Induction of Glutathione Synthesis Provides Cardioprotection Regulating NO, AMPK and PPARa Signaling in Ischemic Rat Hearts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Isolated Hearts by Langendorff Preparation
2.3. Oxygen Consumption by Myocardium of Isolated Rat Heart
2.4. Glutathione Assay
2.5. Measurement of H2O2
2.6. Measurement of Superoxide Radical (˙O2−) Generation Rate
2.7. Measurement of Diene Conjugates
2.8. Measurement of NOS Activity
2.9. Measurement of Nitrite Content
2.10. Measurement of Nitrate Content
2.11. Measurement of H2S
2.12. Measurementof CSE + CBS Activity
2.13. Western Blotting
2.14. Data Analysis
3. Results
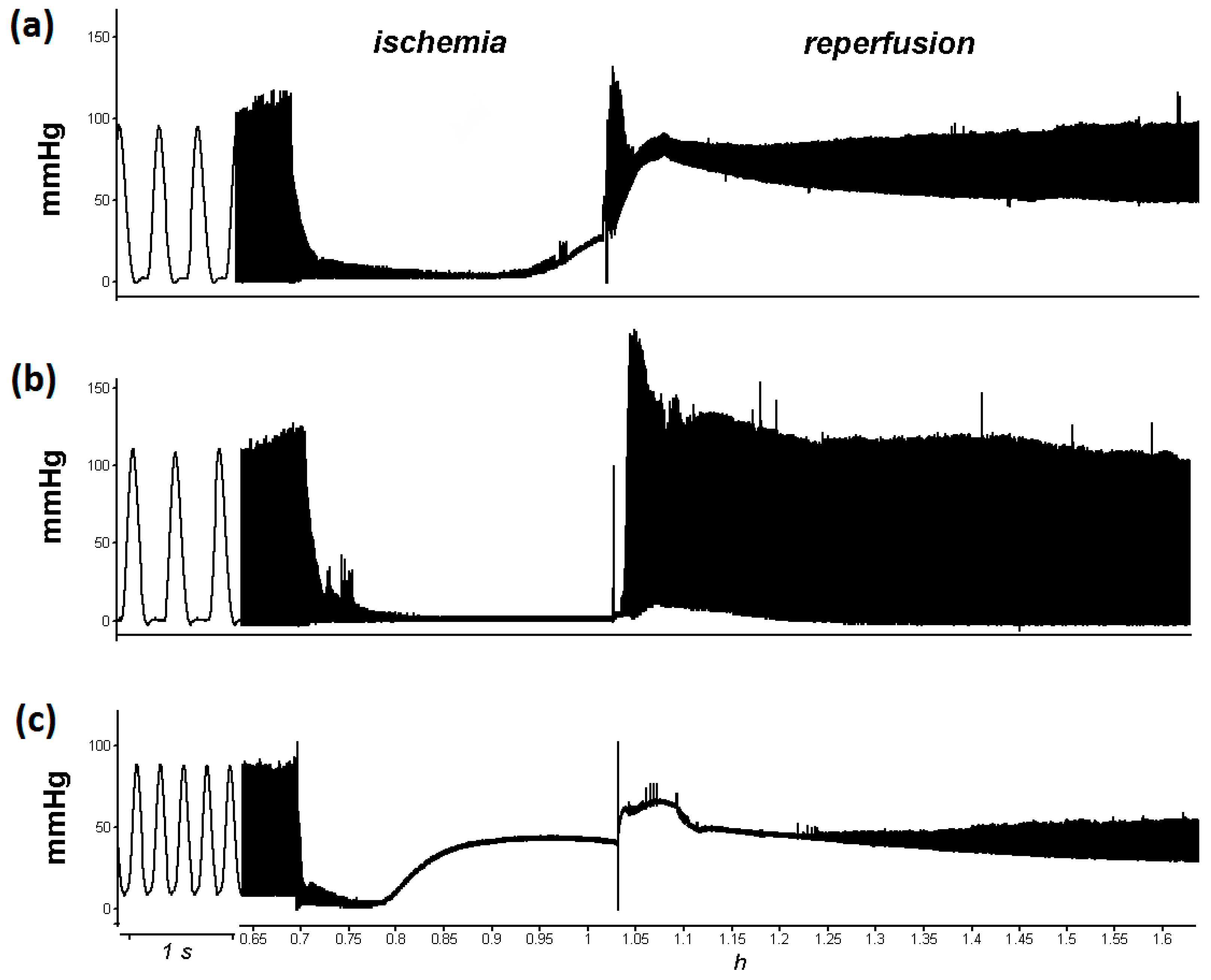
3.1. L-cysteine Improved Relaxation of Heart after Ischemia
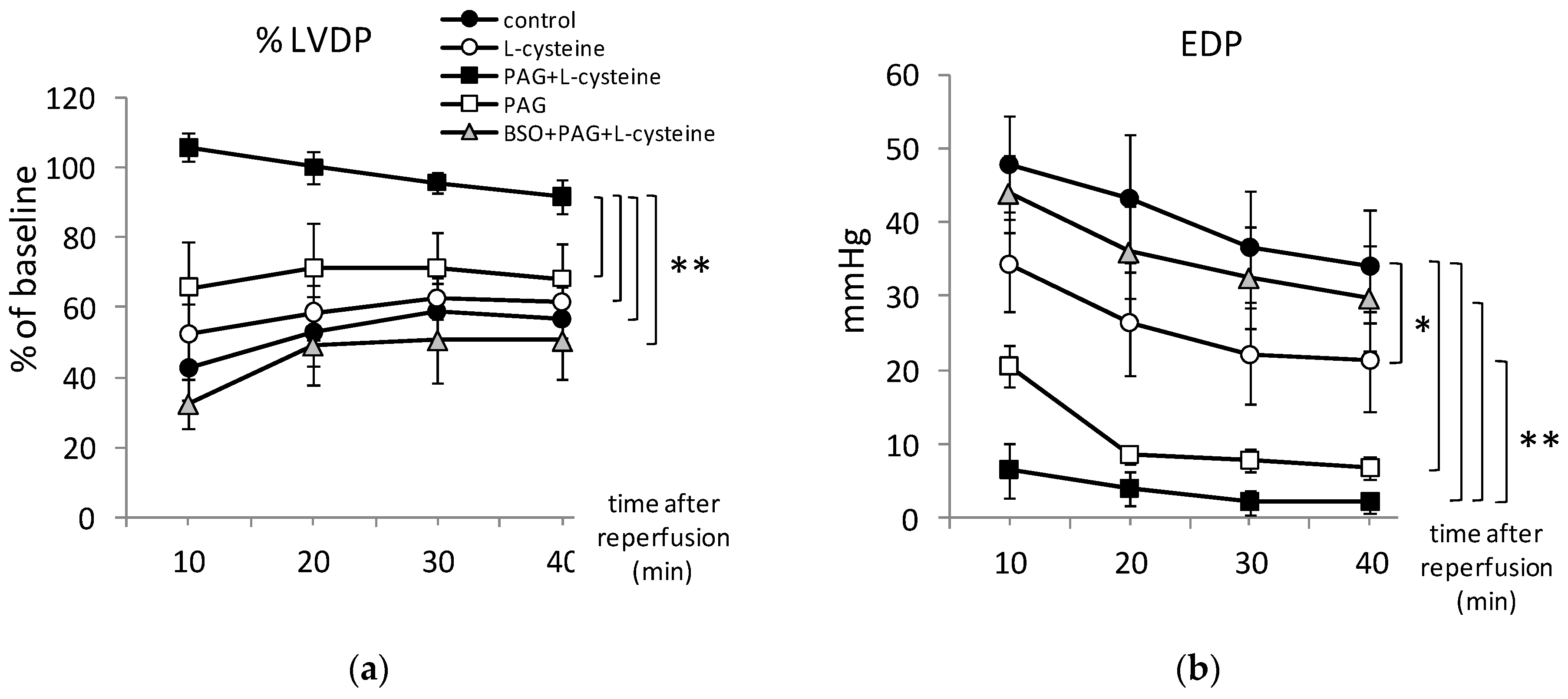
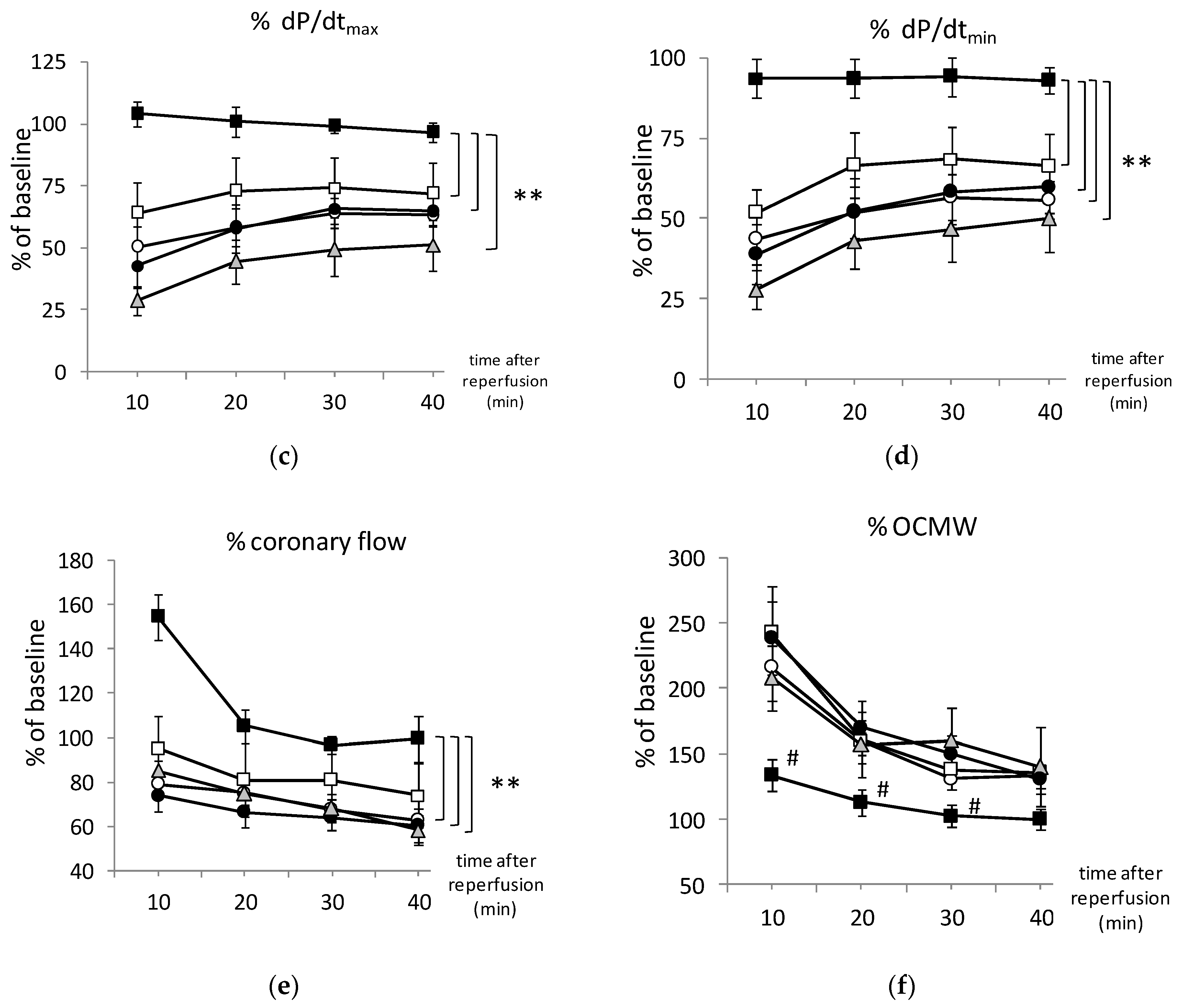
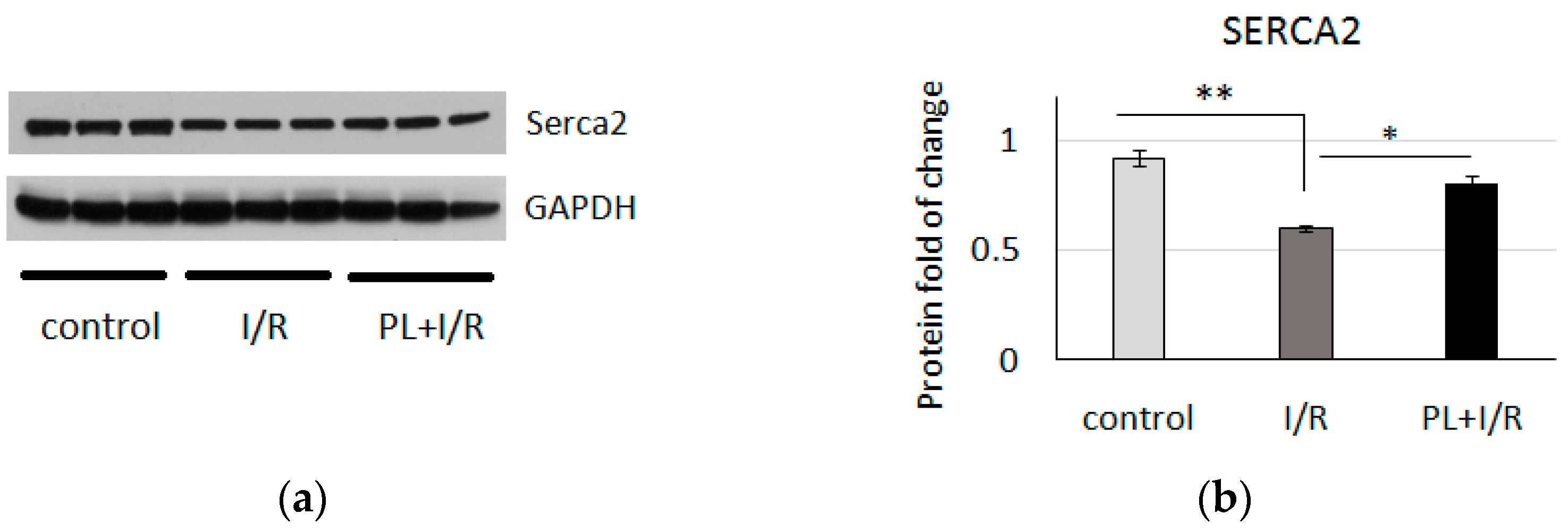
3.2. PAG + L-Cysteine Pretreatment Improved Heart Function Recovery and Preserved SERCA2 Level after Ischemia
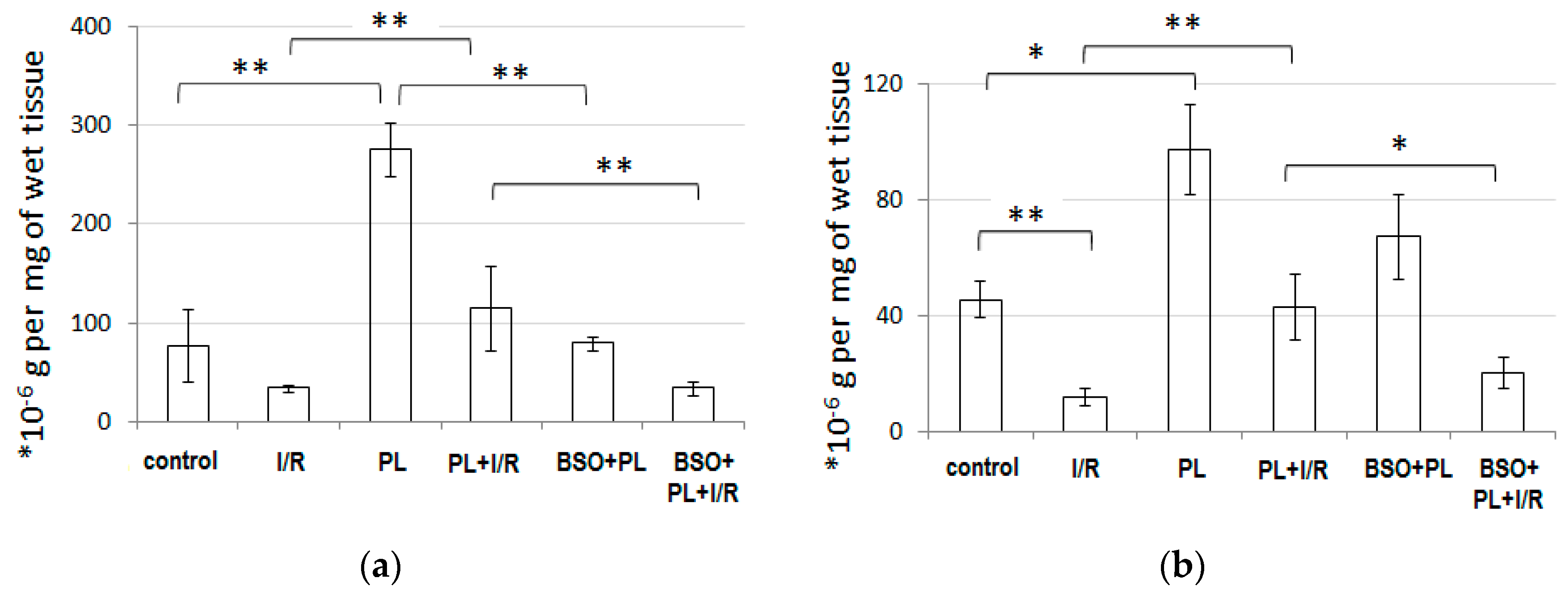
3.3. PAG + L-Cysteine Pretreatment Induced Glutathione Synthesis
3.4. PAG + L-Cysteine Pretreatment Prevented I/R-Induced Oxidative and Nitrosative Stress
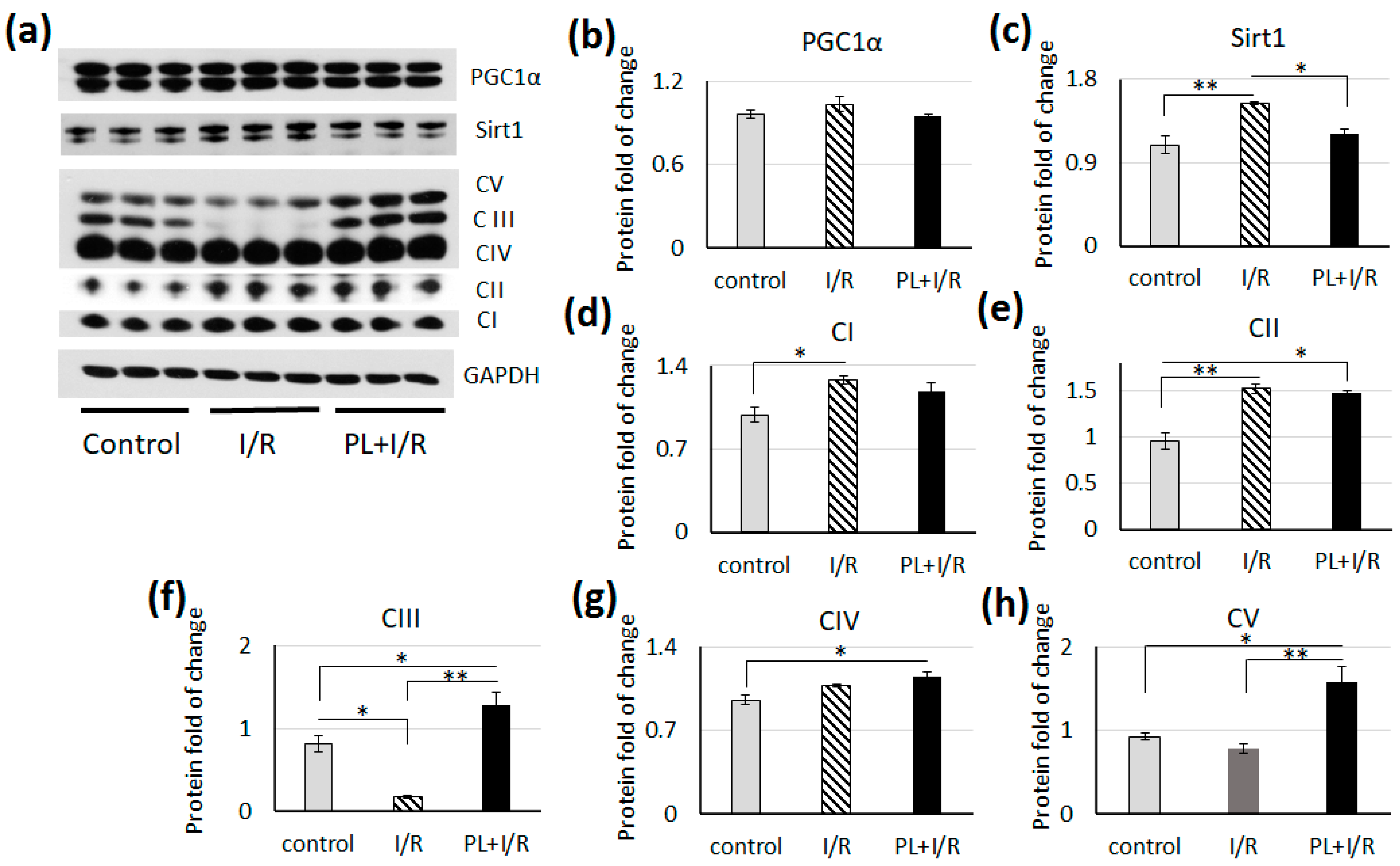
3.5. PAG + L-Cysteine Pretreatment Inhibited Fatty Acids β-oxidation in Ischemized Heart
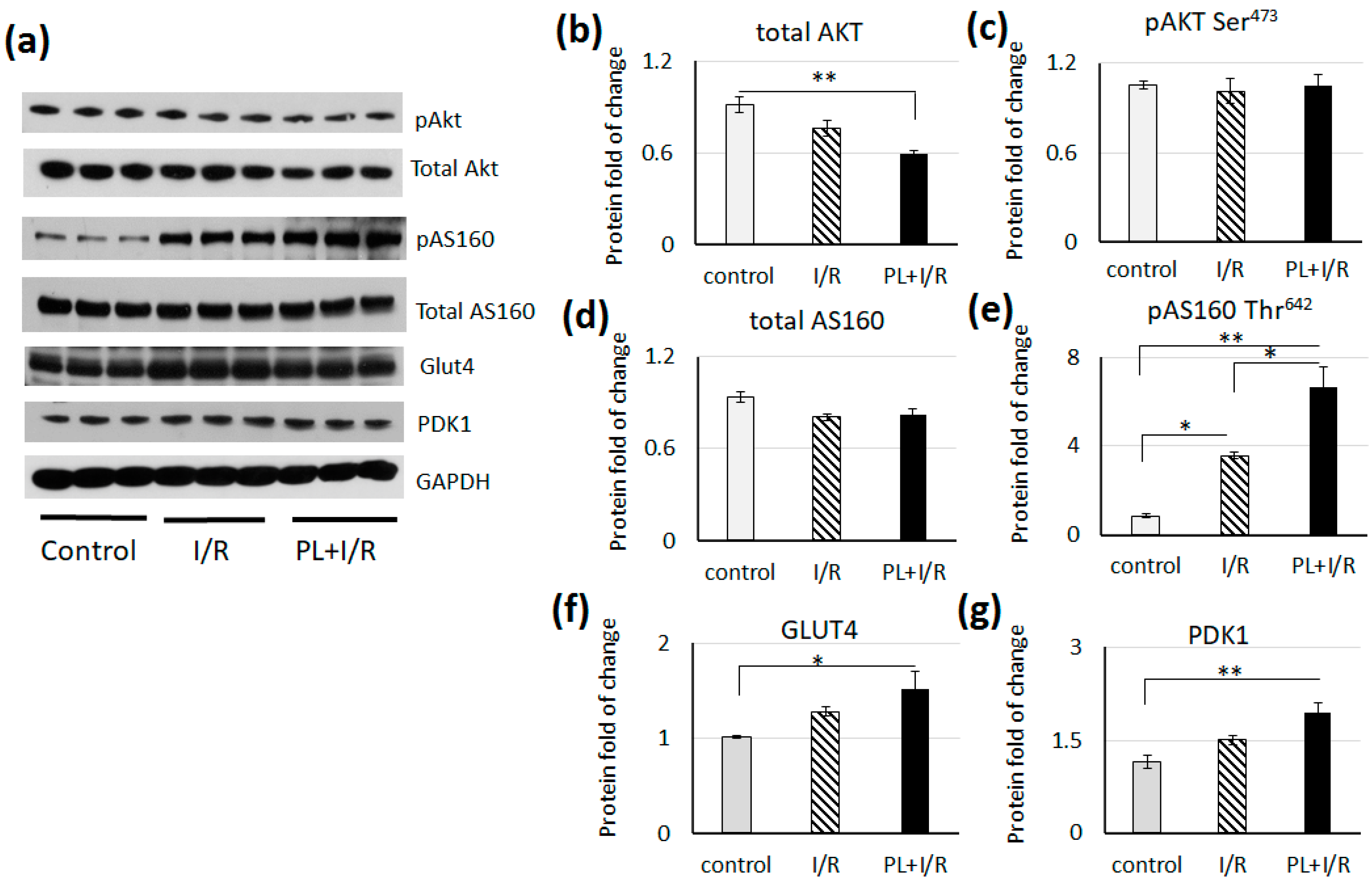
3.6. PAG + L-cysteinepretreatment Stimulated Glucose Consumption and Anaerobic Glycolysis in Ischemized Heart
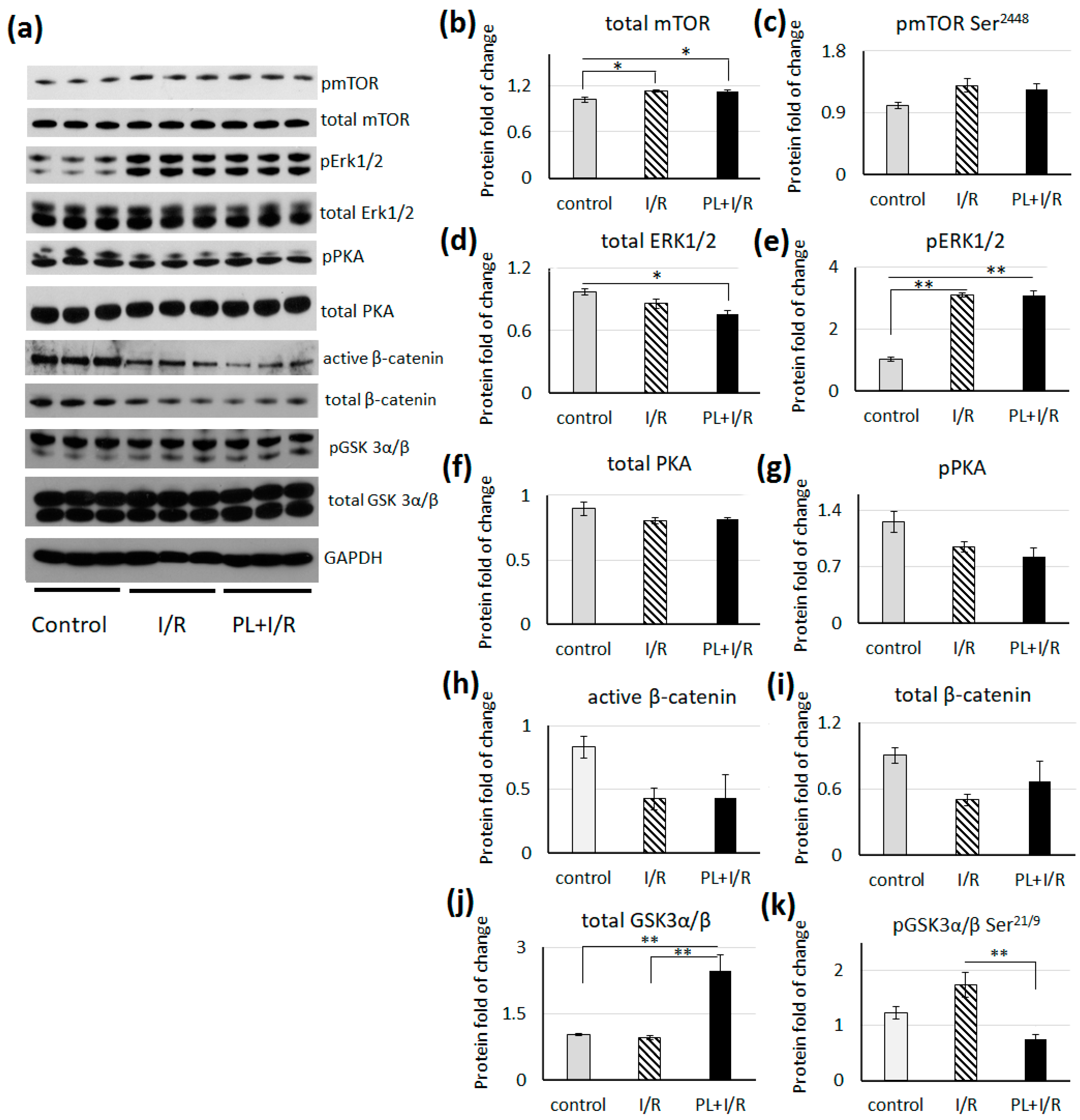
3.7. mTOR, MAPK/Erk1/2 and Canonical Wnt Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK Activators: Mechanisms of Action and Physiological Activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Wang, H.; Wu, X.; He, L.; Zhou, Q.; Wang, F.; Chen, S.; Huang, L.; Chen, J.; Wang, H.; et al. ROS-Mediated Inactivation of the PI3K/AKT Pathway Is Involved in the Antigastric Cancer Effects of Thioredoxin Reductase-1 Inhibitor Chaetocin. Cell Death Dis. 2019, 10, 809. [Google Scholar] [CrossRef] [Green Version]
- Caliceti, C.; Nigro, P.; Rizzo, P.; Ferrari, R. ROS, Notch, and Wnt Signaling Pathways: Crosstalk between Three Major Regulators of Cardiovascular Biology. BioMed Res. Int. 2014, 2014, 318714. [Google Scholar] [CrossRef] [Green Version]
- Balatskyi, V.V.; Palchevska, O.L.; Bortnichuk, L.; Gan, A.-M.; Myronova, A.; Macewicz, L.L.; Navrulin, V.O.; Tumanovska, L.V.; Olichwier, A.; Dobrzyn, P.; et al. β-Catenin Regulates Cardiac Energy Metabolism in Sedentary and Trained Mice. Life 2020, 10, 357. [Google Scholar] [CrossRef]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial Ischemia Reperfusion Injury. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar] [CrossRef]
- Halestrap, A.P. A Pore Way to Die: The Role of Mitochondria in Reperfusion Injury and Cardioprotection. Biochem. Soc. Trans. 2010, 38, 841–860. [Google Scholar] [CrossRef] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Förstermann, U. Uncoupling of Endothelial NO Synthase in Atherosclerosis and Vascular Disease. Curr. Opin. Pharmacol. 2013, 13, 161–187. [Google Scholar] [CrossRef]
- Mieyal, J.J.; Gallogly, M.M.; Qanungo, S.; Sabens, E.A.; Shelton, M.D. Molecular Mechanisms and Clinical Implications of Reversible Protein S-Glutathionylation. Antioxid. Redox Signal. 2008, 10, 1941–1988. [Google Scholar] [CrossRef]
- Aracena-Parks, P.; Goonasekera, S.A.; Gilman, C.P.; Dirksen, R.T.; Hidalgo, C.; Hamilton, S.L. Identification of Cysteines Involved in S-Nitrosylation, S-Glutathionylation, and Oxidation to Disulfides in Ryanodine Receptor Type 1. J. Biol. Chem. 2006, 281, 40354–40368. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-A.; Wang, T.-Y.; Varadharaj, S.; Reyes, L.A.; Hemann, C.; Talukder, M.A.H.; Chen, Y.-R.; Druhan, L.J.; Zweier, J.L. S-Glutathionylation Uncouples ENOS and Regulates Its Cellular and Vascular Function. Nature 2010, 468, 1115–1118. [Google Scholar] [CrossRef] [Green Version]
- Circu, M.L.; Aw, T.Y. Glutathione and Modulation of Cell Apoptosis. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 1767–1777. [Google Scholar] [CrossRef] [Green Version]
- Adamy, C.; Mulder, P.; Khouzami, L.; Andrieu-abadie, N.; Defer, N.; Candiani, G.; Pavoine, C.; Caramelle, P.; Souktani, R.; Le Corvoisier, P.; et al. Neutral Sphingomyelinase Inhibition Participates to the Benefits of N-Acetylcysteine Treatment in Post-Myocardial Infarction Failing Heart Rats. J. Mol. Cell. Cardiol. 2007, 43, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating Oxidative Stress in Heart Failure: Past, Present and Future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Muda, P.; Kampus, P.; Zilmer, M.; Zilmer, K.; Kairane, C.; Ristimäe, T.; Fischer, K.; Teesalu, R. Homocysteine and Red Blood Cell Glutathione as Indices for Middle-Aged Untreated Essential Hypertension Patients. J. Hypertens. 2003, 21, 2329–2333. [Google Scholar] [CrossRef]
- Anderson, M.F.; Sims, N.R. The Effects of Focal Ischemia and Reperfusion on the Glutathione Content of Mitochondria from Rat Brain Subregions. J. Neurochem. 2002, 81, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, T.; Tsutsui, H.; Matsusaka, H.; Murakami, K.; Hayashidani, S.; Ikeuchi, M.; Wen, J.; Kubota, T.; Utsumi, H.; Takeshita, A. Overexpression of Glutathione Peroxidase Prevents Left Ventricular Remodeling and Failure After Myocardial Infarction in Mice. Circulation 2004, 109, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Chuah, S.C.; Moore, P.K.; Zhu, Y.Z. S-Allylcysteine Mediates Cardioprotection in an Acute Myocardial Infarction Rat Model via a Hydrogen Sulfide-Mediated Pathway. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2693–H2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Cui, J.; Xia, W.; Li, Y.; Qian, L.B.; Ye, Z.G.; Wang, H.P.; Xia, Q. Effect of S-Allyl-L-Cysteine on Isolate Heart Subject to Ischemia/Reperfusion. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2011, 27, 13–17. [Google Scholar]
- Beltran, C.; Pardo, R.; Bou-Teen, D.; Ruiz-Meana, M.; Villena, J.A.; Ferreira-González, I.; Barba, I. Enhancing Glycolysis Protects against Ischemia-Reperfusion Injury by Reducing ROS Production. Metabolites 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Elsey, D.J.; Fowkes, R.C.; Baxter, G.F. L-Cysteine Stimulates Hydrogen Sulfide Synthesis in Myocardium Associated With Attenuation of Ischemia-Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, M.M.; Snyder, S.H. Hydrogen Sulfide as a Gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.; Zhao, M.; Jiang, H.; Tan, G.; Pan, S.; Sun, X. Role of Hydrogen Sulfide in Hepatic Ischemia-Reperfusion-Induced Injury in Rats. Liver Transplant. 2009, 15, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, Y.; Nakayama, J.; Watanabe, Y. Sex Difference in Susceptibility to Acetaminophen Hepatotoxicity Is Reversed by Buthionine Sulfoximine. Toxicology 2011, 287, 54–60. [Google Scholar] [CrossRef]
- Neely, J.R.; Liebermeister, H.; Battersby, E.; Morgan, H. Effect of Pressure Development on Oxygen Consumption by Isolated Rat Heart. Am. J. Physiol. Leg. Content 1967, 212, 804–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for Quantitative Determination of Glutathione and Glutathione Disulfide Levels Using Enzymatic Recycling Method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Huwiler, M.; Kohler, H. Pseudo-Catalytic Degradation of Hydrogen Peroxide in the Lactoperoxidase/H2O2/Iodide System. Eur. J. Biochem. 1984, 141, 69–74. [Google Scholar] [CrossRef]
- Kuthan, H.; Ullrich, V.; Estabrook, R.W. A Quantitative Test for Superoxide Radicals Produced in Biological Systems. Biochem. J. 1982, 203, 551–558. [Google Scholar] [CrossRef] [Green Version]
- Gavrilov, V.B.; Gavrilova, A.R.; Khmara, N.F. Measurement of Diene Conjugates in Blood Plasma Using the UV Absorption of Heptane and Isopropanol Extracts. Lab Delo 1988, 2, 60–64. [Google Scholar]
- Chin, S.Y.; Pandey, K.N.; Shi, S.-J.; Kobori, H.; Moreno, C.; Navar, L.G. Increased Activity and Expression of Ca2+ -Dependent NOS in Renal Cortex of ANG II-Infused Hypertensive Rats. Am. J. Physiol. Ren. Physiol. 1999, 277, F797–F804. [Google Scholar] [CrossRef]
- Salter, M.; Knowles, R.G.; Moncada, S. Widespread Tissue Distribution, Species Distribution and Changes in Activity of Ca2+-Dependent and Ca2+-Independent Nitric Oxide Synthases. FEBS Lett. 1991, 291, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N]Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Gorren, A.C.F.; Schmidt, K.; Werner, E.R.; Hansert, B.; Bohle, D.S.; Mayer, B. Metabolic Fate of Peroxynitrite in Aqueous Solution. J. Biol. Chem. 1997, 272, 3465–3470. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.-H.; Zhang, N.; Li, L.-F.; Zhang, Q.-Z.; Xie, L.-J.; Jiang, H.; Li, L.-P.; Hao, N.; Zhang, J.-X. Hydrogen Sulfide Reduces Regional Myocardial Ischemia Injury through Protection of Mitochondrial Function. Mol. Med. Rep. 2014, 10, 1907–1914. [Google Scholar] [CrossRef] [Green Version]
- Mel’nyk, A.V.; Pentiuk, O.O. Activity of Hydrogen Sulfide Production Enzymes in Kidneys of Rats. Ukrains’ Kyi Biokhimichnyi Zhurnal 1999, 81, 12–22. [Google Scholar]
- Bednarski, T.; Olichwier, A.; Opasinska, A.; Pyrkowska, A.; Gan, A.-M.; Ntambi, J.M.; Dobrzyn, P. Stearoyl-CoA Desaturase 1 Deficiency Reduces Lipid Accumulation in the Heart by Activating Lipolysis Independently of Peroxisome Proliferator-Activated Receptor α. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2016, 1861, 2029–2037. [Google Scholar] [CrossRef]
- Kramer, H.F.; Witczak, C.A.; Fujii, N.; Jessen, N.; Taylor, E.B.; Arnolds, D.E.; Sakamoto, K.; Hirshman, M.F.; Goodyear, L.J. Distinct Signals Regulate AS160 Phosphorylation in Response to Insulin, AICAR, and Contraction in Mouse Skeletal Muscle. Diabetes 2006, 55, 2067–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Szabo, C. Both the H2S Biosynthesis Inhibitor Aminooxyacetic Acid and the Mitochondrially Targeted H2S Donor AP39 Exert Protective Effects in a Mouse Model of Burn Injury. Pharmacol. Res. 2016, 113. [Google Scholar] [CrossRef]
- Nadtochiy, S.M.; Wang, Y.T.; Nehrke, K.; Munger, J.; Brookes, P.S. Cardioprotection by Nicotinamide Mononucleotide (NMN): Involvement of Glycolysis and Acidic PH. J. Mol. Cell. Cardiol. 2018, 121, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.L.; Lopaschuk, G.D. Fuels, Aerobic and Anaerobic Metabolism. In Heart Physiology, from Cell to Circulation, 4th ed.; Opie, L.L., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Zhou, Y.; Wang, S.; Li, Y.; Yu, S.; Zhao, Y. SIRT1/PGC-1α Signaling Promotes Mitochondrial Functional Recovery and Reduces Apoptosis after Intracerebral Hemorrhage in Rats. Front. Mol. Neurosci. 2018, 10, 443. [Google Scholar] [CrossRef]
- Klaus, A.; Zorman, S.; Berthier, A.; Polge, C.; Ramirez, S.; Michelland, S.; Sève, M.; Vertommen, D.; Rider, M.; Lentze, N.; et al. Glutathione S-Transferases Interact with AMP-Activated Protein Kinase: Evidence for S-Glutathionylation and Activation In Vitro. PLoS ONE 2013, 8, e62497. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, X.; Ren, D.; Tan, Y.; Chen, J.; Yang, L.; Chen, R.; Li, J.; Zhu, P. The Cardioprotective Effects of Carvedilol on Ischemia and Reperfusion Injury by AMPK Signaling Pathway. Biomed. Pharmacother. 2019, 117, 109106. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Peck, G.R.; Kettenbach, A.N.; Gerber, S.A.; Lienhard, G.E. Insulin-Stimulated GLUT4 Protein Translocation in Adipocytes Requires the Rab10 Guanine Nucleotide Exchange Factor Dennd4C. J. Biol. Chem. 2011, 286, 16541–16545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, Y.; Luckett, C.; Lu, S.; Li, J. Aspirin Protects Heart Against Ischemia-Reperfusion Injury via LKB1-Sestrn2-AMPK Signaling Cascade. Circulation 2017, 136, A15274. [Google Scholar]

| Index | Control (n = 8) | I/R (n = 10) | PAG + L-cysteine (n = 5) | PAG + L-cysteine + I/R (n = 7) |
|---|---|---|---|---|
| O2−, nmol mg−1 min−1 | 2.63 ± 0.08 | 8.72 ± 0.57 *** | 1.61 ± 0.05 *** | 2.37 ± 0.11 ### |
| H2O2, pmol mg−1 | 0.79 ± 0.04 | 2.27 ± 0.26 *** | 0.69 ± 0.02 | 1.15 ± 0.11 ## |
| Diene conjugates, ng mg−1 | 3.59 ± 0.25 | 8.84 ± 0.48 *** | 0.92 ± 0.12 *** | 5.24 ± 0.63 ### |
| cNOS activity, pmol mg−1 min−1 | 7.51 ± 0.19 | 1.36 ± 0.09 *** | 8.57 ± 0.30 * | 2.38 ± 0.34 ## |
| iNOS activity, pmol mg−1 min−1 | 2.64 ± 0.15 | 7.07 ± 0.20 *** | 1.68 ± 0.04 *** | 3.86 ± 0.26 ### |
| NO2−, pmol mg−1 | 361.8 ± 17.9 | 131.5 ± 12.2 *** | 440.1 ± 5.2 ** | 343.4 ± 5.17 ### |
| NO3−, nmol mg−1 | 10.92 ± 0.21 | 22.27 ± 0.88 *** | 5.24 ± 0.13 *** | 9.95 ± 0.40 ### |
| H2S, pmol mg−1 | 17.56 ± 1.26 | 96.80 ± 7.90 *** | 14.56 ± 0.77 | 29.23 ± 0.86 ### |
| CSE + CBS activity, pmol of H2S mg−1 min−1 | 8.80 ± 0.22 | 30.15 ± 2.95 *** | 7.71 ± 0.11 ** | 16.48 ± 0.81 ## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goshovska, Y.V.; Fedichkina, R.A.; Balatskyi, V.V.; Piven, O.O.; Dobrzyn, P.; Sagach, V.F. Induction of Glutathione Synthesis Provides Cardioprotection Regulating NO, AMPK and PPARa Signaling in Ischemic Rat Hearts. Life 2021, 11, 631. https://doi.org/10.3390/life11070631
Goshovska YV, Fedichkina RA, Balatskyi VV, Piven OO, Dobrzyn P, Sagach VF. Induction of Glutathione Synthesis Provides Cardioprotection Regulating NO, AMPK and PPARa Signaling in Ischemic Rat Hearts. Life. 2021; 11(7):631. https://doi.org/10.3390/life11070631
Chicago/Turabian StyleGoshovska, Yulia V., Raisa A. Fedichkina, Volodymyr V. Balatskyi, Oksana O. Piven, Pawel Dobrzyn, and Vadym F. Sagach. 2021. "Induction of Glutathione Synthesis Provides Cardioprotection Regulating NO, AMPK and PPARa Signaling in Ischemic Rat Hearts" Life 11, no. 7: 631. https://doi.org/10.3390/life11070631
APA StyleGoshovska, Y. V., Fedichkina, R. A., Balatskyi, V. V., Piven, O. O., Dobrzyn, P., & Sagach, V. F. (2021). Induction of Glutathione Synthesis Provides Cardioprotection Regulating NO, AMPK and PPARa Signaling in Ischemic Rat Hearts. Life, 11(7), 631. https://doi.org/10.3390/life11070631






