In Vitro Measurements of Cellular Forces and their Importance in the Lung—From the Sub- to the Multicellular Scale
Abstract
1. Introduction
2. Forces on Lung Cells and Tissue
3. Cellular Force Measurement
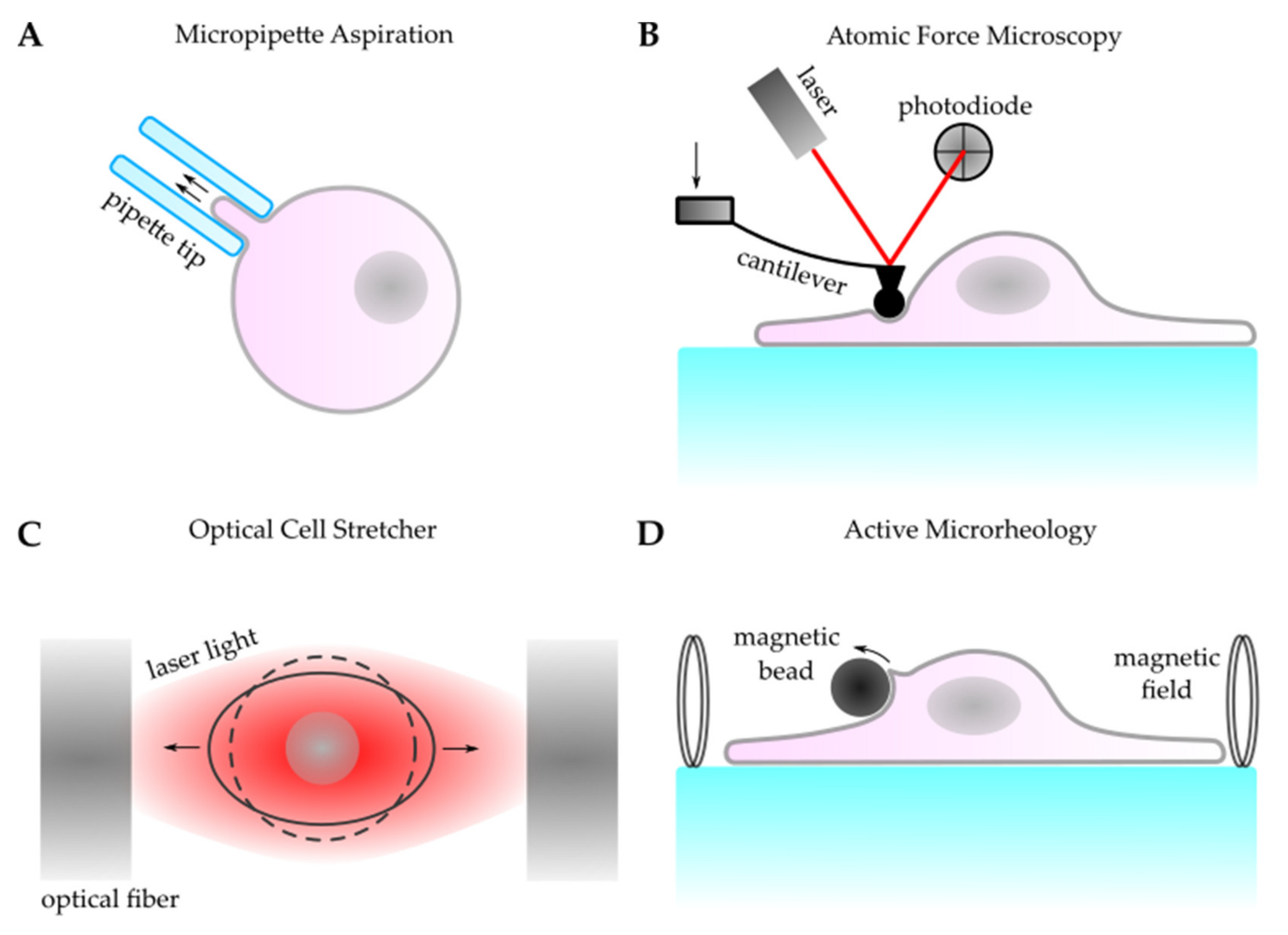
3.1. Active Cellular Force Probing
3.2. Passive Cellular Force Mapping
4. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tschumperlin, D.J.; Boudreault, F.; Liu, F. Recent advances and new opportunities in lung mechanobiology. J. Biomech. 2010, 43, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mariano, C.A.; Sattari, S.; Maghsoudi-Ganjeh, M.; Tartibi, M.; Lo, D.D.; Eskandari, M. Novel Mechanical Strain Characterization of Ventilated ex vivo Porcine and Murine Lung using Digital Image Correlation. Front. Physiol. 2020, 11, 1536. [Google Scholar] [CrossRef]
- Arora, H.; Mitchell, R.L.; Johnston, R.; Manolesos, M.; Howells, D.; Sherwood, J.M.; Bodey, A.J.; Wanelik, K. Correlating local volumetric tissue strains with global lung mechanics measurements. Materials 2021, 14, 439. [Google Scholar] [CrossRef] [PubMed]
- Looney, M.R.; Bhattacharya, J. Live imaging of the lung. Annu. Rev. Physiol. 2014, 76, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Grune, J.; Tabuchi, A.; Kuebler, W.M. Alveolar dynamics during mechanical ventilation in the healthy and injured lung. Intensive Care Med. Exp. 2019, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Perlman, C.E.; Bhattacharya, J. Alveolar expansion imaged by optical sectioning microscopy. J. Appl. Physiol. 2007, 103, 1037–1044. [Google Scholar] [CrossRef]
- Sera, T.; Uesugi, K.; Yagi, N. Localized morphometric deformations of small airways and alveoli in intact mouse lungs under quasi-static inflation. Respir. Physiol. Neurobiol. 2005, 147, 51–63. [Google Scholar] [CrossRef]
- Lovric, G.; Mokso, R.; Arcadu, F.; Vogiatzis Oikonomidis, I.; Schittny, J.C.; Roth-Kleiner, M.; Stampanoni, M. Tomographic in vivo microscopy for the study of lung physiology at the alveolar level. Sci. Rep. 2017, 7, 12545. [Google Scholar] [CrossRef]
- Sera, T.; Yokota, H.; Tanaka, G.; Uesugi, K.; Yagi, N.; Schroter, R.C. Murine pulmonary acinar mechanics during quasi-static inflation using synchrotron refraction-enhanced computed tomography. J. Appl. Physiol. 2013, 115, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Goss, B.C.; McGee, K.P.; Ehman, E.C.; Manduca, A.; Ehman, R.L. Magnetic resonance elastography of the lung: Technical feasibility. Magn. Reson. Med. 2006, 56, 1060–1066. [Google Scholar] [CrossRef]
- Bu, R.; Balakrishnan, S.; Price, H.; Zdanski, C.; Mitran, S.; Oldenburg, A.L. Localized compliance measurement of the airway wall using anatomic optical coherence elastography. Opt. Express 2019, 27, 16751–16766. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.; Hendricks, P.; Weir, J.; Somasundaram, R.; Sittampalam, G.S.; Nirmalanandhan, V.S. Stretching mechanotransduction from the lung to the lab: Approaches and physiological relevance in drug discovery. Assay Drug Dev. Technol. 2012, 10, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hubmayr, R.D.; Kallet, R.H. Understanding pulmonary stress-strain relationships in severe ARDS and its implications for designing a safer approach to setting the ventilator. Respir. Care 2018, 63, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Tschumperlin, D.J.; Lagares, D. Mechano-therapeutics: Targeting Mechanical Signaling in Fibrosis and Tumor Stroma. Pharmacol. Ther. 2020, 212, 107575. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.; Ochs, M. The micromechanics of lung alveoli: Structure and function of surfactant and tissue components. Histochem. Cell Biol. 2018, 150, 661–676. [Google Scholar] [CrossRef]
- Liu, M.; Post, M. Invited review: Mechanochemical signal transduction in the fetal lung. J. Appl. Physiol. 2000, 89, 2078–2084. [Google Scholar] [CrossRef]
- Liu, M.; Xu, J.; Tanswell, A.K.; Post, M. Stretch-induced growth-promoting activities stimulate fetal rat lung epithelial cell proliferation. Exp. Lung Res. 1993, 19, 505–517. [Google Scholar] [CrossRef]
- Foster, C.D.; Varghese, L.S.; Gonzales, L.W.; Margulies, S.S.; Guttentag, S.H. The Rho pathway mediates transition to an alveolar type i cell phenotype during static stretch of alveolar type II cells. Pediatr. Res. 2010, 67, 585–590. [Google Scholar] [CrossRef]
- Sanchez-Esteban, J.; Cicchiello, L.A.; Wang, Y.; Tsai, S.W.; Williams, L.K.; Torday, J.S.; Rubin, L.P. Mechanical stretch promotes alveolar epithelial type II cell differentiation. J. Appl. Physiol. 2001, 91, 589–595. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Chu, Q.; Jiang, K.; Li, J.; Tang, N. The Strength of Mechanical Forces Determines the Differentiation of Alveolar Epithelial Cells. Dev. Cell 2018, 44, 297–312. [Google Scholar] [CrossRef]
- Tang, Z.; Hu, Y.; Wang, Z.; Jiang, K.; Zhan, C.; Marshall, W.F.; Tang, N. Mechanical Forces Program the Orientation of Cell Division during Airway Tube Morphogenesis. Dev. Cell 2018, 44, 313–325. [Google Scholar] [CrossRef]
- Edwards, Y.S. Stretch stimulation: Its effects on alveolar type II cell function in the lung. Comp. Biochem. Physiol. Part A 2001, 129, 245–260. [Google Scholar] [CrossRef]
- Wirtz, H.R.W.; Dobbs, L.G. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 1990, 250, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Frick, M.; Bertocchi, C.; Jennings, P.; Haller, T.; Mair, N.; Singer, W.; Pfaller, W.; Ritsch-Marte, M.; Dietl, P. Ca2+ entry is essential for cell strain-induced lamellar body fusion in isolated rat type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L210–L220. [Google Scholar] [CrossRef] [PubMed]
- Ashino, Y.; Ying, X.; Dobbs, L.G.; Bhattacharya, J. Ca(2+)(i) oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L5–L13. [Google Scholar] [CrossRef]
- Patel, A.S.; Reigada, D.; Mitchell, C.H.; Bates, S.R.; Margulies, S.S.; Koval, M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L489–L496. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.; Boudreault, F.; Adam, D.; Brochiero, E.; Grygorczyk, R. Type 2 secretory cells are primary source of ATP release in mechanically stretched lung alveolar cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L49–L58. [Google Scholar] [CrossRef] [PubMed]
- Furuya, K.; Tan, J.J.; Boudreault, F.; Sokabe, M.; Berthiaume, Y.; Grygorczyk, R. Real-time imaging of inflation-induced ATP release in the ex vivo rat lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L956–L969. [Google Scholar] [CrossRef]
- Diem, K.; Fauler, M.; Fois, G.; Hellmann, A.; Winokurow, N.; Schumacher, S.; Kranz, C.; Frick, M. Mechanical stretch activates piezo1 in caveolae of alveolar type I cells to trigger ATP release and paracrine stimulation of surfactant secretion from alveolar type II cells. FASEB J. 2020, 34, 12785–12804. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.R.; Oswari, J.; Margulies, S.S. Role of stretch on tight junction structure in alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001, 25, 584–591. [Google Scholar] [CrossRef]
- Cohen, T.S.; Cavanaugh, K.J.; Margulies, S.S. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur. Respir. J. 2008, 32, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Correll, K.; Schiel, J.A.; Finigan, J.H.; Prekeris, R.; Mason, R.J. Lung fibroblasts accelerate wound closure in human alveolar epithelial cells through hepatocyte growth factor/c-Met signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L94–L105. [Google Scholar] [CrossRef] [PubMed]
- Tschumperlin, D.J.; Oswari, J.; Margulies, S.S. Deformation-induced injury of alveolar epithelial cells: Effect of frequency, duration, and amplitude. Am. J. Respir. Crit. Care Med. 2000, 162, 357–362. [Google Scholar] [CrossRef]
- Hammerschmidt, S.; Kuhn, H.; Gessner, C.; Seyfarth, H.J.; Wirtz, H. Stretch-induced alveolar type II cell apoptosis: Role of endogenous bradykinin and PI3K-Akt signaling. Am. J. Respir. Cell Mol. Biol. 2007, 37, 699–705. [Google Scholar] [CrossRef]
- Iwaki, M.; Ito, S.; Morioka, M.; Iwata, S.; Numaguchi, Y.; Ishii, M.; Kondo, M.; Kume, H.; Naruse, K.; Sokabe, M.; et al. Mechanical stretch enhances IL-8 production in pulmonary microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2009, 389, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, I.; Santos, C.L.; Huhle, R.; Ferreira, J.M.C.; Koch, T.; Schnabel, C.; Koch, E.; Pelosi, P.; Rocco, P.R.M.; de Abreu, M.G. Variable stretch reduces the pro-inflammatory response of alveolar epithelial cells. PLoS ONE 2017, 12, e0182369. [Google Scholar] [CrossRef]
- Valentine, M.S.; Link, P.A.; Herbert, J.A.; Kamga Gninzeko, F.J.; Schneck, M.B.; Shankar, K.; Nkwocha, J.; Reynolds, A.M.; Heise, R.L. Inflammation and Monocyte Recruitment Due to Aging and Mechanical Stretch in Alveolar Epithelium are Inhibited by the Molecular Chaperone 4-Phenylbutyrate. Cell. Mol. Bioeng. 2018, 11, 495–508. [Google Scholar] [CrossRef]
- Felder, M.; Trueeb, B.; Stucki, A.O.; Borcard, S.; Stucki, J.D.; Schnyder, B.; Geiser, T.; Guenat, O.T. Impaired wound healing of alveolar lung epithelial cells in a breathing lung-on-a-chip. Front. Bioeng. Biotechnol. 2019, 7, 3. [Google Scholar] [CrossRef]
- Desai, L.P.; Chapman, K.E.; Waters, C.M. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L958–L965. [Google Scholar] [CrossRef] [PubMed]
- Vlahakis, N.E.; Hubmayr, R.D. Cellular stress failure in ventilator-injured lungs. Am. J. Respir. Crit. Care Med. 2005, 171, 1328–1342. [Google Scholar] [CrossRef]
- Perlman, C.E.; Lederer, D.J.; Bhattacharya, J. Micromechanics of alveolar edema. Am. J. Respir. Cell Mol. Biol. 2010, 44, 34–39. [Google Scholar] [CrossRef]
- Mertens, M.; Tabuchi, A.; Meissner, S.; Krueger, A.; Schirrmann, K.; Kertzscher, U.; Pries, A.R.; Slutsky, A.S.; Koch, E.; Kuebler, W.M. Alveolar dynamics in acute lung injury: Heterogeneous distension rather than cyclic opening and collapse. Crit. Care Med. 2009, 37, 2604–2611. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2018, 307, 2526–2533. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis: An integral model. Am. J. Respir. Crit. Care Med. 2014, 189, 1161–1172. [Google Scholar] [CrossRef]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef]
- Nonomura, K.; Woo, S.H.; Chang, R.B.; Gillich, A.; Qiu, Z.; Francisco, A.G.; Ranade, S.S.; Liberles, S.D.; Patapoutian, A. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 2017, 541, 176–181. [Google Scholar] [CrossRef]
- Noble, P.B.; Sharma, A.; McFawn, P.K.; Mitchell, H.W. Elastic properties of the bronchial mucosa: Epithelial unfolding and stretch in response to airway inflation. J. Appl. Physiol. 2005, 99, 2061–2066. [Google Scholar] [CrossRef]
- Yu, J. Airway mechanosensors. Respir. Physiol. Neurobiol. 2005, 148, 217–243. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Fredberg, J.J.; Drazen, J.M. Putting the squeeze on airway epithelia. Physiology 2015, 30, 293–303. [Google Scholar] [CrossRef]
- Li, N.; He, Y.; Yang, G.; Yu, Q.; Li, M. Role of TRPC1 channels in pressure-mediated activation of airway remodeling. Respir. Res. 2019, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Tschumperlin, D.J.; Drazen, J.M. Mechanical stimuli to airway remodeling. Am. J. Respir. Crit. Care Med. 2001, 164, S90–S94. [Google Scholar] [CrossRef]
- Copland, I.B.; Kavanagh, B.P.; Engelberts, D.; McKerlie, C.; Belik, J.; Post, M. Early Changes in Lung Gene Expression due to High Tidal Volume. Am. J. Respir. Crit. Care Med. 2003, 168, 1051–1059. [Google Scholar] [CrossRef]
- Faisy, C.; Pinto, F.M.; Le Guen, M.; Naline, E.; Delyle, S.G.; Sage, E.; Candenas, M.L.; Devillier, P. Airway response to acute mechanical stress in a human bronchial model of stretch. Crit. Care 2011, 15, R208. [Google Scholar] [CrossRef] [PubMed]
- Wells, N.; Roesler, A.M.; Ravix, J.; Teske, J.J.; Pabelick, C.M.; Prakash, Y.S. Mechanical Stretch Contributes to Airway Hyperresponsiveness and Remodeling in Human Fetal Airway Smooth Muscle Cells via the Calcium Sensing Receptor. In A30. Contract and Relax: What’s New in Airway Smooth Muscle Mechanisms; American Thoracic Society: New York, NY, USA, 2020. [Google Scholar]
- Nucci, G.; Suki, B.; Lutchen, K. Modeling airflow-related shear stress during heterogeneous constriction and mechanical ventilation. J. Appl. Physiol. 2003, 95, 348–356. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Sesma, J.I.; Seminario-Vidal, L.; Kreda, S.M. Chapter 8—Molecular Mechanisms of Purine and Pyrimidine Nucleotide Release. In Advances in Pharmacology: Pharmacology of Purine and Pyrimidine Receptors; Jacobson, K.A., Linden, J., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 221–261. ISBN 1054-3589. [Google Scholar]
- Tarran, R.; Button, B.; Picher, M.; Paradiso, A.M.; Ribeiro, C.M.; Lazarowski, E.R.; Zhang, L.; Collins, P.L.; Pickles, R.J.; Fredberg, J.J.; et al. Normal and cystic fibrosis airway surface liquid homeostasis: The effects of phasic shear stress and viral infections. J. Biol. Chem. 2005, 280, 35751–35759. [Google Scholar] [CrossRef]
- Zhu, Y.; Abdullah, L.H.; Doyle, S.P.; Nguyen, K.; Ribeiro, C.M.P.; Vasquez, P.A.; Forest, M.G.; Lethem, M.I.; Dickey, B.F.; Davis, C.W. Baseline goblet cell mucin secretion in the airways exceeds stimulated secretion over extended time periods, and is sensitive to shear stress and intracellular mucin stores. PLoS ONE 2015, 10, e0127267. [Google Scholar] [CrossRef] [PubMed]
- Sidhaye, V.K.; Schweitzer, K.S.; Caterina, M.J.; Shimoda, L.; King, L.S. Shear stress regulates aquaporin-5 and airway epithelial barrier function. Proc. Natl. Acad. Sci. USA 2008. [Google Scholar] [CrossRef] [PubMed]
- Bilek, A.M.; Dee, K.C.; Gaver, D.P. Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J. Appl. Physiol. 2003, 94, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Ravasio, A.; Hobi, N.; Bertocchi, C.; Jesacher, A.; Dietl, P.; Haller, T. Interfacial sensing by alveolar type II cells: A new concept in lung physiology? Am. J. Physiol. Cell Physiol. 2011, 300, C1456–C1465. [Google Scholar] [CrossRef]
- Hobi, N.; Ravasio, A.; Haller, T. Interfacial stress affects rat alveolar type II cell signaling and gene expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L117–L129. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Bhattacharya, J.; Matthay, M.A.; Malik, A.B. Integrated control of lung fluid balance. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L1081–L1090. [Google Scholar] [CrossRef] [PubMed]
- Szulcek, R.; Happe, C.M.; Rol, N.; Fontijn, R.D.; Dickhoff, C.; Hartemink, K.J.; Grünberg, K.; Tu, L.; Timens, W.; Nossent, G.D.; et al. Delayed microvascular shear adaptation in pulmonary arterial hypertension: Role of platelet endothelial cell adhesion molecule-1 cleavage. Am. J. Respir. Crit. Care Med. 2016, 193, 1410–1420. [Google Scholar] [CrossRef]
- Dickinson, M.G.; Bartelds, B.; Borgdorff, M.A.J.; Berger, R.M.F. The role of disturbed blood flow in the development of pulmonary arterial hypertension: Lessons from preclinical animal models. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L1–L14. [Google Scholar] [CrossRef]
- Liu, F.; Mih, J.D.; Shea, B.S.; Kho, A.T.; Sharif, A.S.; Tager, A.M.; Tschumperlin, D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010, 190, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Polio, S.R.; Stasiak, S.E.; Jamieson, R.R.; Balestrini, J.L.; Krishnan, R.; Parameswaran, H. Extracellular matrix stiffness regulates human airway smooth muscle contraction by altering the cell-cell coupling. Sci. Rep. 2019, 9, 9564. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Lagares, D. Matrix Stiffness: The Conductor of Organ Fibrosis. Curr. Rheumatol. Rep. 2018, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Tissue stiffness, latent TGF-β1 Activation, and mechanical signal transduction: Implications for the pathogenesis and treatment of fibrosis. Curr. Rheumatol. Rep. 2009, 11, 120–126. [Google Scholar] [CrossRef]
- Freeberg, M.A.T.; Perelas, A.; Rebman, J.K.; Phipps, R.P.; Thatcher, T.H.; Sime, P.J. Mechanical Feed-Forward Loops Contribute to Idiopathic Pulmonary Fibrosis. Am. J. Pathol. 2021, 191, 18–25. [Google Scholar] [CrossRef]
- Chanda, D.; Otoupalova, E.; Smith, S.R.; Volckaert, T.; de Langhe, S.P.; Thannickal, V.J. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Asp. Med. 2019, 65, 56–69. [Google Scholar] [CrossRef]
- Marinković, A.; Liu, F.; Tschumperlin, D.J. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am. J. Respir. Cell Mol. Biol. 2013, 48, 422–430. [Google Scholar] [CrossRef]
- Zhou, Y.; Horowitz, J.C.; Naba, A.; Ambalavanan, N.; Atabai, K.; Balestrini, J.; Bitterman, P.B.; Corley, R.A.; Ding, B.-S.; Engler, A.J.; et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 2018, 73, 77–104. [Google Scholar] [CrossRef]
- Huse, M. Mechanical forces in the immune system. Nat. Rev. Immunol. 2017, 17, 679–690. [Google Scholar] [CrossRef]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.D.; Yun, S.; de Zoete, M.R.; Warnock, J.N.; To, S.D.F.; York, A.G.; et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 2019, 573, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Button, B.; Boucher, R.C. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir. Physiol. Neurobiol. 2008, 163, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Tschumperlin, D.J.; Drazen, J.M. Chronic effects of mechanical force on airways. Annu. Rev. Physiol. 2006, 68, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Veerati, P.C.; Mitchel, J.A.; Reid, A.T.; Knight, D.A.; Bartlett, N.W.; Park, J.-A.; Grainge, C.L. Airway mechanical compression: Its role in asthma pathogenesis and progression. Eur. Respir. Rev. 2020, 29, 190123. [Google Scholar] [CrossRef]
- Waters, C.M.; Roan, E.; Navajas, D. Mechanobiology in lung epithelial cells: Measurements, perturbations, and responses. Compr. Physiol. 2012, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Stamenović, D.; Wang, N. Stress transmission within the cell. Compr. Physiol. 2011, 1, 499. [Google Scholar] [CrossRef]
- Mathieu, S.; Manneville, J.-B. Intracellular mechanics: Connecting rheology and mechanotransduction. Curr. Opin. Cell Biol. 2019, 56, 34–44. [Google Scholar] [CrossRef]
- Hu, X.; Margadant, F.M.; Yao, M.; Sheetz, M.P. Molecular stretching modulates mechanosensing pathways. Protein Sci. 2017, 26, 1337–1351. [Google Scholar] [CrossRef]
- Rajan, S.; Schremmer, C.; Weber, J.; Alt, P.; Geiger, F.; Dietrich, A. Ca2+ Signaling by TRPV4 Channels in Respiratory Function and Disease. Cells 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, N.M.; Ravindran, K.; Kuebler, W.M. TRPV4: Physiological role and therapeutic potential in respiratory diseases. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 421–436. [Google Scholar] [CrossRef]
- Zhong, M.; Komarova, Y.; Rehman, J.; Malik, A.B. Mechanosensing Piezo channels in tissue homeostasis including their role in lungs. Pulm. Circ. 2018, 8, 2045894018767393. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lagares, D.; Choi, K.M.; Stopfer, L.; Marinković, A.; Vrbanac, V.; Probst, C.K.; Hiemer, S.E.; Sisson, T.H.; Horowitz, J.C.; et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L344–L357. [Google Scholar] [CrossRef]
- Huang, X.; Yang, N.; Fiore, V.F.; Barker, T.H.; Sun, Y.; Morris, S.W.; Ding, Q.; Thannickal, V.J.; Zhou, Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am. J. Respir. Cell Mol. Biol. 2012, 47, 340–348. [Google Scholar] [CrossRef]
- Zheng, X.R.; Zhang, X. Microsystems for cellular force measurement: A review. J. Micromech. Microeng. 2011, 21, 54003. [Google Scholar] [CrossRef]
- Campàs, O. A toolbox to explore the mechanics of living embryonic tissues. Semin. Cell Dev. Biol. 2016, 55, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, I.; Marcq, P.; Bosveld, F.; Fetler, L.; Bellaïche, Y.; Graner, F. Mechanical state, material properties and continuous description of an epithelial tissue. J. R. Soc. Interface 2012, 9, 2614–2623. [Google Scholar] [CrossRef]
- Guirao, B.; Rigaud, S.U.; Bosveld, F.; Bailles, A.; López-Gay, J.; Ishihara, S.; Sugimura, K.; Graner, F.; Bellaïche, Y. Unified quantitative characterization of epithelial tissue development. Elife 2015, 4, e08519. [Google Scholar] [CrossRef]
- Evans, E.A.; Kwok, R.; McCown, T. Calibration of beam deflection produced by cellular forces in the 10 −9 –10 −6 gram range. Cell Biophys. 1980, 2, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Yeung, A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys. J. 1989, 56, 151–160. [Google Scholar] [CrossRef]
- Hochmuth, R.M. Micropipette aspiration of living cells. J. Biomech. 2000, 33, 15–22. [Google Scholar] [CrossRef]
- Guevorkian, K.; Maître, J.L. Micropipette aspiration: A unique tool for exploring cell and tissue mechanics in vivo. Methods Cell Biol. 2017, 139, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Maître, J.-L.; Niwayama, R.; Turlier, H.; Nédélec, F.; Hiiragi, T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat. Cell Biol. 2015, 17, 849–855. [Google Scholar] [CrossRef]
- Cook, T.; Alexander, H.; Cohen, M. Experimental method for determining the 2-dimensional mechanical properties of living human skin. Med. Biol. Eng. Comput. 1977, 15, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Ohashi, T.; Matsumoto, T.; Sato, M. The pipette aspiration applied to the local stiffness measurement of soft tissues. Ann. Biomed. Eng. 1997, 25, 581–587. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930. [Google Scholar] [CrossRef]
- Franz, C.M.; Puech, P.-H. Atomic Force Microscopy: A Versatile Tool for Studying Cell Morphology, Adhesion and Mechanics. Cell. Mol. Bioeng. 2008, 1, 289–300. [Google Scholar] [CrossRef]
- Fotiadis, D.; Scheuring, S.; Müller, S.A.; Engel, A.; Müller, D.J. Imaging and manipulation of biological structures with the AFM. Micron 2002, 33, 385–397. [Google Scholar] [CrossRef]
- Dufrêne, Y.F. Atomic force microscopy, a powerful tool in microbiology. J. Bacteriol. 2002, 184, 5205–5213. [Google Scholar] [CrossRef] [PubMed]
- Radmacher, M.; Fritz, M.; Hansma, P.K. Imaging soft samples with the atomic force microscope: Gelatin in water and propanol. Biophys. J. 1995, 69, 264–270. [Google Scholar] [CrossRef]
- Radmacher, M.; Fritz, M.; Kacher, C.M.; Cleveland, J.P.; Hansma, P.K. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys. J. 1996, 70, 556–567. [Google Scholar] [CrossRef]
- Hassan, E.; Heinz, W.F.; Antonik, M.D.; D’Costa, N.P.; Nageswaran, S.; Schoenenberger, C.-A.; Hoh, J.H. Relative Microelastic Mapping of Living Cells by Atomic Force Microscopy. Biophys. J. 1998, 74, 1564–1578. [Google Scholar] [CrossRef]
- Benoit, M.; Gaub, H.E. Measuring cell adhesion forces with the atomic force microscope at the molecular level. Cells Tissues Organs 2002, 172, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Drake, B.; Prater, C.B.; Weisenhorn, A.L.; Gould, S.A.; Albrecht, T.R.; Quate, C.F.; Cannell, D.S.; Hansma, H.G.; Hansma, P.K. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science 1989, 243, 1586–1589. [Google Scholar] [CrossRef]
- Alexander, S.; Hellemans, L.; Marti, O.; Schneir, J.; Elings, V.; Hansma, P.K.; Longmire, M.; Gurley, J. An atomic-resolution atomic-force microscope implemented using an optical lever. J. Appl. Phys. 1989, 65, 164–167. [Google Scholar] [CrossRef]
- Meyer, G.; Amer, N.M. Novel optical approach to atomic force microscopy. Appl. Phys. Lett. 1988, 53, 1045–1047. [Google Scholar] [CrossRef]
- Moeendarbary, E.; Valon, L.; Fritzsche, M.; Harris, A.R.; Moulding, D.A.; Thrasher, A.J.; Stride, E.; Mahadevan, L.; Charras, G.T. The cytoplasm of living cells behaves as a poroelastic material. Nat. Mater. 2013, 12, 253–261. [Google Scholar] [CrossRef]
- Haase, K.; Pelling, A.E. Investigating cell mechanics with atomic force microscopy. J. R. Soc. Interface 2015, 12, 20140970. [Google Scholar] [CrossRef]
- Haase, K.; Pelling, A.E. Resiliency of the plasma membrane and actin cortex to large-scale deformation. Cytoskeleton 2013, 70, 494–514. [Google Scholar] [CrossRef]
- Kuznetsova, T.G.; Starodubtseva, M.N.; Yegorenkov, N.I.; Chizhik, S.A.; Zhdanov, R.I. Atomic force microscopy probing of cell elasticity. Micron 2007, 38, 824–833. [Google Scholar] [CrossRef]
- Darling, E.M.; Topel, M.; Zauscher, S.; Vail, T.P.; Guilak, F. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J. Biomech. 2008, 41, 454–464. [Google Scholar] [CrossRef]
- Lutz, A.; Jung, D.; Diem, K.; Fauler, M.; Port, F.; Gottschalk, K.; Felder, E. Acute effects of cell stretch on keratin filaments in A549 lung cells. FASEB J. 2020, 34, 11227–11242. [Google Scholar] [CrossRef]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef]
- Li, Q.S.; Lee, G.Y.H.; Ong, C.N.; Lim, C.T. AFM indentation study of breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 374, 609–613. [Google Scholar] [CrossRef]
- Petersen, N.O.; McConnaughey, W.B.; Elson, E.L. Dependence of locally measured cellular deformability on position on the cell, temperature, and cytochalasin B. Proc. Natl. Acad. Sci. USA 1982, 79, 5327–5331. [Google Scholar] [CrossRef]
- McConnaughey, W.B.; Petersen, N.O. Cell poker: An apparatus for stress-strain measurements on living cells. Rev. Sci. Instrum. 2008, 51, 575. [Google Scholar] [CrossRef]
- Duszyk, M.; Schwab, B.; Zahalak, G.I.; Qian, H.; Elson, E.L. Cell poking: Quantitative analysis of indentation of thick viscoelastic layers. Biophys. J. 1989, 55, 683–690. [Google Scholar] [CrossRef]
- Daily, B.; Elson, E.L.; Zahalak, G.I. Cell poking. Determination of the elastic area compressibility modulus of the erythrocyte membrane. Biophys. J. 1984, 45, 671–682. [Google Scholar] [CrossRef]
- Albrecht-Buehler, G. Role of cortical tension in fibroblast shape and movement. Cell Motil. Cytoskelet. 1987, 7, 54–67. [Google Scholar] [CrossRef]
- Kolega, J. Effects of mechanical tension on protrusive activity and microfilament and intermediate filament organization in an epidermal epithelium moving in culture. J. Cell Biol. 1986, 102, 1400–1411. [Google Scholar] [CrossRef]
- Dennerll, T.J.; Lamoureux, P.; Buxbaum, R.E.; Heidemann, S.R. The cytomechanics of axonal elongation and retraction. J. Cell Biol. 1989, 109, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Felder, S.; Elson, E.L. Mechanics of fibroblast locomotion: Quantitative analysis of forces and motions at the leading lamellas of fibroblasts. J. Cell Biol. 1990, 111, 2513–2526. [Google Scholar] [CrossRef]
- Moore, S.W. A fiber optic system for measuring dynamic mechanical properties of embryonic tissues. IEEE Trans. Biomed. Eng. 1994, 41, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zamir, E.A.; Srinivasan, V.; Perucchio, R.; Taber, L.A. Mechanical Asymmetry in the Embryonic Chick Heart during Looping. Ann. Biomed. Eng. 2003, 31, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Thoumine, O.; Ott, A. Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. J. Cell Sci. 1997, 110, 2109–2116. [Google Scholar] [CrossRef]
- Thoumine, O.; Ott, A.; Cardoso, O.; Meister, J.-J. Microplates: A new tool for manipulation and mechanical perturbation of individual cells. J. Biochem. Biophys. Methods 1999, 39, 47–62. [Google Scholar] [CrossRef]
- Marmottant, P.; Mgharbel, A.; Käfer, J.; Audren, B.; Rieu, J.-P.; Vial, J.-C.; van der Sanden, B.; Marée, A.F.M.; Graner, F.; Delanoë-Ayari, H. The role of fluctuations and stress on the effective viscosity of cell aggregates. Proc. Natl. Acad. Sci. USA 2009, 106, 17271–17275. [Google Scholar] [CrossRef]
- Kalantarian, A.; Ninomiya, H.; Saad, S.M.I.; David, R.; Winklbauer, R.; Neumann, A.W. Axisymmetric drop shape analysis for estimating the surface tension of cell aggregates by centrifugation. Biophys. J. 2009, 96, 1606–1616. [Google Scholar] [CrossRef]
- Phillips, H.M.; Steinberg, M.S. Equilibrium Measurements of Embryonic Chick Cell Adhesiveness, I. Shape Equilibrium in Centrifugal Fields. Proc. Natl. Acad. Sci. USA 1969, 64, 121–127. [Google Scholar] [CrossRef]
- Ashkin, A. Acceleration and Trapping of Particles by Radiation Pressure. Phys. Rev. Lett. 1970, 24, 156. [Google Scholar] [CrossRef]
- Svoboda, K.; Block, S.M. Biological Applications of Optical Forces. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 247–285. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.C.; Sheetz, M.P. Optical tweezers in cell biology. Trends Cell Biol. 1992, 2, 116–118. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Chemla, Y.R.; Smith, S.B.; Bustamante, C. Recent advances in optical tweezers. Annu. Rev. Biochem. 2008, 77, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, K.; Schmidt, C.F.; Schnapp, B.J.; Block, S.M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 1993, 365, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Finer, J.T.; Simmons, R.M.; Spudich, J.A. Single myosin molecule mechanics: Piconewton forces and nanometre steps. Nature 1994, 368, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kucik, D.F.; Kuo, S.C.; Elson, E.L.; Sheetz, M.P. Preferential attachment of membrane glycoproteins to the cytoskeleton at the leading edge of lamella. J. Cell Biol. 1991, 114, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Sheetz, M.P. Mechanical properties of neuronal growth cone membranes studied by tether formation with laser optical tweezers. Biophys. J. 1995, 68, 988–996. [Google Scholar] [CrossRef]
- Nawaz, S.; Sánchez, P.; Bodensiek, K.; Li, S.; Simons, M.; Schaap, I.A.T. Cell Visco-Elasticity Measured with AFM and Optical Trapping at Sub-Micrometer Deformations. PLoS ONE 2012, 7, e45297. [Google Scholar] [CrossRef]
- Guck, J.; Ananthakrishnan, R.; Mahmood, H.; Moon, T.J.; Cunningham, C.C.; Käs, J. The Optical Stretcher: A Novel Laser Tool to Micromanipulate Cells. Biophys. J. 2001, 81, 767–784. [Google Scholar] [CrossRef]
- Guck, J.; Ananthakrishnan, R.; Cunningham, C.C.; Käs, J. Stretchingbiological cells with light. J. Phys. Condens. Matter 2002, 14, 4843. [Google Scholar] [CrossRef]
- Yang, T.; Bragheri, F.; Minzioni, P. A Comprehensive Review of Optical Stretcher for Cell Mechanical Characterization at Single-Cell Level. Micromachines 2016, 7, 90. [Google Scholar] [CrossRef]
- Remmerbach, T.W.; Wottawah, F.; Dietrich, J.; Lincoln, B.; Wittekind, C.; Guck, J. Oral Cancer Diagnosis by Mechanical Phenotyping. Cancer Res. 2009, 69, 1728–1732. [Google Scholar] [CrossRef]
- Guck, J.; Schinkinger, S.; Lincoln, B.; Wottawah, F.; Ebert, S.; Romeyke, M.; Lenz, D.; Erickson, H.M.; Ananthakrishnan, R.; Mitchell, D.; et al. Optical Deformability as an Inherent Cell Marker for Testing Malignant Transformation and Metastatic Competence. Biophys. J. 2005, 88, 3689–3698. [Google Scholar] [CrossRef]
- Maloney, J.M.; Nikova, D.; Lautenschläger, F.; Clarke, E.; Langer, R.; Guck, J.; van Vliet, K.J. Mesenchymal Stem Cell Mechanics from the Attached to the Suspended State. Biophys. J. 2010, 99, 2479–2487. [Google Scholar] [CrossRef]
- Bambardekar, K.; Clément, R.; Blanc, O.; Chardès, C.; Lenne, P.-F. Direct laser manipulation reveals the mechanics of cell contacts in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 1416–1421. [Google Scholar] [CrossRef]
- Fabry, B.; Maksym, G.N.; Butler, J.P.; Glogauer, M.; Navajas, D.; Fredberg, J.J. Scaling the microrheology of living cells. Phys. Rev. Lett. 2001, 87, 148102. [Google Scholar] [CrossRef]
- Valberg, P.A.; Feldman, H.A. Magnetic particle motions within living cells. Measurement of cytoplasmic viscosity and motile activity. Biophys. J. 1987, 52, 551–561. [Google Scholar] [CrossRef]
- Bausch, A.R.; Möller, W.; Sackmann, E. Measurement of Local Viscoelasticity and Forces in Living Cells by Magnetic Tweezers. Biophys. J. 1999, 76, 573–579. [Google Scholar] [CrossRef]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Crecea, V.; Ahmad, A.; Boppart, S.A. Magnetomotive optical coherence elastography for microrheology of biological tissues. JBO 2013, 18, 121504. [Google Scholar] [CrossRef][Green Version]
- Liang, X.; Michael, M.; Gomez, G.A. Measurement of Mechanical Tension at Cell-cell Junctions Using Two-photon Laser Ablation. Bio Protoc. 2016, 6, e2068. [Google Scholar] [CrossRef]
- Ma, X.; Lynch, H.E.; Scully, P.C.; Hutson, M.S. Probing embryonic tissue mechanics with laser hole drilling. Phys. Biol. 2009, 6, 36004. [Google Scholar] [CrossRef]
- Colombelli, J.; Solon, J. Force communication in multicellular tissues addressed by laser nanosurgery. Cell Tissue Res. 2013, 352, 133–147. [Google Scholar] [CrossRef]
- Beloussov, L.V.; Dorfman, J.G.; Cherdantzev, V.G. Mechanical stresses and morphological patterns in amphibian embryos. Development 1975, 34, 559–574. [Google Scholar] [CrossRef]
- Nobis, U.; Pries, A.R.; Cokelet, G.R.; Gaehtgens, P. Radial distribution of white cells during blood flow in small tubes. Microvasc. Res. 1985, 29, 295–304. [Google Scholar] [CrossRef]
- Dewey, C.F.; Bussolari, S.R.; Gimbrone, M.A.; Davies, P.F. The Dynamic Response of Vascular Endothelial Cells to Fluid Shear Stress. J. Biomech. Eng. 1981, 103, 177–185. [Google Scholar] [CrossRef]
- Bacci, C.; Wong, V.; Barahona, V.; Merna, N. Cardiac and lung endothelial cells in response to fluid shear stress on physiological matrix stiffness and composition. Microcirculation 2021, 28, e12659. [Google Scholar] [CrossRef]
- Dong, C.; Cao, J.; Struble, E.J.; Lipowsky, H.H. Mechanics of Leukocyte Deformation and Adhesion to Endothelium in Shear Flow. Ann. Biomed. Eng. 1999, 27, 298–312. [Google Scholar] [CrossRef]
- Du, V.; Luciani, N.; Richard, S.; Mary, G.; Gay, C.; Mazuel, F.; Reffay, M.; Menasché, P.; Agbulut, O.; Wilhelm, C. A 3D magnetic tissue stretcher for remote mechanical control of embryonic stem cell differentiation. Nat. Commun. 2017, 8, 400. [Google Scholar] [CrossRef]
- Gorfien, S.F.; Winston, F.K.; Thibault, L.E.; Macarak, E.J. Effects of biaxial deformation on pulmonary artery endothelial cells. J. Cell. Physiol. 1989, 139, 492–500. [Google Scholar] [CrossRef]
- Kulik, T.J.; Alvarado, S.P. Effect of stretch on growth and collagen synthesis in cultured rat and lamb pulmonary arterial smooth muscle cells. J. Cell. Physiol. 1993, 157, 615–624. [Google Scholar] [CrossRef]
- Kim, B.-S.; Nikolovski, J.; Bonadio, J.; Mooney, D.J. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat. Biotechnol. 1999, 17, 979–983. [Google Scholar] [CrossRef]
- Harris, A.K.; Wild, P.; Stopak, D. Silicone rubber substrata: A new wrinkle in the study of cell locomotion. Science 1980, 208, 177–179. [Google Scholar] [CrossRef]
- Hur, S.S.; Jeong, J.H.; Ban, M.J.; Park, J.H.; Yoon, J.K.; Hwang, Y. Traction Force Microscopy for Understanding Cellular Mechanotransduction. BMB Rep. 2020, 53, 74–81. [Google Scholar] [CrossRef]
- Burton, K.; Taylor, D.L. Traction forces of cytokinesis measured with optically modified elastic substrata. Nature 1997, 385, 450–454. [Google Scholar] [CrossRef]
- Burton, K.; Park, J.H.; Taylor, D.L. Keratocytes generate traction forces in two phases. Mol. Biol. Cell 1999, 10, 3745–3769. [Google Scholar] [CrossRef]
- Lee, J.; Leonard, M.; Oliver, T.; Ishihara, A.; Jacobson, K. Traction forces generated by locomoting keratocytes. J. Cell Biol. 1994, 127, 1957–1964. [Google Scholar] [CrossRef]
- Dembo, M.; Wang, Y.-L. Stresses at the Cell-to-Substrate Interface during Locomotion of Fibroblasts. Biophys. J. 1999, 76, 2307–2316. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Schwarz, U.S.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; et al. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef]
- Tan, J.L.; Tien, J.; Pirone, D.M.; Gray, D.S.; Bhadriraju, K.; Chen, C.S. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA 2003, 100, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.T.; Sniadecki, N.J.; Chen, C.S. Geometric Considerations of Micro- to Nanoscale Elastomeric Post Arrays to Study Cellular Traction Forces. Adv. Mater. 2007, 19, 3119–3123. [Google Scholar] [CrossRef]
- Du Roure, O.; Saez, A.; Buguin, A.; Austin, R.H.; Chavrier, P.; Siberzan, P.; Ladoux, B. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA 2005, 102, 2390–2395. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wen, X.; Tan, X.H.M.; Chiou, P.-Y. Plasmonic micropillars for precision cell force measurement across a large field-of-view. Appl. Phys. Lett. 2018, 112, 33701. [Google Scholar] [CrossRef]
- Galbraith, C.G.; Sheetz, M.P. A micromachined device provides a new bend on fibroblast traction forces. Proc. Natl. Acad. Sci. USA 1997, 94, 9114–9118. [Google Scholar] [CrossRef] [PubMed]
- German, G.K.; Engl, W.C.; Pashkovski, E.; Banerjee, S.; Xu, Y.; Mertz, A.F.; Hyland, C.; Dufresne, E.R. Heterogeneous Drying Stresses in Stratum Corneum. Biophys. J. 2012, 102, 2424–2432. [Google Scholar] [CrossRef]
- Gjorevski, N.; Nelson, C.M. Mapping of Mechanical Strains and Stresses around Quiescent Engineered Three-Dimensional Epithelial Tissues. Biophys. J. 2012, 103, 152–162. [Google Scholar] [CrossRef]
- Cho, Y.; Park, E.Y.; Ko, E.; Park, J.-S.; Shin, J.H. Recent advances in biological uses of traction force microscopy. Int. J. Precis. Eng. Manuf. 2016, 17, 1401–1412. [Google Scholar] [CrossRef]
- Hur, S.S.; del Álamo, J.C.; Park, J.S.; Li, Y.-S.; Nguyen, H.A.; Teng, D.; Wang, K.-C.; Flores, L.; Alonso-Latorre, B.; Lasheras, J.C.; et al. Roles of cell confluency and fluid shear in 3-dimensional intracellular forces in endothelial cells. Proc. Natl. Acad. Sci. USA 2012, 109, 11110–11115. [Google Scholar] [CrossRef]
- Lam, R.H.W.; Sun, Y.; Chen, W.; Fu, J. Elastomeric microposts integrated into microfluidics for flow-mediated endothelial mechanotransduction analysis. Lab Chip 2012, 12, 1865–1873. [Google Scholar] [CrossRef]
- Gavara, N.; Roca-Cusachs, P.; Sunyer, R.; Farré, R.; Navajas, D. Mapping Cell-Matrix Stresses during Stretch Reveals Inelastic Reorganization of the Cytoskeleton. Biophys. J. 2008, 95, 464–471. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; Pal, S.; Maiti, S.; Davidson, L.A. Force production and mechanical accommodation during convergent extension. Development 2015, 142, 692–701. [Google Scholar] [CrossRef]
- Campàs, O.; Mammoto, T.; Hasso, S.; Sperling, R.A.; O’Connell, D.; Bischof, A.G.; Maas, R.; Weitz, D.A.; Mahadevan, L.; Ingber, D.E. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Methods 2014, 11, 183–189. [Google Scholar] [CrossRef]
- Miyawaki, A. Development of probes for cellular functions using fluorescent proteins and fluorescence resonance energy transfer. Annu. Rev. Biochem. 2011, 80, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Suchyna, T.M.; Sachs, F. A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ. FEBS J. 2008, 275, 3072–3087. [Google Scholar] [CrossRef]
- Grashoff, C.; Hoffman, B.D.; Brenner, M.D.; Zhou, R.; Parsons, M.; Yang, M.T.; McLean, M.A.; Sligar, S.G.; Chen, C.S.; Ha, T.; et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 2010, 466, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Polte, T.R.; Alsberg, E.; Mooney, D.J. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc. Natl. Acad. Sci. USA 2005, 102, 4300–4305. [Google Scholar] [CrossRef] [PubMed]
- Borghi, N.; Sorokina, M.; Shcherbakova, O.G.; Weis, W.I.; Pruitt, B.L.; Nelson, W.J.; Dunn, A.R. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell–cell contacts upon externally applied stretch. Proc. Natl. Acad. Sci. USA 2012, 109, 12568–12573. [Google Scholar] [CrossRef]
- Freikamp, A.; Cost, A.-L.; Grashoff, C. The Piconewton Force Awakens: Quantifying Mechanics in Cells. Trends Cell Biol. 2016, 26, 838–847. [Google Scholar] [CrossRef]
- Poh, Y.-C.; Shevtsov, S.P.; Chowdhury, F.; Wu, D.C.; Na, S.; Dundr, M.; Wang, N. Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nat. Commun. 2012, 3, 866. [Google Scholar] [CrossRef]
- Göhring, J.; Kellner, F.; Schrang, L.; Platzer, R.; Klotzsch, E.; Stockinger, H.; Huppa, J.B.; Schütz, G.J. Temporal analysis of T-cell receptor-imposed forces via quantitative single molecule FRET measurements. Nat. Commun. 2021, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ehrlicher, A.J.; Jensen, M.H.; Renz, M.; Moore, J.R.; Goldman, R.D.; Lippincott-Schwartz, J.; Mackintosh, F.C.; Weitz, D.A. Probing the Stochastic, Motor-Driven Properties of the Cytoplasm Using Force Spectrum Microscopy. Cell 2014, 158, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Granek, R.; Elbaum, M. Enhanced Diffusion in Active Intracellular Transport. Phys. Rev. Lett. 2000, 85, 5655–5658. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.W.C.; Hoffman, B.D.; Davies, A.; Crocker, J.C.; Lubensky, T.C. Microrheology, stress fluctuations, and active behavior of living cells. Phys. Rev. Lett. 2003, 91, 198101. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Wirtz, D.; Kuo, S.C. Mechanics of Living Cells Measured by Laser Tracking Microrheology. Biophys. J. 2000, 78, 1736–1747. [Google Scholar] [CrossRef]
- Weihs, D.; Mason, T.G.; Teitell, M.A. Bio-microrheology: A frontier in microrheology. Biophys. J. 2006, 91, 4296–4305. [Google Scholar] [CrossRef]
- Mandal, K.; Asnacios, A.; Goud, B.; Manneville, J.-B. Mapping intracellular mechanics on micropatterned substrates. Proc. Natl. Acad. Sci. USA 2016, 113, E7159–E7168. [Google Scholar] [CrossRef]
- Curtis, A.; Sokolikova-Csaderova, L.; Aitchison, G. Measuring Cell Forces by a Photoelastic Method. Biophys. J. 2007, 92, 2255–2261. [Google Scholar] [CrossRef]
- Sugimura, K.; Lenne, P.-F.; Graner, F. Measuring forces and stresses in situ in living tissues. Development 2016, 143, 186–196. [Google Scholar] [CrossRef]
- Prevedel, R.; Diz-Muñoz, A.; Ruocco, G.; Antonacci, G. Brillouin microscopy: An emerging tool for mechanobiology. Nat. Methods 2019, 16, 969–977. [Google Scholar] [CrossRef]
- Scarcelli, G.; Yun, S.H. Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nat. Photon 2008, 2, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.B.; Gordon, R. Epithelia as bubble rafts: A new method for analysis of cell shape and intercellular adhesion in embryonic and other epithelia. J. Theor. Biol. 1982, 97, 625–639. [Google Scholar] [CrossRef]
- Brodland, G.W.; Veldhuis, J.H.; Kim, S.; Perrone, M.; Mashburn, D.; Hutson, M.S. CellFIT: A cellular force-inference toolkit using curvilinear cell boundaries. PLoS ONE 2014, 9, e99116. [Google Scholar] [CrossRef]
- Chiou, K.K.; Hufnagel, L.; Shraiman, B.I. Mechanical Stress Inference for Two Dimensional Cell Arrays. PLOS Comput. Biol. 2012, 8, e1002512. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, K.; Ishihara, S. The mechanical anisotropy in a tissue promotes ordering in hexagonal cell packing. Development 2013, 140, 4091–4101. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Sugimura, K. Bayesian inference of force dynamics during morphogenesis. J. Theor. Biol. 2012, 313, 201–211. [Google Scholar] [CrossRef]
- Bachofen, H.; Schürch, S. Alveolar surface forces and lung architecture. Comp. Biochem. Physiol. Part A 2001, 129, 183–193. [Google Scholar] [CrossRef]
- Vogel, V. Unraveling the Mechanobiology of Extracellular Matrix. Annu. Rev. Physiol. 2018, 80, 353–387. [Google Scholar] [CrossRef] [PubMed]
- Uto, K.; Tsui, J.H.; DeForest, C.A.; Kim, D.-H. Dynamically tunable cell culture platforms for tissue engineering and mechanobiology. Prog. Polym. Sci. 2017, 65, 53–82. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, T.; Mammoto, A.; Ingber, D.E. Mechanobiology and Developmental Control. Annu. Rev. Cell Dev. Biol. 2013, 29, 27–61. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.R.; Cho, S.; Discher, D.E. Stem Cell Differentiation is Regulated by Extracellular Matrix Mechanics. Physiology 2018, 33, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Uroz, M.; Bays, J.L.; Chen, C.S. Harnessing Mechanobiology for Tissue Engineering. Dev. Cell 2021, 56, 180–191. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolb, P.; Schundner, A.; Frick, M.; Gottschalk, K.-E. In Vitro Measurements of Cellular Forces and their Importance in the Lung—From the Sub- to the Multicellular Scale. Life 2021, 11, 691. https://doi.org/10.3390/life11070691
Kolb P, Schundner A, Frick M, Gottschalk K-E. In Vitro Measurements of Cellular Forces and their Importance in the Lung—From the Sub- to the Multicellular Scale. Life. 2021; 11(7):691. https://doi.org/10.3390/life11070691
Chicago/Turabian StyleKolb, Peter, Annika Schundner, Manfred Frick, and Kay-E. Gottschalk. 2021. "In Vitro Measurements of Cellular Forces and their Importance in the Lung—From the Sub- to the Multicellular Scale" Life 11, no. 7: 691. https://doi.org/10.3390/life11070691
APA StyleKolb, P., Schundner, A., Frick, M., & Gottschalk, K.-E. (2021). In Vitro Measurements of Cellular Forces and their Importance in the Lung—From the Sub- to the Multicellular Scale. Life, 11(7), 691. https://doi.org/10.3390/life11070691





