mTOR signaling. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H231–H243. [Google Scholar] [CrossRef]
- James, J.; Zhang, Y.; Osinska, H.; Sanbe, A.; Klevitsky, R.; Hewett, T.E.; Robbins, J. Transgenic modeling of a cardiac troponin I mutation linked to familial hypertrophic cardiomyopathy. Circ. Res. 2000, 87, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.; Lerman, I.; Kranias, E.G.; Bohlmeyer, T.; Bristow, M.R.; Lefkowitz, R.J.; Iaccarino, G.; Koch, W.J.; Leinwand, L.A. Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J. Clin. Investig. 2001, 107, 967–974. [Google Scholar] [CrossRef]
- Martins, A.S.; Parvatiyar, M.S.; Feng, H.Z.; Bos, J.M.; Gonzalez-Martinez, D.; Vukmirovic, M.; Turna, R.S.; Sanchez-Gonzalez, M.A.; Badger, C.D.; Zorio, D.A.R.; et al. In vivo analysis of troponin C knock-in (A8V) mice: Evidence that TNNC1 is a hypertrophic cardiomyopathy susceptibility gene. Circ. Cardiovasc. Genet. 2015, 8, 653–664. [Google Scholar] [CrossRef]
- Bi, X.; Song, Y.; Song, Y.; Yuan, J.; Cui, J.; Zhao, S.; Qiao, S. Collagen cross-linking is associated with cardiac remodeling in hypertrophic obstructive cardiomyopathy. J. Am. Heart Assoc. 2021, 10, e017752. [Google Scholar] [CrossRef]
- Roldán, V.; Marín, F.; Gimeno, J.R.; Ruiz-Espejo, F.; González, J.; Feliu, E.; García-Honrubia, A.; Saura, D.; de la Morena, G.; Valdés, M.; et al. Matrix metalloproteinases and tissue remodeling in hypertrophic cardiomyopathy. Am. Heart J. 2008, 156, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; López, B.; Coelho-Filho, O.R.; Lakdawala, N.K.; Cirino, A.L.; Jarolim, P.; Kwong, R.; González, A.; Colan, S.D.; Seidman, J.G.; et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N. Engl. J. Med. 2010, 363, 552–563. [Google Scholar] [CrossRef]
- Kawano, H.; Toda, G.; Nakamizo, R.; Koide, Y.; Seto, S.; Yano, K. Valsartan decreases type I collagen synthesis in patients with hypertrophic cardiomyopathy. Circ. J. 2005, 69, 1244–1248. [Google Scholar] [CrossRef]
- Arteaga, E.; De Araújo, A.Q.; Bernstein, M.; Ramires, F.J.A.; Ianni, B.M.; Fernandes, F.; Mady, C. Prognostic value of the collagen volume fraction in hypertrophic cardiomyopathy. Arq. Bras. Cardiol. 2009, 92, 216–220. [Google Scholar]
- Lim, D.S.; Lutucuta, S.; Bachireddy, P.; Youker, K.; Evans, A.; Entman, M.; Roberts, R.; Marian, A.J. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 2001, 103, 789–791. [Google Scholar] [CrossRef]
- Bolca, O.; Özer, N.; Eren, M.; Dagdeviren, B.; Norgaz, T.; Akdemir, O.; Tezel, T. Dobutamine induced dynamic left ventricular outflow tract obstruction in patients with hypertrophic nonobstructive cardiomyopathy. Tohoku J. Exp. Med. 2002, 198, 79–87. [Google Scholar] [CrossRef]
- Tower-Rader, A.; Ramchand, J.; Nissen, S.E.; Desai, M.Y. Mavacamten: A novel small molecule modulator of β-cardiac myosin for treatment of hypertrophic cardiomyopathy. Expert Opin. Investig. Drugs 2020, 29, 1171–1178. [Google Scholar] [CrossRef]
- Heitner, S.B.; Jacoby, D.; Lester, S.J.; Owens, A.; Wang, A.; Zhang, D.; Lambing, J.; Lee, J.; Semigran, M.; Sehnert, A.J. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: A clinical trial. Ann. Intern. Med. 2019, 170, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Oreziak, A.; Barriales-Villa, R.; Abraham, T.P.; Masri, A.; Garcia-Pavia, P.; Saberi, S.; Lakdawala, N.K.; Wheeler, M.T.; Owens, A.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 396, 759–769. [Google Scholar] [CrossRef]
- Higashikuse, Y.; Mittal, N.; Arimura, T.; Yoon, S.H.; Oda, M.; Enomoto, H.; Kaneda, R.; Hattori, F.; Suzuki, T.; Kawakami, A.; et al. Perturbation of the titin/MURF1 signaling complex is associated with hypertrophic cardiomyopathy in a fish model and in human patients. Dis. Model Mech. 2019, 12, dmm041103. [Google Scholar] [CrossRef]
- Abraham, T.P.; Jones, M.; Kazmierczak, K.; Liang, H.Y.; Pinheiro, A.C.; Wagg, C.S.; Lopaschuk, G.D.; Szczesna-Cordary, D. Diastolic dysfunction in familial hypertrophic cardiomyopathy transgenic model mice. Cardiovasc. Res. 2009, 82, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, V.; Bertero, E.; Maack, C. Energetic drain driving hypertrophic cardiomyopathy. FEBS Lett. 2019, 593, 1616–1626. [Google Scholar] [CrossRef]
- Wijnker, P.J.M.; Sequeira, V.; Kuster, D.W.D.; Velden, J.V. Hypertrophic cardiomyopathy: A vicious cycle triggered by sarcomere mutations and secondary disease hits. Antioxid. Redox Signal. 2019, 31, 318–358. [Google Scholar] [CrossRef]
- Wu, H.; Yang, H.; Rhee, J.W.; Zhang, J.Z.; Lam, C.K.; Sallam, K.; Chang, A.C.Y.; Ma, N.; Lee, J.; Zhang, H.; et al. Modelling diastolic dysfunction in induced pluripotent stem cell-derived cardiomyocytes from hypertrophic cardiomyopathy patients. Eur. Heart J. 2019, 40, 3685–3695. [Google Scholar] [CrossRef]
- Sequeira, V.; Najafi, A.; Wijnker, P.J.M.; Dos Remedios, C.G.; Michels, M.; Kuster, D.W.D.; van der Velden, J. ADP-stimulated contraction: A predictor of thin-filament activation in cardiac disease. Proc. Natl. Acad. Sci. USA 2015, 112, E7003–E7012. [Google Scholar] [CrossRef] [PubMed]
- Teekakirikul, P.; Eminaga, S.; Toka, O.; Alcalai, R.; Wang, L.; Wakimoto, H.; Nayor, M.; Konno, T.; Gorham, J.M.; Wolf, C.M.; et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-β. J. Clin. Investig. 2010, 120, 3520–3529. [Google Scholar] [CrossRef] [PubMed]
- Dweck, D.; Sanchez-Gonzalez, M.A.; Chang, A.N.; Dulce, R.A.; Badger, C.D.; Koutnik, A.P.; Ruiz, E.L.; Griffin, B.; Liang, J.; Kabbaj, M.; et al. Long term ablation of protein kinase A (PKA)-mediated cardiac troponin I phosphorylation leads to excitation-contraction uncoupling and diastolic dysfunction in a knock-in mouse model of hypertrophic cardiomyopathy. J. Biol. Chem. 2014, 289, 23097–23111. [Google Scholar] [CrossRef]
- Alves, M.L.; Dias, F.A.L.; Gaffin, R.D.; Simon, J.N.; Montminy, E.M.; Biesiadecki, B.J.; Hinken, A.C.; Warren, C.M.; Utter, M.S.; Davis, R.T.; et al. Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ. Cardiovasc. Genet. 2014, 7, 132–143. [Google Scholar] [CrossRef]
- Granzier, H.L.; Radke, M.H.; Peng, J.; Westermann, D.; Nelson, O.L.; Rost, K.; King, N.M.P.; Yu, Q.; Tschöpe, C.; McNabb, M.; et al. Truncation of titin’s elastic PEVK region leads to cardiomyopathy with diastolic dysfunction. Circ. Res. 2009, 105, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Bongini, C.; Ferrantini, C.; Girolami, F.; Coppini, R.; Arretini, A.; Targetti, M.; Bardi, S.; Castelli, G.; Torricelli, F.; Cecchi, F.; et al. Impact of genotype on the occurrence of atrial fibrillation in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2016, 117, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Ogimoto, A.; Okayama, H.; Ohtsuka, T.; Shigematsu, Y.; Hamada, M.; Miki, T.; Higaki, J. A985G polymorphism of the endothelin-2 gene and atrial fibrillation in patients with hypertrophic cardiomyopathy. Circ. J. 2007, 71, 1932–1936. [Google Scholar] [CrossRef][Green Version]
- Okuda, S.; Sufu-Shimizu, Y.; Kato, T.; Fukuda, M.; Nishimura, S.; Oda, T.; Kobayashi, S.; Yamamoto, T.; Morimoto, S.; Yano, M. CaMKII-mediated phosphorylation of RyR2 plays a crucial role in aberrant Ca2+ release as an arrhythmogenic substrate in cardiac troponin T-related familial hypertrophic cardiomyopathy. Biochem. Biophys. Res. Commun. 2018, 496, 1250–1256. [Google Scholar] [CrossRef]
- Lan, F.; Lee, A.S.; Liang, P.; Sanchez-Freire, V.; Nguyen, P.K.; Wang, L.; Han, L.; Yen, M.; Wang, Y.; Sun, N.; et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 2013, 12, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Tsoutsman, T.; Lam, L.; Semsarian, C. Genes, calcium and modifying factors in hypertrophic cardiomyopathy. Clin. Exp. Pharmacol. Physiol. 2006, 33, 139–145. [Google Scholar] [CrossRef]
- Han, L.; Li, Y.; Tchao, J.; Kaplan, A.D.; Lin, B.; Li, Y.; Mich-Basso, J.; Lis, A.; Hassan, N.; London, B.; et al. Study familial hypertrophic cardiomyopathy using patient-specific induced pluripotent stem cells. Cardiovasc. Res. 2014, 104, 258–269. [Google Scholar] [CrossRef]
- Coppini, R.; Santini, L.; Olivotto, I.; Ackerman, M.J.; Cerbai, E. Abnormalities in sodium current and calcium homoeostasis as drivers of arrhythmogenesis in hypertrophic cardiomyopathy. Cardiovasc. Res. 2020, 116, 1585–1599. [Google Scholar] [CrossRef]
- Parvatiyar, M.S.; Landstrom, A.P.; Figueiredo-Freitas, C.; Potter, J.D.; Ackerman, M.J.; Pinto, J.R. A mutation in TNNC1-encoded cardiac troponin C, TNNC1-A31S, predisposes to hypertrophic cardiomyopathy and ventricular fibrillation. J. Biol. Chem. 2012, 287, 31845–31855. [Google Scholar] [CrossRef]
- Chung, W.K.; Kitner, C.; Maron, B.J. Novel frameshift mutation in Troponin C (TNNC1) associated with hypertrophic cardiomyopathy and sudden death. Cardiol. Young 2011, 21, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Nemer, G.; Bitar, F.F.; Arnaout, S.; Abchee, A.B.; Batrawi, M.; Khalil, A.; Abou Hassan, O.K.; DePalma, S.R.; McDonough, B.; et al. Founder mutation in N terminus of cardiac troponin I causes malignant hypertrophic cardiomyopathy. Circ. Genom. Precis. Med. 2020, 13, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, F.; Syrris, P.; Kaski, J.P.; Mogensen, J.; McKenna, W.J.; Elliott, P. Long-term outcomes in hypertrophic cardiomyopathy caused by mutations in the cardiac troponin T gene. Circ. Cardiovasc. Genet. 2012, 5, 10–17. [Google Scholar] [CrossRef]
- Karabina, A.; Kazmierczak, K.; Szczesna-Cordary, D.; Moore, J.R. Myosin regulatory light chain phosphorylation enhances cardiac β-myosin in vitro motility under load. Arch. Biochem. Biophys. 2015, 580, 14–21. [Google Scholar] [CrossRef]
- Roderick, H.L.; Berridge, M.J.; Bootman, M.D. Calcium-induced calcium release. Curr. Biol. 2003, 13, R425. [Google Scholar] [CrossRef]
- Knöll, R. Myosin binding protein C: Implications for signal-transduction. J. Muscle Res. Cell Motil. 2012, 33, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Nabavizadeh, P.; Song, T.; Desai, D.; Singh, R.; Bazrafshan, S.; Kumar, M.; Wang, Y.; Gilbert, R.J.; Dhandapany, P.S.; et al. Genetic, clinical, molecular, and pathogenic aspects of the South Asian–specific polymorphic MYBPC3Δ25bp variant. Biophys. Rev. 2020, 12, 1065–1084. [Google Scholar] [CrossRef] [PubMed]
- Kissopoulou, A.; Trinks, C.; Green, A.; Karlsson, J.E.; Jonasson, J.; Gunnarsson, C. Homozygous missense MYBPC3 Pro873His mutation associated with increased risk for heart failure development in hypertrophic cardiomyopathy. ESC Hear Fail. 2018, 5, 716–723. [Google Scholar] [CrossRef]
- Li, X.; Lu, W.J.; Li, Y.; Wu, F.; Bai, R.; Ma, S.; Dong, T.; Zhang, H.; Lee, A.S.; Wang, Y.; et al. MLP-deficient human pluripotent stem cell derived cardiomyocytes develop hypertrophic cardiomyopathy and heart failure phenotypes due to abnormal calcium handling. Cell Death Dis. 2019, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A review of the molecular mechanisms underlying the development and progression of cardiac remodeling. Oxid. Med. Cell. Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef]
- Liu, T.; Song, D.; Dong, J.; Zhu, P.; Liu, J.; Liu, W.; Ma, X.; Zhao, L.; Ling, S. Current Understanding of the pathophysiology of myocardial fibrosis and its quantitative assessment in heart failure. Front Physiol. 2017, 8, 238. [Google Scholar] [CrossRef]
- Jordà, P.; García-Álvarez, A. Hypertrophic cardiomyopathy: Sudden cardiac death risk stratification in adults. Glob. Cardiol. Sci. Pract. 2018, 2018, 25. [Google Scholar] [CrossRef]
- Waldmann, V.; Jouven, X.; Narayanan, K.; Piot, O.; Chugh, S.S.; Albert, C.M.; Marijon, E. Association between atrial fibrillation and sudden cardiac death. Circ. Res. 2020, 127, 301–309. [Google Scholar] [CrossRef]
- O’Mahony, C.; Elliott, P.; McKenna, W. Sudden cardiac death in hypertrophic cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2013, 6, 443–451. [Google Scholar] [CrossRef]
- Petersen, S.E.; Jerosch-Herold, M.; Hudsmith, L.E.; Robson, M.D.; Francis, J.M.; Doll, H.A.; Selvanayagam, J.B.; Neubauer, S.; Watkins, H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy. Circulation 2007, 115, 2418–2425. [Google Scholar] [CrossRef]
- Cecchi, F.; Olivotto, I.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Camici, P.G. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N. Engl. J. Med. 2003, 349, 1027–1035. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Maron, B.J.; Prasad, S.K.; Cecchi, F.; Udelson, J.E.; Camici, P.G. The case for myocardial ischemia in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 866–875. [Google Scholar] [CrossRef]
- Raphael, C.E.; Mitchell, F.; Kanaganayagam, G.S.; Liew, A.C.; Di Pietro, E.; Vieira, M.S.; Kanapeckaite, L.; Newsome, S.; Gregson, J.; Owen, R.; et al. Cardiovascular magnetic resonance predictors of heart failure in hypertrophic cardiomyopathy: The role of myocardial replacement fibrosis and the microcirculation. J. Cardiovasc. Magn. Reson. 2021, 23, 26. [Google Scholar] [CrossRef]
- Marian, A.J.; Braunwald, E. Hypertrophic cardiomyopathy: Genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef]
- Repetti, G.G.; Kim, Y.; Pereira, A.C.; Ingles, J.; Russell, M.W.; Lakdawala, N.K.; Ho, C.Y.; Day, S.; Semsarian, C.; McDonough, B.; et al. Discordant clinical features of identical hypertrophic cardiomyopathy twins. Proc. Natl. Acad. Sci. USA 2021, 118, e2021717118. [Google Scholar] [CrossRef]
- Pérez-Sánchez, I.; Romero-Puche, A.J.; García-Molina Sáez, E.; Sabater-Molina, M.; López-Ayala, J.M.; Muñoz-Esparza, C.; López-Cuenca, D.; de la Morena, G.; Castro-García, F.J.; Gimeno-Blanes, J.R. Factors influencing the phenotypic expression of hypertrophic cardiomyopathy in genetic carriers. Rev. Esp. Cardiol. 2018, 71, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Barefield, D.; Kumar, M.; Gorham, J.; Seidman, J.G.; Seidman, C.E.; de Tombe, P.P.; Sadayappan, S. Haploinsufficiency of MYBPC3 exacerbates the development of hypertrophic cardiomyopathy in heterozygous mice. J. Mol. Cell Cardiol. 2015, 79, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Stern, J.A. A one health approach to hypertrophic cardiomyopathy. Yale J. Biol. Med. 2017, 90, 433–448. [Google Scholar] [PubMed]
- Tye, C.; Runicles, A.K.; Whitehouse, A.J.O.; Alvares, G.A. Characterizing the interplay between autism spectrum disorder and comorbid medical conditions: An integrative review. Front. Psychiatry 2018, 9, 751. [Google Scholar] [CrossRef]
- Hoyt, C.T.; Domingo-Fernández, D.; Balzer, N.; Güldenpfennig, A.; Hofmann-Apitius, M. A systematic approach for identifying shared mechanisms in epilepsy and its comorbidities. Database 2018, 2018, bay050. [Google Scholar] [CrossRef]
- Ko, Y.; Cho, M.; Lee, J.S.; Kim, J. Identification of disease comorbidity through hidden molecular mechanisms. Sci. Rep. 2016, 6, 39433. [Google Scholar] [CrossRef]
- Meng, Z.Q.; Wu, J.R.; Zhu, Y.L.; Zhou, W.; Fu, C.G.; Liu, X.K.; Liu, S.Y.; Ni, M.W.; Guo, S.Y. Revealing the common mechanisms of scutellarin in angina pectoris and ischemic stroke treatment via a network pharmacology approach. Chin. J. Integr. Med. 2021, 27, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Gokuladhas, S.; Schierding, W.; Cameron-Smith, D.; Wake, M.; Scotter, E.L.; O’Sullivan, J. Shared regulatory pathways reveal novel genetic correlations between grip strength and neuromuscular disorders. Front. Genet. 2020, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Costa Sa, A.C.; Madsen, H.; Brown, J.R. Shared molecular signatures across neurodegenerative diseases and herpes virus infections highlights potential mechanisms for maladaptive innate immune responses. Sci. Rep. 2019, 9, 8795. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Shang, Z.; Teng, Y.; Chen, X.; Zhang, M.; Lv, H.; Zhang, R. The shared and specific mechanism of four autoimmune diseases. Oncotarget 2017, 8, 108355–108374. [Google Scholar] [CrossRef] [PubMed]
- Landolt, L.; Spagnoli, G.C.; Hertig, A.; Brocheriou, I.; Marti, H.-P. Fibrosis and cancer: Shared features and mechanisms suggest common targeted therapeutic approaches. Nephrol. Dial. Transplant. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Ormstad, H.; Simonsen, C.S.; Broch, L.; Maes, D.M.; Anderson, G.; Celius, E.G. Chronic fatigue and depression due to multiple sclerosis: Immune-inflammatory pathways, tryptophan catabolites and the gut-brain axis as possible shared pathways. Mult. Scler. Relat. Disord. 2020, 46, 102533. [Google Scholar] [CrossRef] [PubMed]
- Tap, L.; Kirkham, F.A.; Mattace-Raso, F.; Joly, L.; Rajkumar, C.; Benetos, A. Unraveling the links underlying arterial stiffness, bone demineralization, and muscle loss. Hypertension 2020, 76, 629–639. [Google Scholar] [CrossRef]
- Yaron, A.; Schuldiner, O. Common and divergent mechanisms in developmental neuronal remodeling and dying back neurodegeneration. Curr. Biol. 2016, 26, R628–R639. [Google Scholar] [CrossRef]
- Inzelberg, R.; Flash, S.; Friedman, E.; Azizi, E. Cutaneous malignant melanoma and Parkinson disease: Common pathways? Ann. Neurol. 2016, 80, 811–820. [Google Scholar] [CrossRef]
- Zhu, Y.; Ding, X.; She, Z.; Bai, X.; Nie, Z.; Wang, F.; Wang, F.; Geng, X. Exploring shared pathogenesis of Alzheimer’s disease and type 2 diabetes mellitus via co-expression networks analysis. Curr. Alzheimer Res. 2020, 17, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Kodamullil, A.T.; Hofmann-Apitius, M. Comorbidity analysis between Alzheimer’s disease and type 2 diabetes mellitus (T2DM) based on shared pathways and the role of T2DM drugs. J. Alzheimer’s Dis. 2017, 60, 721–731. [Google Scholar] [CrossRef] [PubMed]
| Pathophysiologic Entity | Number of Articles Read Automatically | Number of INDRA Statements Extracted |
|---|---|---|
| hypertrophic cardiomyopathy | 8111 | 7559 |
| cardiomyocyte hypertrophy | 1337 | 2500 |
| myofibrillar disarray | 51 | 356 |
| cardiomyocyte disarray | 11 | 22 |
| myocardial remodeling | 967 | 1500 |
| cardiac remodeling | 4572 | 5432 |
| myocardial fibrosis | 3634 | 4978 |
| left ventricular outflow tract obstruction | 1023 | 177 |
| myocardial hypercontractility | 3 | 3 |
| impaired myocardial relaxation | 31 | 33 |
| impaired cardiac relaxation | 12 | 28 |
| myocardial stiffness | 257 | 500 |
| diastolic dysfunction | 6342 | 6101 |
| atrial fibrillation | 54,117 | 25,842 |
| sudden cardiac death | 10,060 | 6770 |
| coronary microvascular dysfunction | 569 | 522 |
| myocardial ischemia | 19,637 | 19,078 |
| heart failure | 111,565 | 98,397 |
| major adverse cardiovascular events | 4700 | 1713 |
| rehospitalization | 3073 | 656 |
| Pathophysiologic Entities | Link to the Network Representing Shared Molecular Mechanisms |
|---|---|
| hypertrophic cardiomyopathy, cardiomyocyte hypertrophy | https://bit.ly/39Yn90x (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myofibrillar disarray | https://bit.ly/2PRnPOz (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, cardiomyocyte disarray | https://bit.ly/3wJsmmy (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial remodeling | https://bit.ly/2Q8dDkD (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, cardiac remodeling | https://bit.ly/31ZG3Qh (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial fibrosis | https://bit.ly/3fZX3hC (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, left ventricular outflow tract obstruction | https://bit.ly/3dN8G8R (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, impaired myocardial relaxation | https://bit.ly/322sU94 (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial stiffness | https://bit.ly/3mxmecq (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, diastolic dysfunction | https://bit.ly/3wHxRCn (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, atrial fibrillation | https://bit.ly/3d31kyT (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, sudden cardiac death | https://bit.ly/3wIN5ao (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, coronary microvascular dysfunction | https://bit.ly/31Xh2VN (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial ischemia | https://bit.ly/31YlC6a (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, heart failure | https://bit.ly/322UjI9 (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, major adverse cardiovascular events | https://bit.ly/3mvZZE1 (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, rehospitalization | https://bit.ly/3myyx8t (accessed on 1 August 2021) |
| Network | Top Nodes Ranked by Centrality Scores |
|---|---|
| hypertrophic cardiomyopathy, cardiomyocyte hypertrophy | https://bit.ly/3fCs1Mq (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myofibrillar disarray | https://bit.ly/2OgKHpM (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, cardiomyocyte disarray | https://bit.ly/31LLUsi (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial remodeling | https://bit.ly/3uj130t (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, cardiac remodeling | https://bit.ly/39CYWgj (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial fibrosis | https://bit.ly/3dc8HUA (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, left ventricular outflow tract obstruction | https://bit.ly/3cJRWzY (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, impaired myocardial relaxation | https://bit.ly/3dubAz7 (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial stiffness | https://bit.ly/2PpsZRM (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, diastolic dysfunction | https://bit.ly/2PQuwju (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, atrial fibrillation | https://bit.ly/2OhvNzE (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, sudden cardiac death | https://bit.ly/3ugy2CI (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, coronary microvascular dysfunction | https://bit.ly/3wiA5YR (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial ischemia | https://bit.ly/39Hexvk (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, heart failure | https://bit.ly/3uwQiYP (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, major adverse cardiovascular events | https://bit.ly/3fHsE7w (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, rehospitalization | https://bit.ly/3dzxLUy (accessed on 1 August 2021) |
| Network | Link to Networks with Different PE-Values Applied |
|---|---|
| hypertrophic cardiomyopathy, cardiomyocyte hypertrophy | https://bit.ly/3sMXLm1 (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myofibrillar disarray | https://bit.ly/39Ebt2N (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, cardiomyocyte disarray | https://bit.ly/3cMpoGd (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial remodeling | https://bit.ly/3duSh8V (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, cardiac remodeling | https://bit.ly/3dBWPdU (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial fibrosis | https://bit.ly/324nVoj (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, left ventricular outflow tract obstruction | https://bit.ly/31GacUC (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, impaired myocardial relaxation | https://bit.ly/31MjKxv (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial stiffness | https://bit.ly/3sMZnfz (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, diastolic dysfunction | https://bit.ly/3cNFNu8 (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, atrial fibrillation | https://bit.ly/3mhXtRv (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, sudden cardiac death | https://bit.ly/3wxjGzZ (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, coronary microvascular dysfunction | https://bit.ly/3uhQQ4l (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, myocardial ischemia | https://bit.ly/31GmOLu (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, heart failure | https://bit.ly/3mi5i9U (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, major adverse cardiovascular events | https://bit.ly/2QZt66N (accessed on 1 August 2021) |
| hypertrophic cardiomyopathy, rehospitalization | https://bit.ly/2QYjedr (accessed on 1 August 2021) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glavaški, M.; Velicki, L. Shared Molecular Mechanisms of Hypertrophic Cardiomyopathy and Its Clinical Presentations: Automated Molecular Mechanisms Extraction Approach. Life 2021, 11, 785. https://doi.org/10.3390/life11080785
Glavaški M, Velicki L. Shared Molecular Mechanisms of Hypertrophic Cardiomyopathy and Its Clinical Presentations: Automated Molecular Mechanisms Extraction Approach. Life. 2021; 11(8):785. https://doi.org/10.3390/life11080785
Chicago/Turabian StyleGlavaški, Mila, and Lazar Velicki. 2021. "Shared Molecular Mechanisms of Hypertrophic Cardiomyopathy and Its Clinical Presentations: Automated Molecular Mechanisms Extraction Approach" Life 11, no. 8: 785. https://doi.org/10.3390/life11080785
APA StyleGlavaški, M., & Velicki, L. (2021). Shared Molecular Mechanisms of Hypertrophic Cardiomyopathy and Its Clinical Presentations: Automated Molecular Mechanisms Extraction Approach. Life, 11(8), 785. https://doi.org/10.3390/life11080785