Rutin Mediated Apoptotic Cell Death in Caski Cervical Cancer Cells via Notch-1 and Hes-1 Downregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. In Vitro Cell Viability Assay
2.4. Determination of Cytotoxicity by Lactate Dehydrogenase (LDH) Release Assay
2.5. Determination of Morphological Changes by Phase-Contrast Microscopy
2.6. Estimation of Apoptosis via DAPI Staining
2.7. Mitochondrial Membrane Potential (MMP) Analysis
2.8. Estimation of Caspase-9 and -3 Activities
2.9. Reactive Oxygen Species (ROS) Generation Analysis
2.10. Effect of NAC (N-acetyl-L-cysteine) on Cell Viability and ROS Generation
2.11. Cell Cycle Analysis
2.12. Real-Time qPCR Analysis
2.13. Statistical Analysis
3. Results
3.1. Antiproliferative and Cytotoxic Effects of Rutin against Cervical Cancer Caski Cells
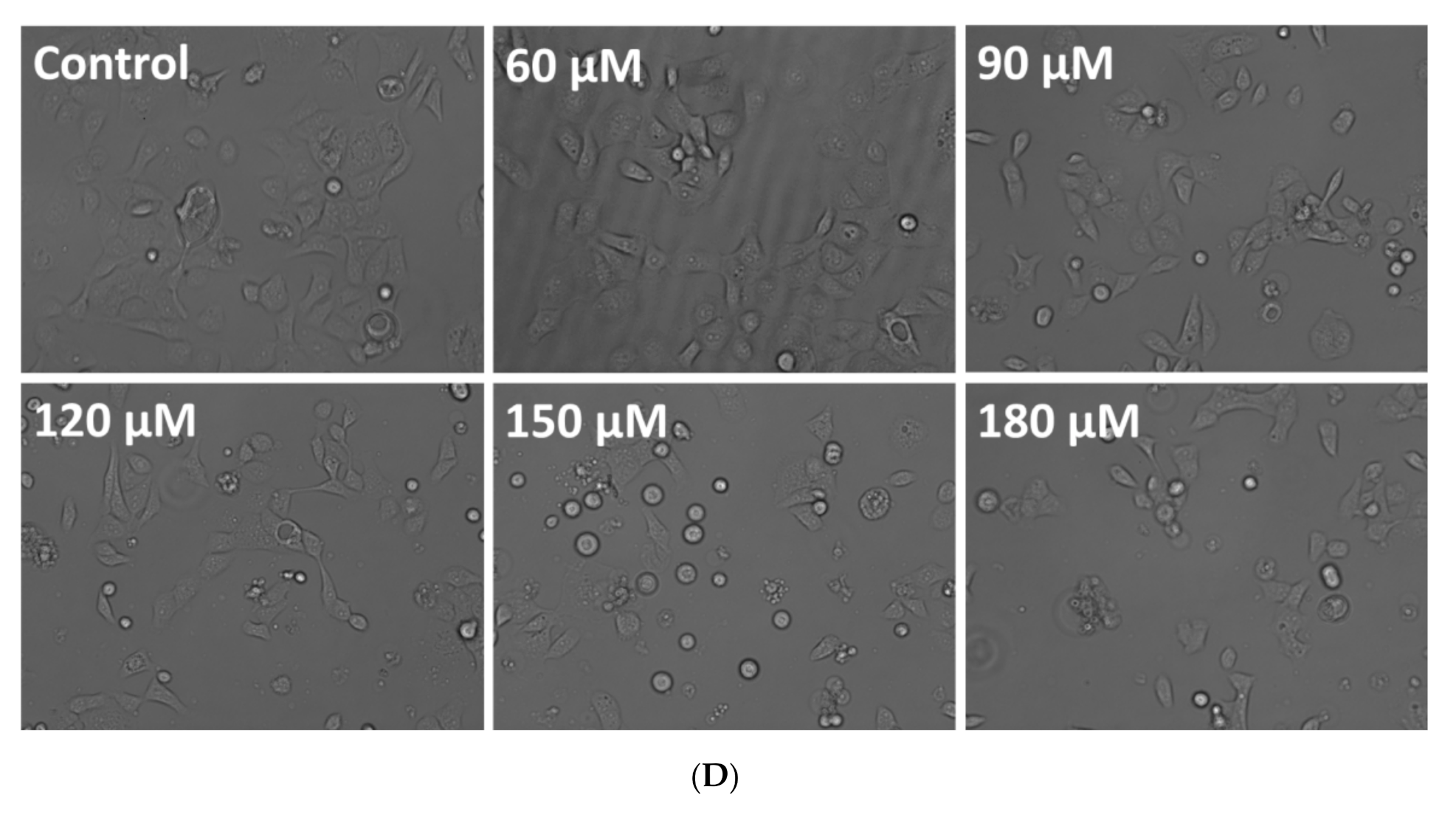
3.2. Effect of Rutin on Caski Cell Morphology
3.3. Rutin-Induced Apoptosis in Caski Cervical Cancer Cells
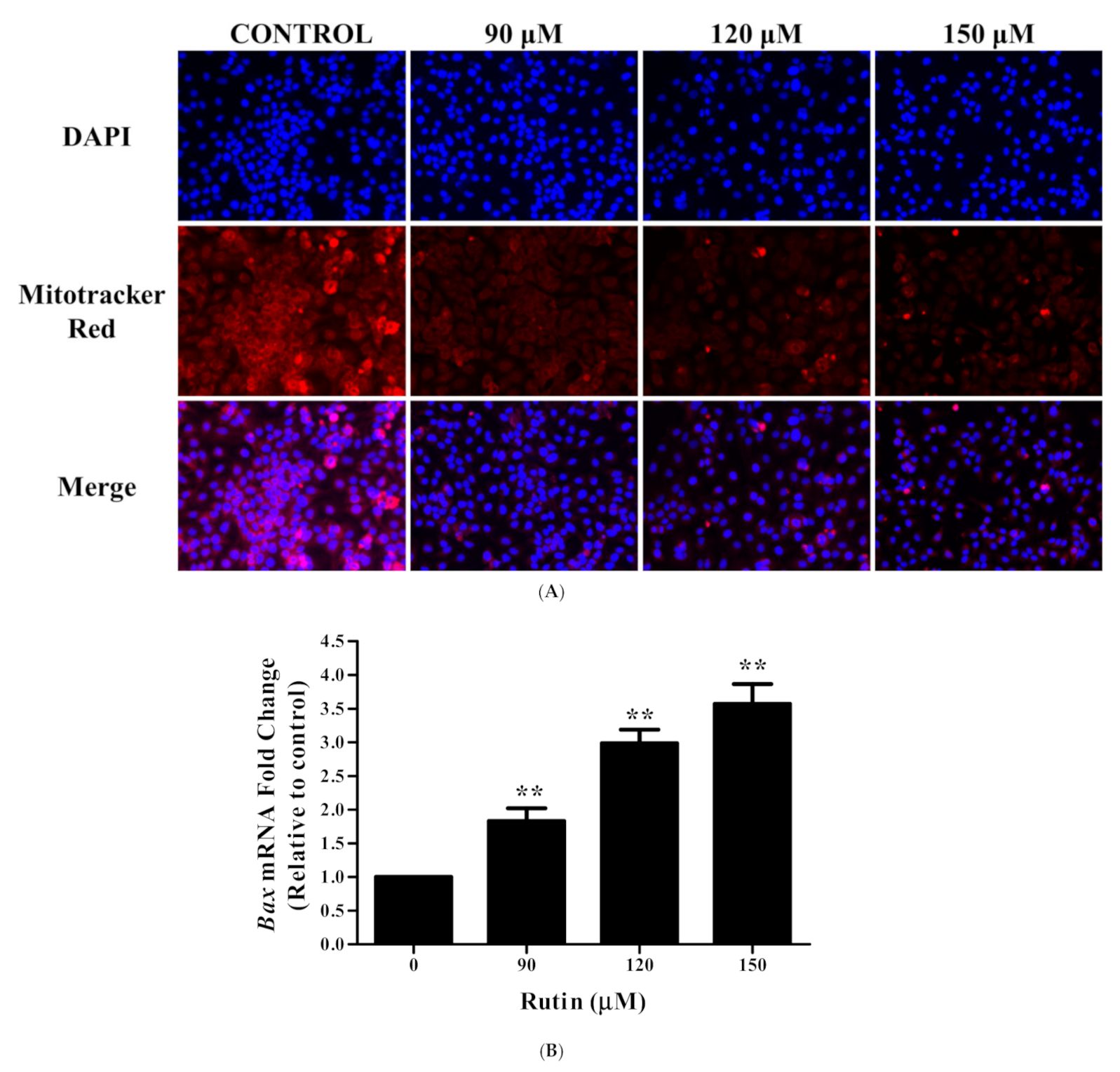
3.4. Rutin Induces Mitochondrial Membrane Depolarization in Caski Cells
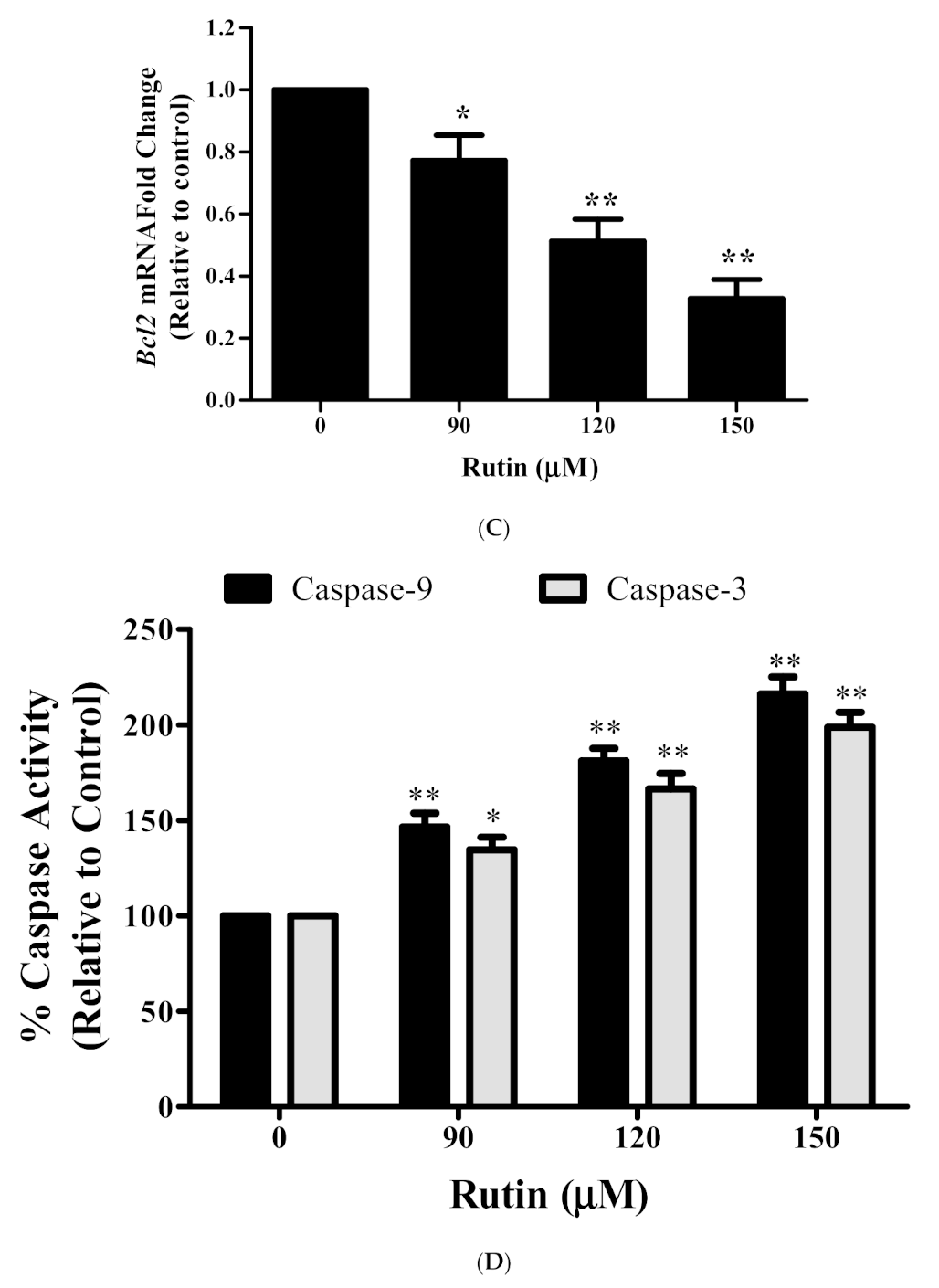
3.5. Rutin Modulated the mRNA Expression Apoptotic-Related Genes
3.6. Rutin Activates Caspase-3/-9 in Caski Cells
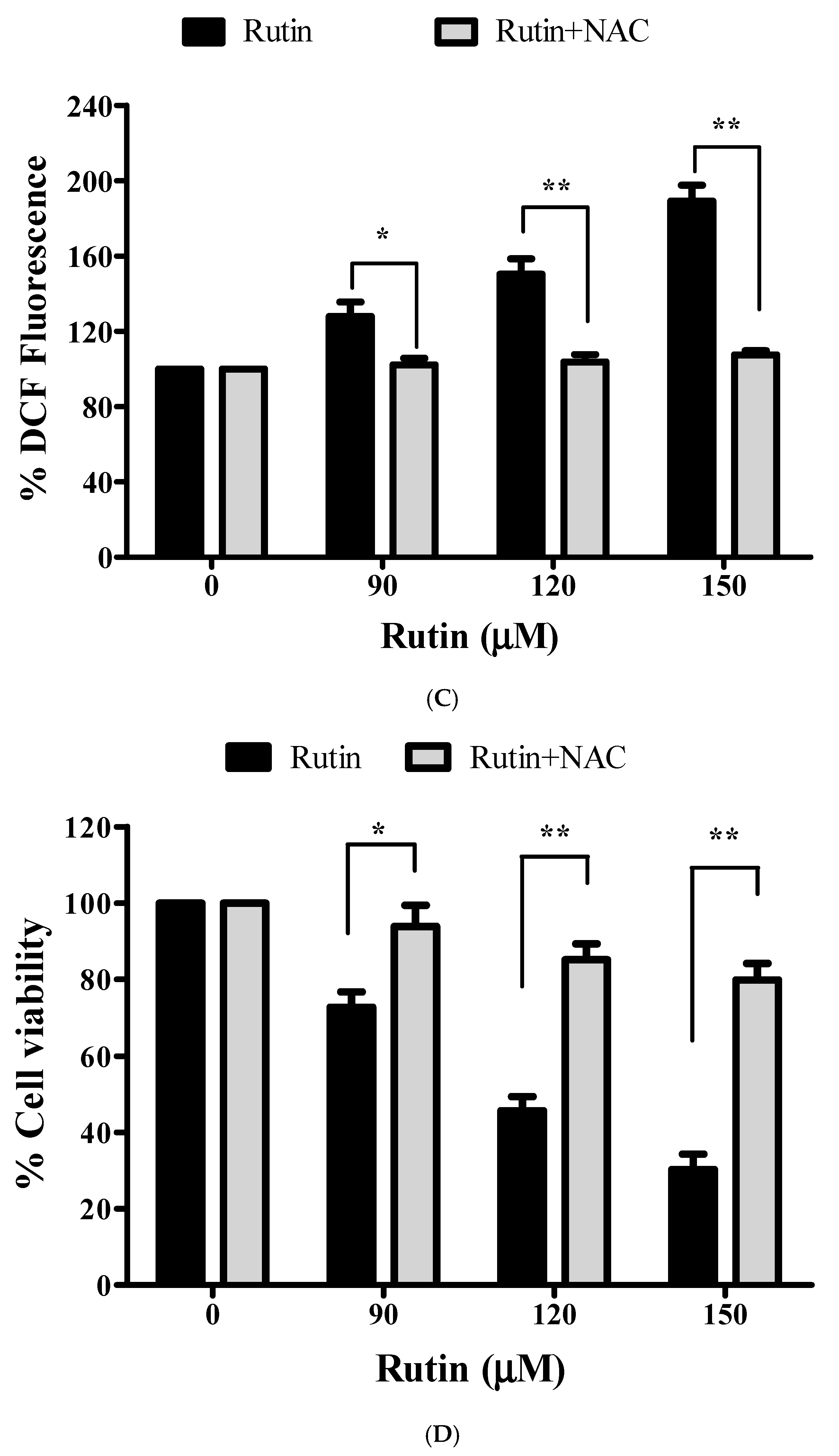
3.7. Augmented ROS Generation by Rutin Contributes to Apoptosis
3.8. Restoration of Cell Viability by N-acetyl-L-cysteine (NAC)
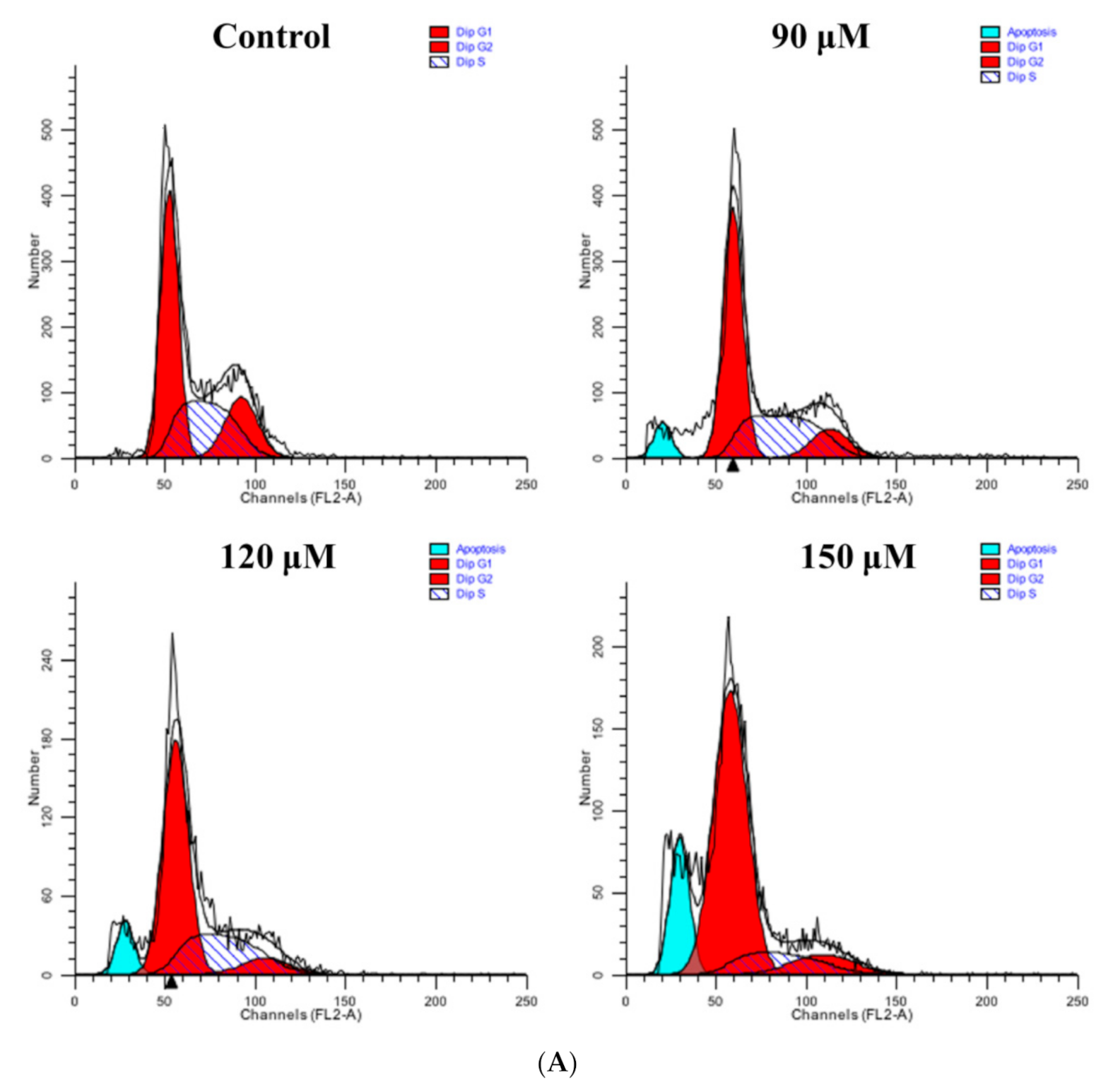
3.9. Rutin Treatment Induces G0/G1 Arrest in Caski Cells
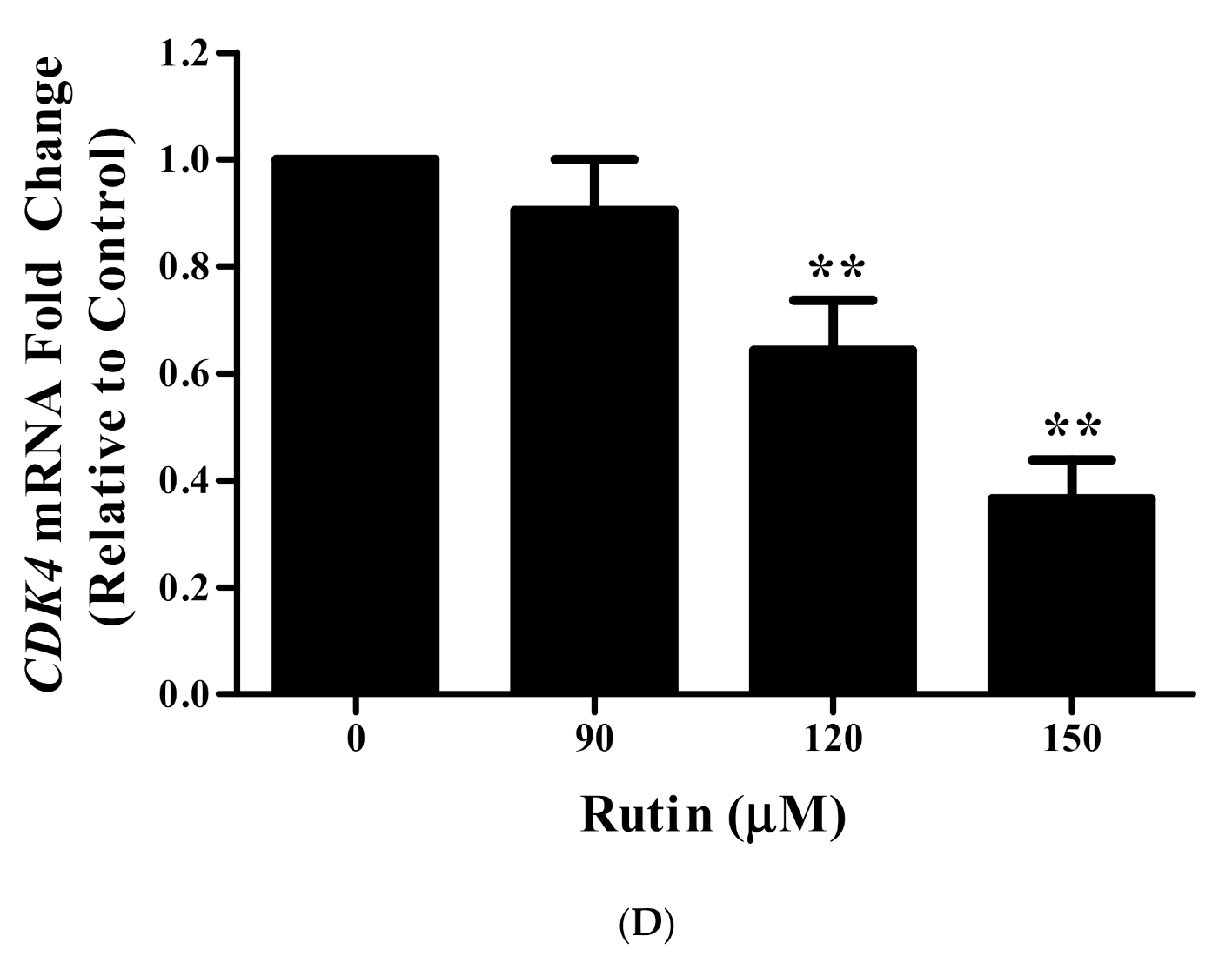
3.10. Rutin Regulates CyclinD1 and CDK4 mRNA Expression
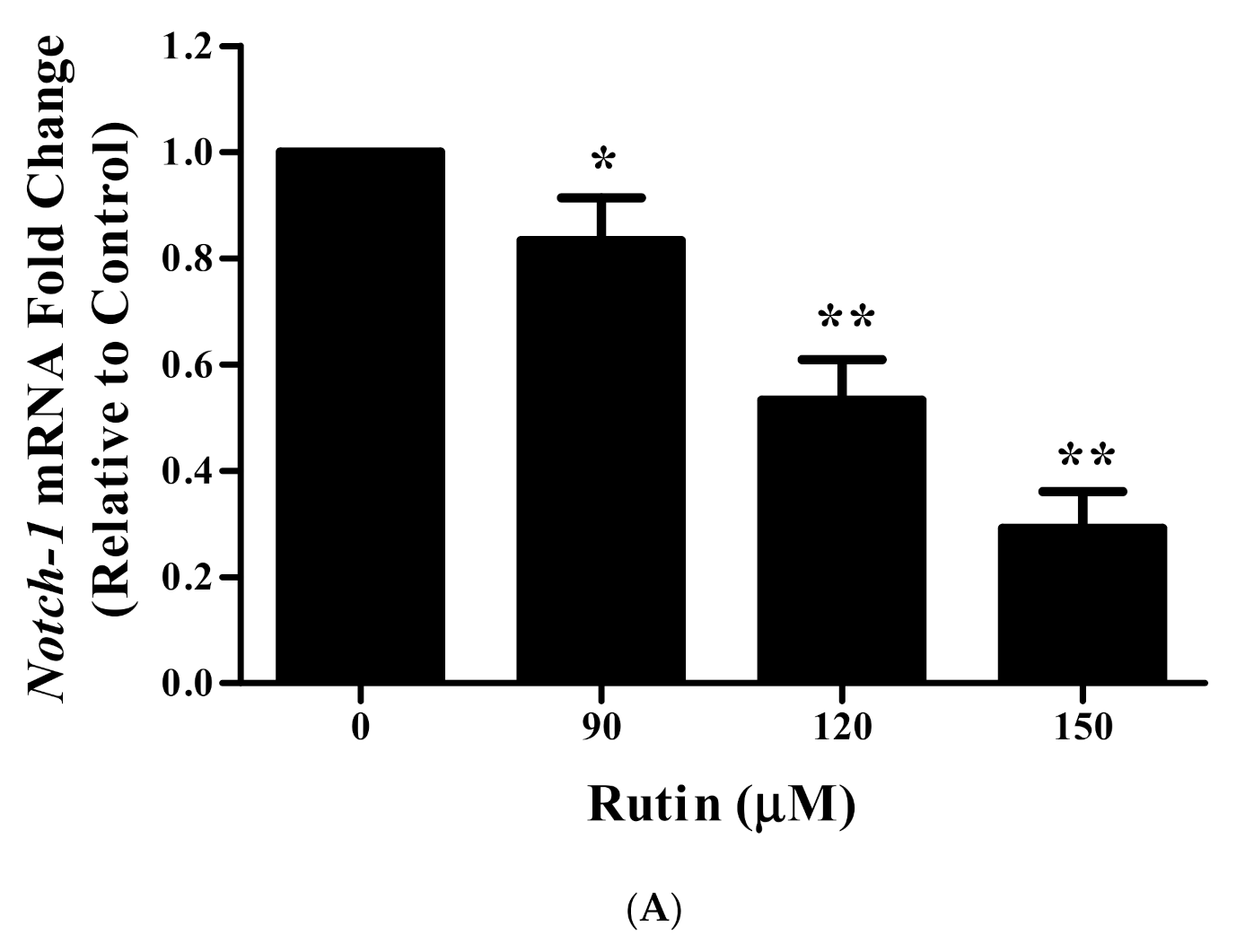
3.11. Rutin Treatment Downregulated mRNA Expression of Notch-1 and Hes-1 in Caski Cells
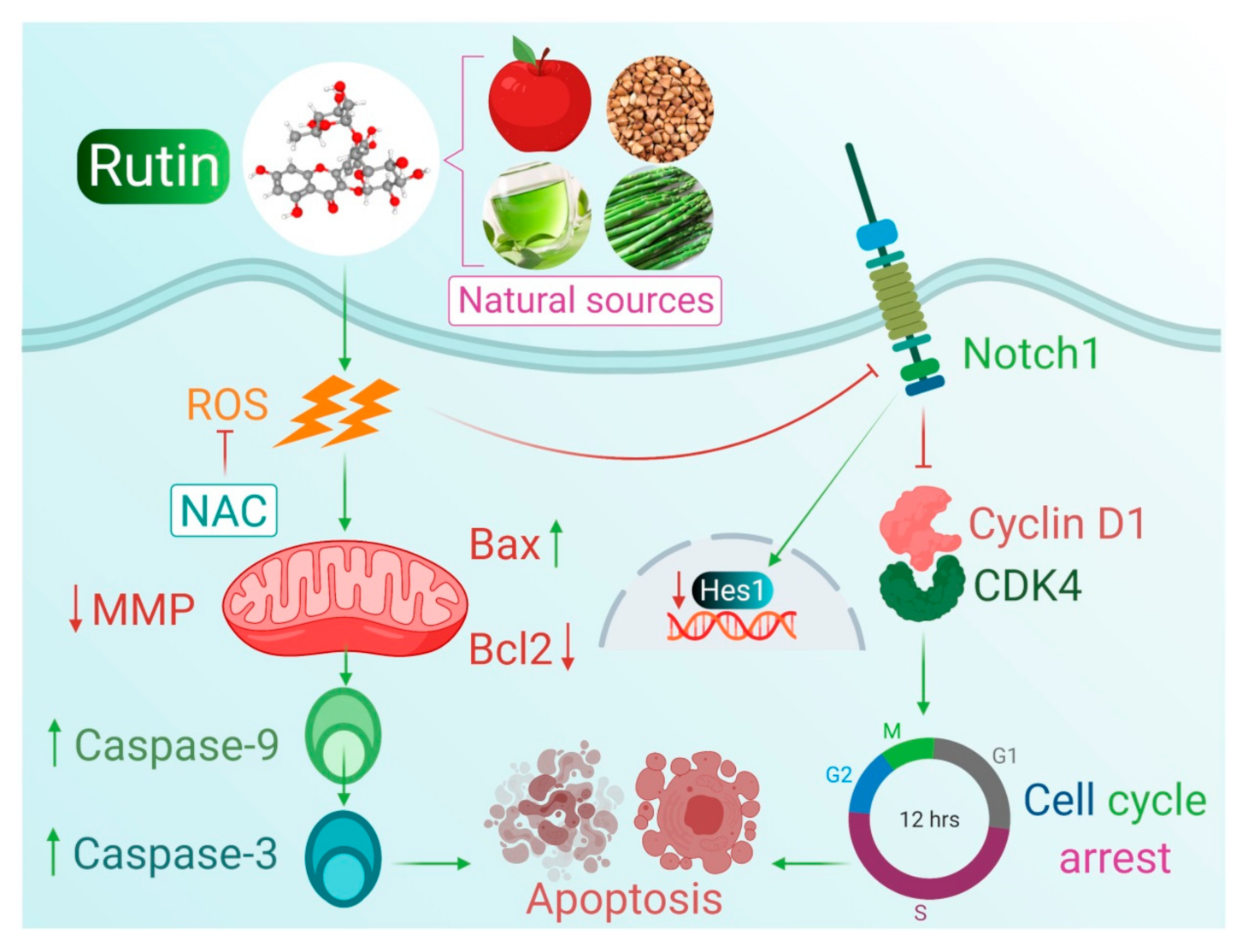
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Cuschieri, K.; Ronco, G.; Lorincz, A.; Smith, L.; Ogilvie, G.; Mirabello, L.; Carozzi, F.; Cubie, H.; Wentzensen, N.; Snijders, P.; et al. Eurogin roadmap 2017: Triage strategies for the management of HPV-positive women in cervical screening programs. Int. J. Cancer 2018, 143, 735–745. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Campos, N.G.; Tsu, V.; Jeronimo, J.; Regan, C.; Resch, S.; Clark, A.; Sy, S.; Kim, J.J. Health impact of delayed implementation of cervical cancer screening programs in India: A modeling analysis. Int. J. Cancer 2019, 144, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Sathishkumar, K.; Chaturvedi, M.; Das, P.; Sudarshan, K.L.; Santhappan, S.; Nallasamy, V.; John, A.; Narasimhan, S. ICMR-NCDIR-NCRP Investigator Group. Cancer Statistics, 2020: Report from national cancer registry programme, India. JCO Glob. Oncol. 2020, 6, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Majumder, S.; Crabtree, J.S.; Golde, T.E.; Minter, L.M.; Osborne, B.A.; Miele, L. Targeting Notch in oncology: The path forward. Nat. Rev. Drug Discov. 2021, 20, 125–144. [Google Scholar] [CrossRef]
- Talora, C.; Sgroi, D.C.; Crum, C.P.; Dotto, G.P. Specific down-modulation of Notch-1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 2002, 16, 2252–2263. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Notch-driven carcinogenesis: The merging of hepatocellular cancer and cholangiocarcinoma into a common molecular liver cancer subtype. J. Hepatol. 2013, 58, 1244–1245. [Google Scholar] [CrossRef]
- Sun, D.W.; Mao, L.; Zhang, J.; Jiang, L.H.; Li, J.; Wu, Y.; Ji, H.; Chen, W.; Wang, J.; Ma, R.; et al. MiR-139-5p inhibits the biological function of breast cancer cells by targeting Notch-1 and mediates chemosensitivity to docetaxel. Biochem. Biophys. Res. Commun. 2015, 465, 702–713. [Google Scholar]
- de Almeida Magalhães, T.; Cruzeiro, G.A.V.; de Sousa, G.R.; da Silva, K.R.; Lira, R.C.P.; Scrideli, C.A.; Tone, L.G.; Valera, E.T.; Borges, K.S. Notch pathway in ependymoma RELA-fused subgroup: Upregulation and association with cancer stem cells markers expression. Gene Ther. 2020, 27, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Kadian, L.K.; Gulshan, G.; Ahuja, P.; Singhal, G.; Sharma, S.; Nanda, S.; Yadav, R. Aberrant promoter methylation of NOTCH-1 and NOTCH3 and its association with cervical cancer risk factors in North Indian population. Am. J. Transl. Res. 2020, 12, 2814–2826. [Google Scholar] [PubMed]
- Grdina, D.J.; Murley, J.S.; Kataoka, Y. Radioprotectants: Current status and new directions. Oncology 2002, 63 (Suppl. 2), 2–10. [Google Scholar] [CrossRef]
- Arora, R.; Gupta, D.; Chawla, R.; Sagar, R.; Sharma, A.; Kumar, R.; Prasad, J.; Singh, S.; Samanta, N.; Sharma, R.K. Radioprotection by plant products: Present status and future prospects. Phytother. Res. 2005, 19, 1–22. [Google Scholar] [CrossRef]
- Weiss, J.F.; Landauer, M.R. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003, 189, 1–20. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Z.; Fan, L.; Meng, S.; Song, C.; Qiu, L.; Xu, P.; Chen, J. Dietary supplementation with rutin has pro-/anti-inflammatory effects in the liver of juvenile GIFT tilapia, Oreochromisniloticus. Fish Shellfish Immunol. 2017, 64, 49–55. [Google Scholar] [CrossRef]
- Arowoogun, J.; Akanni, O.O.; Adefisan, A.O.; Owumi, S.E.; Tijani, A.S.; Adaramoye, O.A. Rutin ameliorates copper sulfate-induced brain damage via antioxidative and anti-inflammatory activities in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22623. [Google Scholar] [CrossRef]
- Saleh, A.; El Fayoumi, H.M.; Youns, M.; Barakat, W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. NaunynSchmiedebergs Arch. Pharmacol. 2019, 392, 165–175. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef]
- Khan, F.; Pandey, P.; Upadhyay, T.K.; Jafri, A.; Jha, N.K.; Mishra, R.; Singh, V. Anti-cancerous effect of rutin against HPV-C33A cervical cancer cells via G0/G1 cell cycle arrest and apoptotic induction. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 409–418. [Google Scholar] [CrossRef]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kaushik, G.; Subramaniam, D.; Dandawate, P.; Neville, K.; Chastain, K.; Anant, S. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J. Hematol. Oncol. 2017, 10, 10. [Google Scholar] [CrossRef]
- Nosrati, N.; Bakovic, M.; Paliyath, G. Molecular mechanisms and pathways as targets for cancer prevention and progression with dietary compounds. Int. J. Mol. Sci. 2017, 18, 2050. [Google Scholar] [CrossRef]
- Khan, F.; Pandey, P.; Ahmad, V.; Upadhyay, T.K. Moringa oleifera methanolic leaves extract induces apoptosis and G0/G1 cell cycle arrest via downregulation of Hedgehog Signaling Pathway in human prostate PC-3 cancer cells. J. Food Biochem. 2020, 44, e13338. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F. Jab1 Inhibition by Methanolic Extract of Moringa Oleifera Leaves in Cervical Cancer Cells: A Potent Targeted Therapeutic Approach. Nutr. Cancer 2020, 1–9. [Google Scholar] [CrossRef]
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive oxygen species and mitochondrial dynamics: The yin and yang of mitochondrial dysfunction and cancer progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef]
- Bilancio, A.; Bontempo, P.; Di Donato, M.; Conte, M.; Giovannelli, P.; Altucci, L.; Migliaccio, A.; Castoria, G. Bisphenol A induces cell cycle arrest in primary and prostate cancer cells through EGFR/ERK/p53 signaling pathway activation. Oncotarget 2017, 8, 115620–115631. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Hmadi, R.; Kareh, M.; Tohme, R.; Darwiche, N. Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis 2015, 20, 1531–1562. [Google Scholar] [CrossRef]
- Saklani, A.; Kutty, S.K. Plant-derived compounds in clinical trials. Drug Discov. Today 2008, 13, 161–171. [Google Scholar] [CrossRef]
- Jucá, M.M.; CysneFilho, F.M.S.; de Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.D.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Yong, D.O.C.; Saker, S.R.; Chellappan, D.K.; Madheswaran, T.; Panneerselvam, J.; Choudhury, H.; Pandey, M.; Chan, Y.L.; Collet, T.; Gupta, G.; et al. Molecular and Immunological Mechanisms Underlying the Various Pharmacological Properties of the Potent Bioflavonoid, Rutin. Endocr. Metab. Immune Disord. Drug Targets. 2020, 20, 1590–1596. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Sharma, S.; Rabbani, S.A.; Narang, J.K.; HyderPottoo, F.; Ali, J.; Kumar, S.; Baboota, S. Role of Rutin Nanoemulsion in Ameliorating Oxidative Stress: Pharmacokinetic and Pharmacodynamics Studies. Chem. Phys. Lipids 2020, 228, 104890. [Google Scholar] [CrossRef] [PubMed]
- Deepika, M.S.; Thangam, R.; Sheena, T.S.; Sasirekha, R.; Sivasubramanian, S.; Babu, M.D.; Jeganathan, K.; Thirumurugan, R. A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomed. Pharmacother. 2019, 109, 1181–1195. [Google Scholar] [CrossRef]
- Farooqui, A.; Khan, F.; Khan, I.; Ansari, I.A. Glycyrrhizin induces reactive oxygen species-dependent apoptosis and cell cycle arrest at G0/G1 in HPV18+ human cervical cancer HeLa cell line. Biomed. Pharmacother. 2018, 97, 752–764. [Google Scholar] [CrossRef]
- Singh, T.; Sharma, S.D.; Katiyar, S.K. Grape Proanthocyanidins Induce Apoptosis by Loss of Mitochondrial Membrane Potential of Human Non-Small Cell Lung Cancer Cells In Vitro and In Vivo. PLoS ONE 2011, 6, e27444. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res. 2019, 27, 629–634. [Google Scholar] [CrossRef]
- Wnęk, A.; Andrzejewska, E.; Kobos, J.; Taran, K.; Przewratil, P. Molecular and immunohistochemical expression of apoptotic proteins Bax, Bcl2 and Caspase 3 in infantile hemangioma tissues as an effect of propranolol treatment. Immunol. Lett. 2017, 185, 27–31. [Google Scholar] [CrossRef]
- Du, L.; Fei, Z.; Song, S.; Wei, N. Antitumor activity of Lobaplatin against esophageal squamous cell carcinoma through caspase-dependent apoptosis and increasing the Bax/Bcl2 ratio. Biomed. Pharmacother. 2017, 95, 447–452. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D’Haese, J.G.; Philippov, P.P.; Werner, J.; Bazhin, A.V. Mitochondria and mitochondrial ROS in cancer: Novel targets for anticancer therapy. J. Cell. Physiol. 2016, 231, 2570–2581. [Google Scholar] [CrossRef]
- Gillissen, B.; Richter, A.; Richter, A.; Preissner, R.; Schulze-Osthoff, K.; Essmann, F.; Daniel, P.T. Bax/Bak-independent mitochondrial depolarization and reactive oxygen species induction by sorafenib overcome resistance to apoptosis in renal cell carcinoma. J. Biol. Chem. 2017, 292, 6478–6492. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Hu, J.J.; Fu, R.Q.; Liu, X.; Zhang, Y.H.; Li, J.; Liu, L.; Li, N.Y.; Deng, Q.; Luo, S.Q.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- de Oliveira, J.M.P.F.; Santos, C.; Fernandes, E. Therapeutic potential of hesperidin and its aglyconehesperetin: Cell cycle regulation and apoptosis induction in cancer models. Phytomedicine 2020, 73, 152887. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Moulder, S.; Keyomarsi, K.; Layman, R.M. Inhibiting CDK in cancer therapy: Current evidence and future directions. Target. Oncol. 2018, 13, 21–38. [Google Scholar] [CrossRef]
- Deshpande, A.; Sicinski, P.; Hinds, P.W. Cyclins and cdks in development and cancer: A perspective. Oncogene 2005, 24, 2909–2915. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef]
- Khan, M.; Maryam, A.; Qazi, J.I.; Ma, T. Targeting apoptosis and multiple signaling pathways with icariside II in cancer cells. Int. J. Biol. Sci. 2015, 11, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Rath, G.; Jawanjal, P.; Sharma, S.; Singhal, P.; Bhambhani, S.; Hussain, S.; Bharadwaj, M. Clinical impact of de-regulated Notch-1 and Notch-3 in the development and progression of HPV-associated different histological subtypes of precancerous and cancerous lesions of human uterine cervix. PLoS ONE 2014, 9, e98642. [Google Scholar] [CrossRef] [PubMed]
- Talora, C.; Cialfi, S.; Segatto, O.; Morrone, S.; Kim Choi, J.; Frati, L.; Paolo Dotto, G.; Gulino, A.; Screpanti, I. Constitutively active Notch-1 induces growth arrest of HPV-positive cervical cancer cells via separate signaling pathways. Exp. Cell Res. 2005, 305, 343–354. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Banerjee, S.; Sarkar, F.H. Exploitation of the Notch signaling pathway as a novel target for cancer therapy. Anticancer Res. 2008, 28, 3621–3630. [Google Scholar]
- Li, M.; Chen, F.; Clifton, N.; Sullivan, D.M.; Dalton, W.S.; Gabrilovich, D.I.; Nefedova, Y. Combined inhibition of Notch signaling and Bcl2/Bcl-xL results in synergistic antimyeloma effect. Mol. Cancer Ther. 2010, 9, 3200–3209. [Google Scholar] [CrossRef]
- Lu, Z.; Ren, Y.; Zhang, M.; Fan, T.; Wang, Y.; Zhao, Q.; Liu, H.M.; Zhao, W.; Hou, G. FLI-06 suppresses proliferation, induces apoptosis and cell cycle arrest by targeting LSD1 and Notch pathway in esophageal squamous cell carcinoma cells. Biomed. Pharmacother. 2018, 107, 1370–1376. [Google Scholar] [CrossRef]
- Ronchini, C.; Capobianco, A.J. Induction of Cyclin D1 transcription and CDK2 activity by Notchic: Implication for cell cycle disruption in transformation by Notchic. Mol. Cell Biol. 2001, 21, 5925–5934. [Google Scholar] [CrossRef]
- Rodrigues, C.; Joy, L.R.; Sachithanandan, S.P.; Krishna, S. Notch signalling in cervical cancer. Exp. Cell Res. 2019, 385, 111682. [Google Scholar] [CrossRef]
- Veeraraghavalu, K.; Pett, M.; Kumar, R.V.; Nair, P.; Rangarajan, A.; Stanley, M.A.; Krishna, S. Papillomavirus-mediated neoplastic progression is associated with reciprocal changes in JAGGED1 and manic fringe expression linked to notch activation. J. Virol. 2004, 78, 8687–8700. [Google Scholar] [CrossRef][Green Version]
- Khan, F.; Pandey, P.; Jha, N.K.; Jafri, A.; Khan, I. Antiproliferative effect of Moringa oleifera methanolic leaf extract by down-regulation of Notch signaling in DU145 prostate cancer cells. Gene Rep. 2020, 19, 100619. [Google Scholar] [CrossRef]
- Khan, F.; Singh, V.K.; Saeed, M.; Kausar, M.A.; Ansari, I.A. Carvacrol Induced Program Cell Death and Cell Cycle Arrest in Androgen-Independent Human Prostate Cancer Cells via Inhibition of Notch Signaling. Anticancer Agents Med. Chem. 2019, 19, 1588–1608. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Farhan, M.; Jafri, A. Elucidation of rutin’s role in inducing caspase-dependent apoptosis via HPV-E6 and E7 down-regulation in cervical cancer HeLa cells. Biosci. Rep. 2021, 41, BSR20210670. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, F.; Pandey, P.; Jha, N.K.; Khalid, M.; Ojha, S. Rutin Mediated Apoptotic Cell Death in Caski Cervical Cancer Cells via Notch-1 and Hes-1 Downregulation. Life 2021, 11, 761. https://doi.org/10.3390/life11080761
Khan F, Pandey P, Jha NK, Khalid M, Ojha S. Rutin Mediated Apoptotic Cell Death in Caski Cervical Cancer Cells via Notch-1 and Hes-1 Downregulation. Life. 2021; 11(8):761. https://doi.org/10.3390/life11080761
Chicago/Turabian StyleKhan, Fahad, Pratibha Pandey, Niraj Kumar Jha, Mohammad Khalid, and Shreesh Ojha. 2021. "Rutin Mediated Apoptotic Cell Death in Caski Cervical Cancer Cells via Notch-1 and Hes-1 Downregulation" Life 11, no. 8: 761. https://doi.org/10.3390/life11080761
APA StyleKhan, F., Pandey, P., Jha, N. K., Khalid, M., & Ojha, S. (2021). Rutin Mediated Apoptotic Cell Death in Caski Cervical Cancer Cells via Notch-1 and Hes-1 Downregulation. Life, 11(8), 761. https://doi.org/10.3390/life11080761








