Recombinase-Aided Amplification Coupled with Lateral Flow Dipstick for Efficient and Accurate Detection of Porcine Parvovirus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus and Sample
2.2. Construction of the Standard Recombinant Plasmid pMD19-T-VP1
2.3. PCR-AGE for PPV
2.4. RAA-AGE for PPV
2.5. PPV RAA-LFD Assay
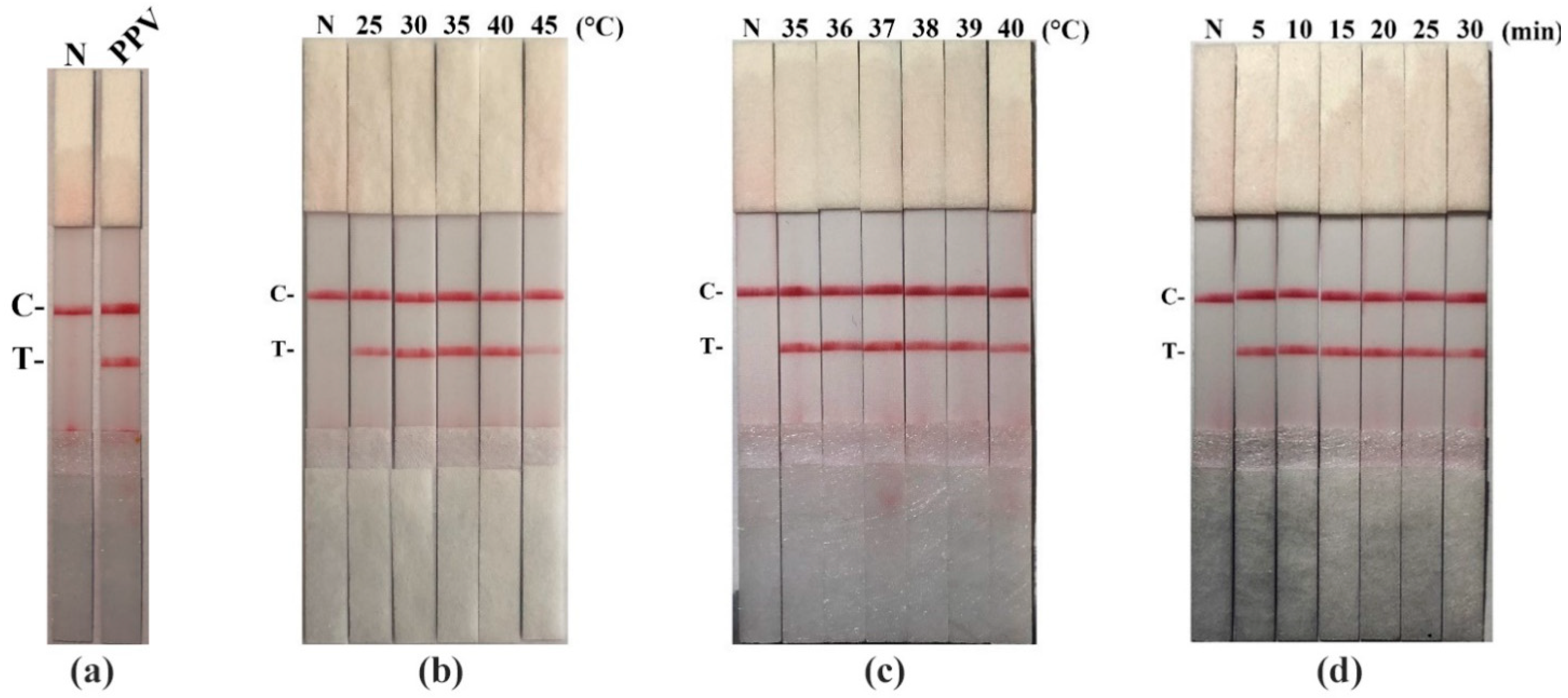
2.6. Optimization of Reaction Conditions for RAA-LFD Assay
2.7. The Specificity and Sensitivity of PPV RAA-LFD Assay
2.8. Evaluation of Clinical Samples
3. Results
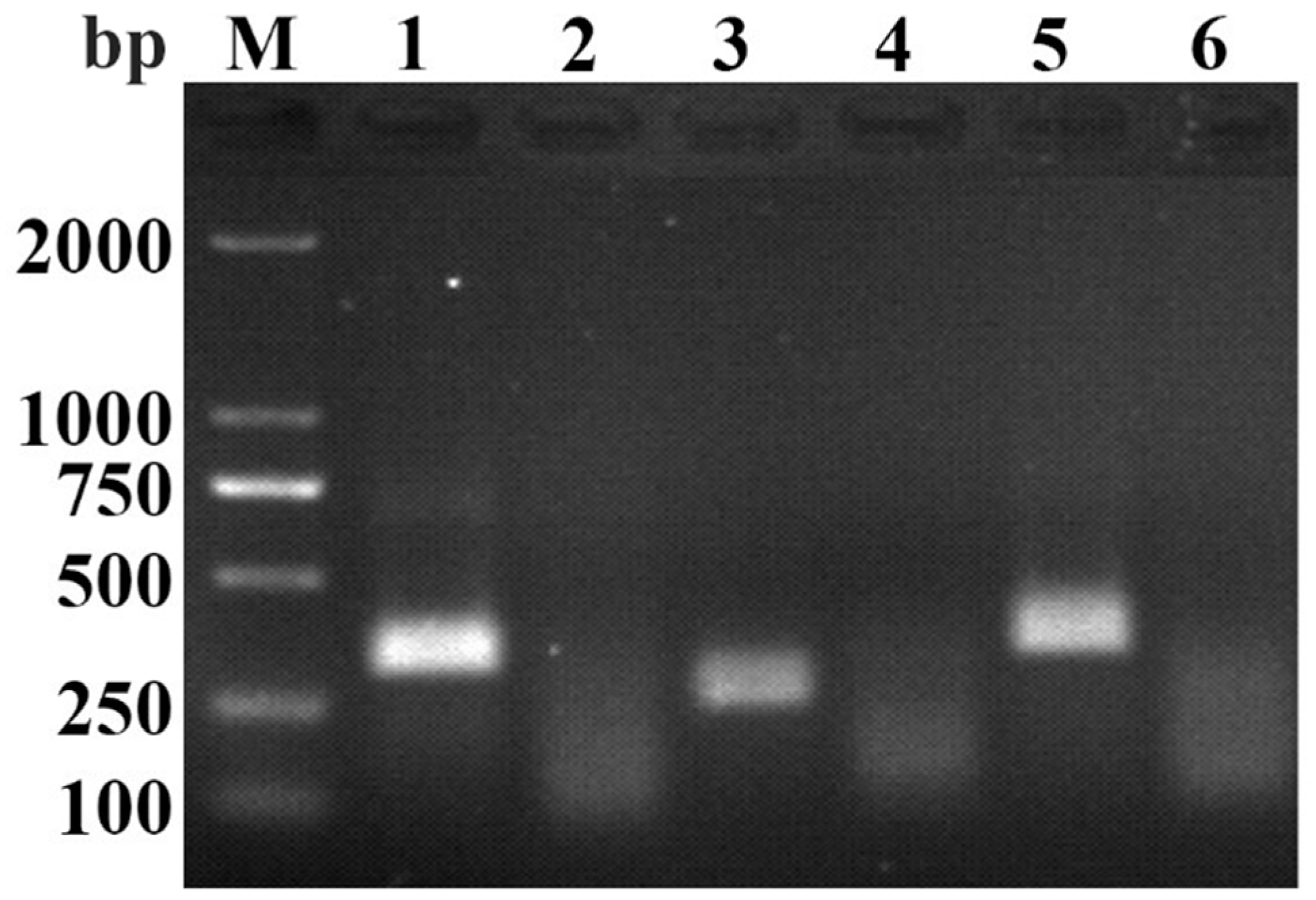
3.1. Primers Screening for RAA-AGE Assay
3.2. The Establishment for RAA-LFD Assay and Optimization of Reaction Conditions
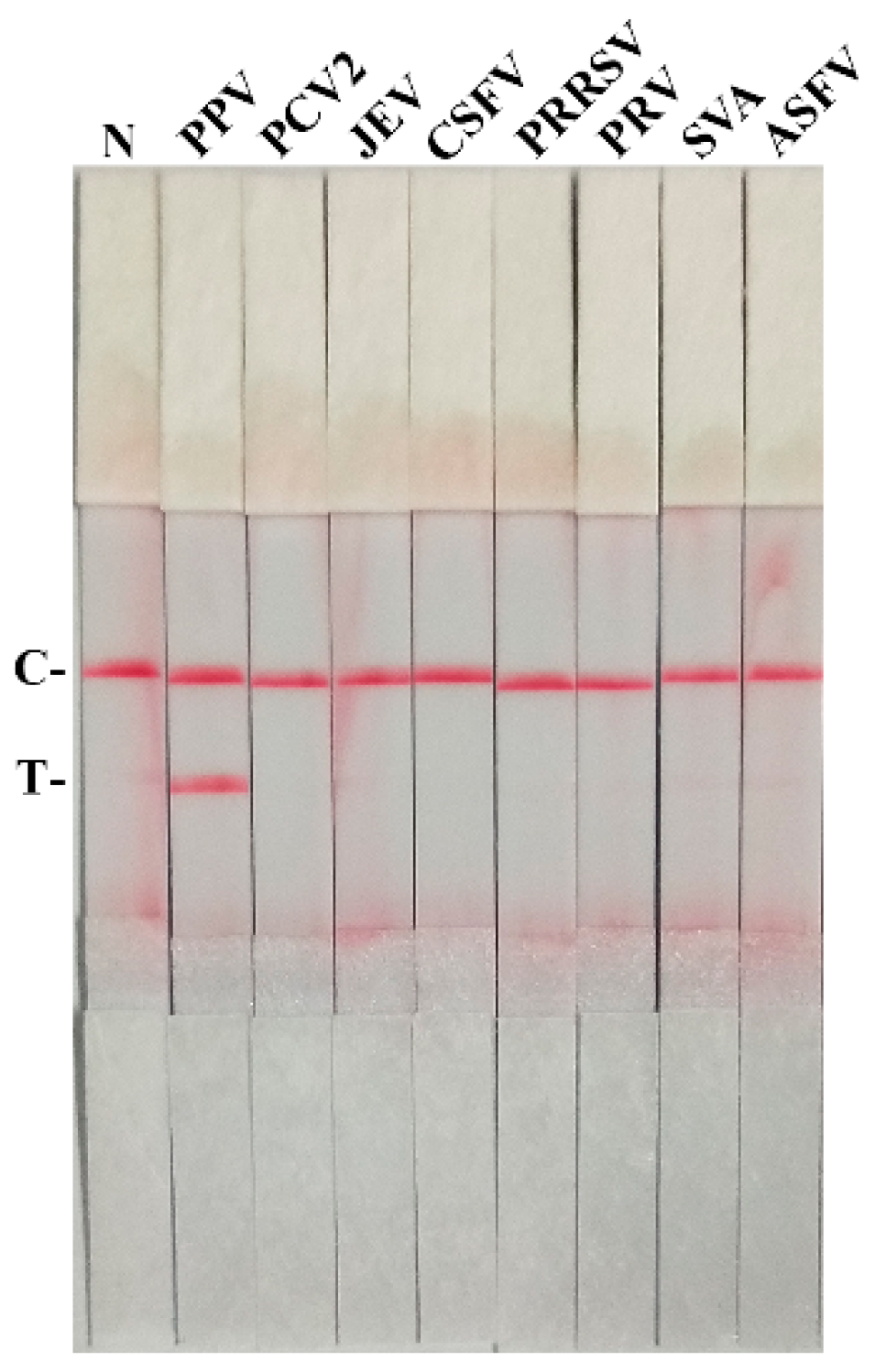
3.3. The Specificity for PPV RAA-LFD Assay
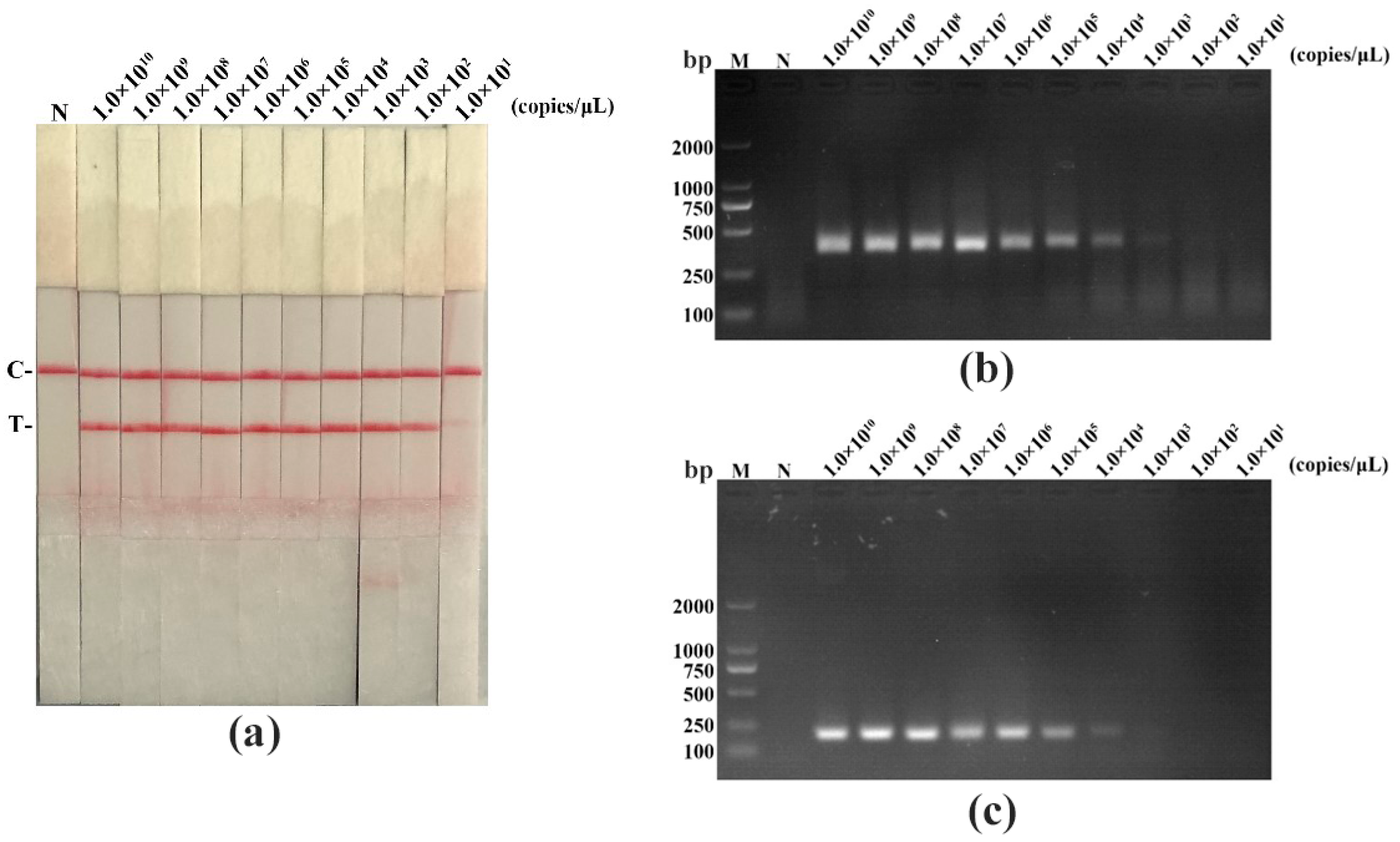
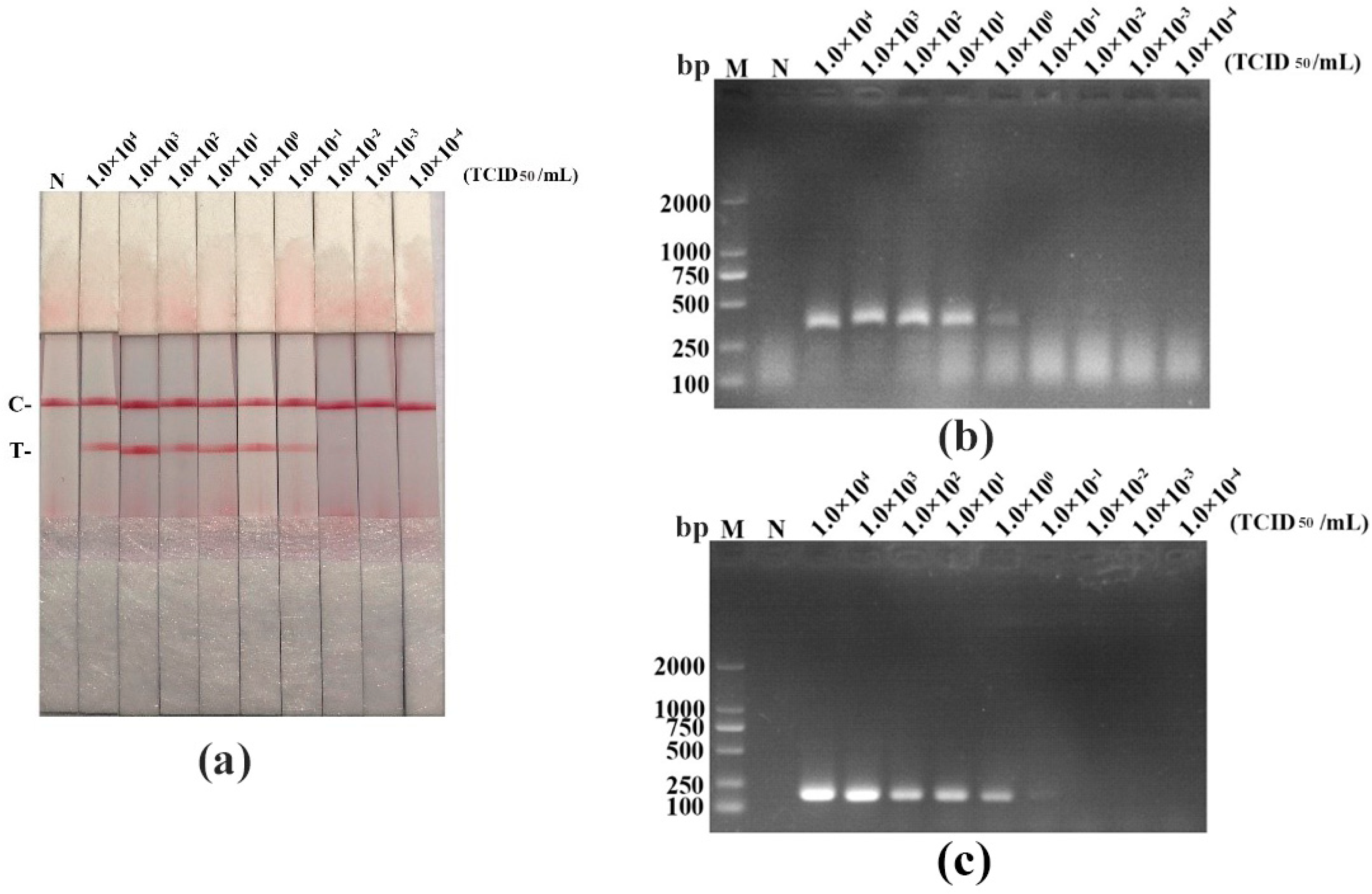
3.4. The Sensitivity for PPV RAA-LFD Assay
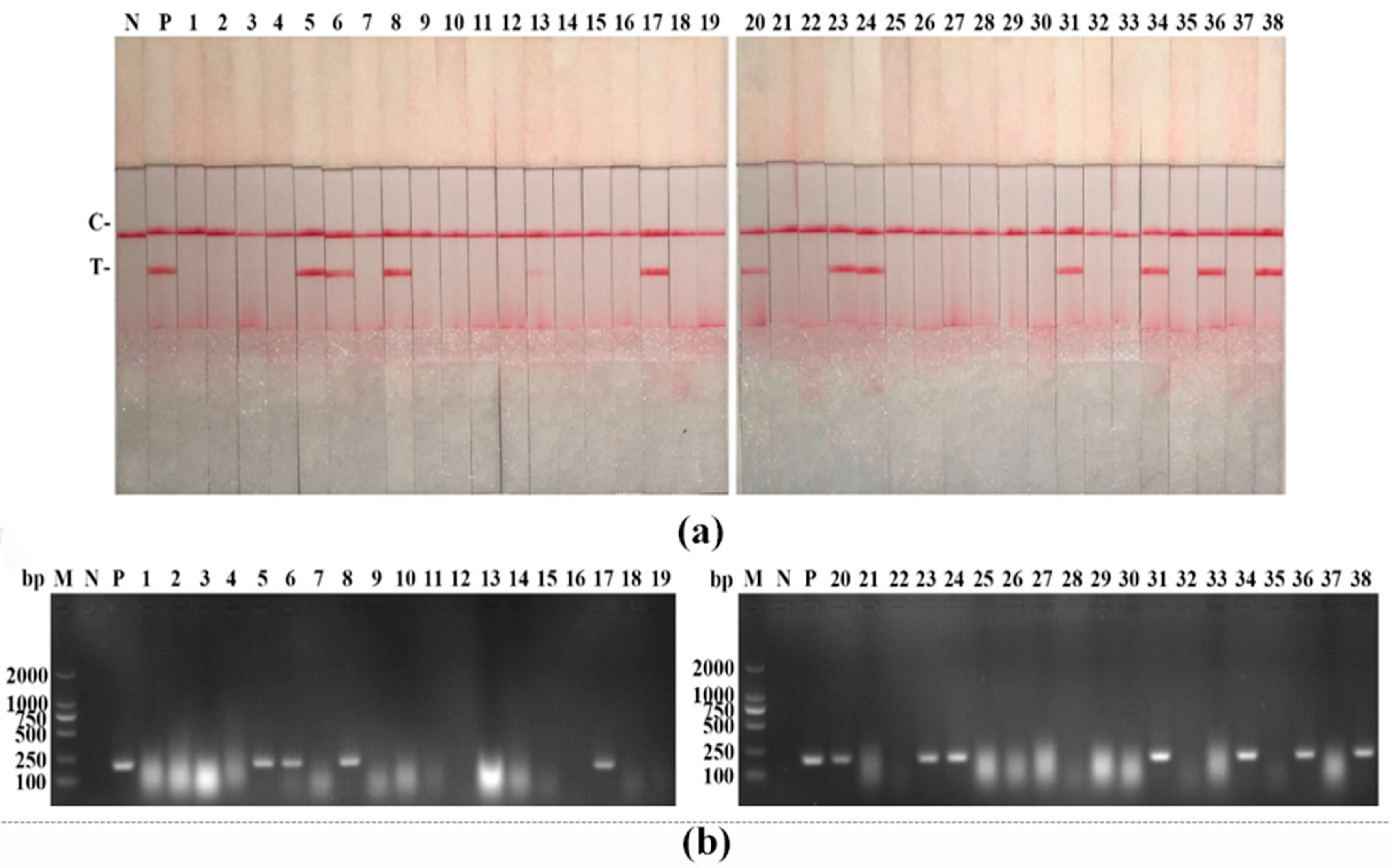
3.5. Clinical Samples Detection of PPV RAA-LFD Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Streck, A.F.; Truyen, U. Porcine Parvovirus. Curr. Issues Mol. Biol. 2020, 37, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Mengeling, W.L.; Lager, K.M.; Vorwald, A.C. The effect of porcine parvovirus and porcine reproductive and respiratory syndrome virus on porcine reproductive performance. Anim. Reprod. Sci. 2000, 60–61, 199–210. [Google Scholar] [CrossRef]
- Xing, X.; Zhou, H.; Tong, L.; Chen, Y.; Sun, Y.; Wang, H.; Zhang, G. First identification of porcine parvovirus 7 in China. Arch. Virol. 2018, 163, 209–213. [Google Scholar] [CrossRef]
- Mayr, A.; Bachmann, P.A.; Siegl, G.; Mahnel, H.; Sheffy, B.E. Characterization of a small porcine DNA virus. Arch. Fur Die Gesamte Virusforsch. 1968, 25, 38–51. [Google Scholar] [CrossRef]
- Cui, J.; Biernacka, K.; Fan, J.; Gerber, P.F.; Stadejek, T.; Opriessnig, T. Circulation of Porcine Parvovirus Types 1 through 6 in Serum Samples Obtained from Six Commercial Polish Pig Farms. Transbound. Emerg. Dis. 2017, 64, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Dea, S.; Elazhary, M.A.; Martineau, G.P.; Vaillancourt, J. Parvovirus-like particles associated with diarrhea in unweaned piglets. Can. J. Comp. Med. Rev. Can. Med. Comp. 1985, 49, 343–345. [Google Scholar]
- Yasuhara, H.; Matsui, O.; Hirahara, T.; Ohgitani, T.; Tanaka, M.; Kodama, K.; Nakai, M.; Sasaki, N. Characterization of a parvovirus isolated from the diarrheic feces of a pig. Nihon Juigaku Zasshi Jpn. J. Vet. Sci. 1989, 51, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Lager, K.M.; Mengeling, W.L. Porcine parvovirus associated with cutaneous lesions in piglets. J. Vet. Diagn. Investig. 1994, 6, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, L.; Zhao, Y.; Liu, Y.; Liu, C.; Wang, Q.; Dong, Y.; Wang, S.; Chi, T.; Song, F. Clinical Validation of Two Recombinase-Based Isothermal Amplification Assays (RPA/RAA) for the Rapid Detection of African Swine Fever Virus. Front. Microbiol. 2020, 11, 1696. [Google Scholar] [CrossRef]
- Kim, J.; Easley, C.J. Isothermal DNA amplification in bioanalysis: Strategies and applications. Bioanalysis 2011, 3, 227–239. [Google Scholar] [CrossRef]
- Ogawa, H.; Taira, O.; Hirai, T.; Takeuchi, H.; Nagao, A.; Ishikawa, Y.; Tuchiya, K.; Nunoya, T.; Ueda, S. Multiplex PCR and multiplex RT-PCR for inclusive detection of major swine DNA and RNA viruses in pigs with multiple infections. J. Virol. Methods 2009, 160, 210–214. [Google Scholar] [CrossRef]
- Krakowka, S.; Ellis, J.A.; Meehan, B.; Kennedy, S.; McNeilly, F.; Allan, G. Viral wasting syndrome of swine: Experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet. Pathol. 2000, 37, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Bolt, D.M.; Hani, H.; Muller, E.; Waldvogel, A.S. Non-suppurative myocarditis in piglets associated with porcine parvovirus infection. J. Comp. Pathol. 1997, 117, 107–118. [Google Scholar] [CrossRef]
- Jozwik, A.; Manteufel, J.; Selbitz, H.J.; Truyen, U. Vaccination against porcine parvovirus protects against disease, but does not prevent infection and virus shedding after challenge infection with a heterologous virus strain. J. Gen. Virol. 2009, 90, 2437–2441. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Li, X.-K.; Cui, B.-A.; Wei, Z.-Y.; Li, X.-S.; Wang, Y.-B.; Zhao, L.; Wang, Z.-Y. A TaqMan-based real-time polymerase chain reaction for the detection of porcine parvovirus. J. Virol. Methods 2009, 156, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.M.; Kennedy, S.; McNeilly, F.; Foster, J.C.; Ellis, J.A.; Krakowka, S.J.; Meehan, B.M.; Adair, B.M. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J. Comp. Pathol. 1999, 121, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chae, C. A comparison of virus isolation, polymerase chain reaction, immunohistochemistry, and in situ hybridization for the detection of porcine circovirus 2 and porcine parvovirus in experimentally and naturally coinfected pigs. J. Vet. Diagn. Investig. 2004, 16, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Qing, L.; Lv, J.; Li, H.; Tan, Y.; Hao, H.; Chen, Z.; Zhao, J.; Chen, H. The recombinant nonstructural polyprotein NS1 of porcine parvovirus (PPV) as diagnostic antigen in ELISA to differentiate infected from vaccinated pigs. Vet. Res. Commun. 2006, 30, 175–190. [Google Scholar] [CrossRef]
- Hohdatsu, T.; Baba, K.; Ide, S.; Tsuchimoto, M.; Nagano, H.; Yamagami, T.; Yamagishi, H.; Fujisaki, Y.; Matumoto, M. Detection of antibodies against porcine parvovirus in swine sera by enzyme-linked immunosorbent assay. Vet. Microbiol. 1988, 17, 11–19. [Google Scholar] [CrossRef]
- Molitor, T.W.; Oraveerakul, K.; Zhang, Q.Q.; Choi, C.S.; Ludemann, L.R. Polymerase chain reaction (PCR) amplification for the detection of porcine parvovirus. J. Virol. Methods 1991, 32, 201–211. [Google Scholar] [CrossRef]
- Song, C.; Zhu, C.; Zhang, C.; Cui, S. Detection of porcine parvovirus using a taqman-based real-time pcr with primers and probe designed for the NS1 gene. Virol. J. 2010, 7, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, K.; Hu, R.; Ni, J.; Liang, J.; He, X.; Du, Y.; Xu, Y.; Zhao, B.; Zhang, Q.; Li, C. Establishment of a porcine parvovirus (PPV) LAMP visual rapid detection method. J. Virol. Methods 2020, 284, 113924. [Google Scholar] [CrossRef] [PubMed]
- Hardinge, P.; Murray, J.A.H. Lack of specificity associated with using molecular beacons in loop mediated amplification assays. BMC Biotechnol. 2019, 19, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.-Z.; Chen, J.-T.; Li, J.; Wu, X.-J.; Wen, J.-Z.; Liu, X.-Z.; Lin, L.-Y.; Liang, X.-Y.; Huang, H.-Y.; Zha, G.-C. Reverse Transcription Recombinase-Aided Amplification Assay With Lateral Flow Dipstick Assay for Rapid Detection of 2019 Novel Coronavirus. Front. Cell. Infect. Microbiol. 2021, 11, 613304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, L.; Ma, R.; Cong, L.; Wu, Z.; Wei, Y.; Xue, S.; Zheng, W.; Tang, S. Rapid detection of Salmonella with Recombinase Aided Amplification. J. Microbiol. Methods 2017, 139, 202–204. [Google Scholar] [CrossRef]
- Song, Z.; Ting, L.; Kun, Y.; Wei, L.; Jian-Feng, Z.; Li-Chuan, G.; Yan-Hong, L.; Yang, D.; Qing-Jie, Y.; Hai-Tao, Y. Establishment of a recombinase-aided isothermal amplification technique to detect Schistosoma japonicum specific gene fragments. Chin. J. Schistosomiasis Control. 2018, 30, 273–277. [Google Scholar]
- Zou, Y.; Mason, M.G.; Wang, Y.; Wee, E.; Turni, C.; Blackall, P.J.; Trau, M.; Botella, J.R. Nucleic acid purification from plants, animals and microbes in under 30 seconds. PLoS Biol. 2017, 15, e2003916. [Google Scholar] [CrossRef] [PubMed]

| Primers | Sequence (5′-3′) | Length (bp) | Gene |
|---|---|---|---|
| PPV-F1 | CACGCATCAAGACTCATACATC | 1226 | VP1 |
| PPV-R1 | TCTGTATCAAGTTCTTTATCCCAT |
| Primers | Sequence (5′-3′) | Length (bp) |
|---|---|---|
| PPV-F2 | CACAGAAGCAACAGCAATTAGG | 203 |
| PPV-R2 | CTAGCTCTTGTGAAGATGTGG |
| RAA Assay | Primers | Sequence (5′-3′) | Length (bp) |
|---|---|---|---|
| PPV-RAA-1 | PPV-RAA-F1 | ACACTGGACAATCACAACAAATAACAGACTCA | 340 |
| PPV-RAA-R1 | CCTACCTGAGCTGGCCTAATTGCTGTTGCTTC | ||
| PPV-RAA-2 | PPV-RAA-F2 | TACAGATATTACCTATCATGCACCAGAAAC | 274 |
| PPV-RAA-R2 | CTGTGGTAGGTTCAGTTAGTAGTTTTGGAG | ||
| PPV-RAA-3 | PPV-RAA-F3 | CATACATCTAAATATGCCAGAACACGAAAC | 354 |
| PPV-RAA-R3 | GGTGTGTATGGAAGTGTGTTATTGGTGTCT |
| Primers/Probe | Sequence (5′-3′) |
|---|---|
| PPV-RAA-F1 | ACACTGGACAATCACAACAAATAACAGACTCA |
| PPV-RAA-R1 | Biotin-CCTACCTGAGCTGGCCTAATTGCTGTTGCTTC |
| PPV-Probe | FAM-ACAGATCTCTAGGACTGCCTCCAAAACTAC-THF-AACTGAACCTACCAC-C3 spacer |
| Template | PPV RAA-LFD | PPV RAA-AGE | PPV PCR-AGE |
|---|---|---|---|
| Recombinant plasmid (copies/μL) | 102 | 104 | 104 |
| DNA (ng/μL) | 6.38 × 10−7 | 6.38 × 10−4 | 6.38 × 10−4 |
| Virus titer (TCID50/mL) | 10−1 | 1 | 1 |
| Method | PPV RAA-LFD | PPV PCR-AGE | ||
|---|---|---|---|---|
| Results | Positive | Negative | Positive | Negative |
| 11 | 27 | 11 | 27 | |
| Total samples | 38 | 38 | ||
| Positive rate | 28.95% | 28.95% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Chen, W.; Fan, J.; Fan, S.; Ding, H.; Chen, J.; Yi, L. Recombinase-Aided Amplification Coupled with Lateral Flow Dipstick for Efficient and Accurate Detection of Porcine Parvovirus. Life 2021, 11, 762. https://doi.org/10.3390/life11080762
He Y, Chen W, Fan J, Fan S, Ding H, Chen J, Yi L. Recombinase-Aided Amplification Coupled with Lateral Flow Dipstick for Efficient and Accurate Detection of Porcine Parvovirus. Life. 2021; 11(8):762. https://doi.org/10.3390/life11080762
Chicago/Turabian StyleHe, Yihong, Wenxian Chen, Jindai Fan, Shuangqi Fan, Hongxing Ding, Jinding Chen, and Lin Yi. 2021. "Recombinase-Aided Amplification Coupled with Lateral Flow Dipstick for Efficient and Accurate Detection of Porcine Parvovirus" Life 11, no. 8: 762. https://doi.org/10.3390/life11080762
APA StyleHe, Y., Chen, W., Fan, J., Fan, S., Ding, H., Chen, J., & Yi, L. (2021). Recombinase-Aided Amplification Coupled with Lateral Flow Dipstick for Efficient and Accurate Detection of Porcine Parvovirus. Life, 11(8), 762. https://doi.org/10.3390/life11080762




