Wireless Capsule Endoscopy for Diagnosis and Management of Post-Operative Recurrence of Crohn’s Disease
Abstract
:1. Introduction
2. POR and Risk Factors
3. Diagnosis of POR in CD
4. WCE and Other Tests to Diagnose EPOR
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roda, G.; Chien, N.S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin, B.L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Primers. 2020, 6, 22. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef] [PubMed]
- Benevento, G.; Avellini, C.; Terrosu, G.; Geraci, M.; Lodolo, I.; Sorrentino, D. Diagnosis and assessment of Crohn’s disease: The present and the future. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 757–766. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin, B.L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Yung, D.E.; Har-Noy, O.; Tham, Y.S.; Ben-Horin, S.; Eliakim, R.; Koulaouzidis, A.; Kopylov, U. Capsule Endoscopy, Magnetic Resonance Enterography, and Small Bowel Ultrasound for Evaluation of Postoperative Recurrence in Crohn’s Disease: Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2017, 24, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, D.; Fogel, S.; Bogaerde, J.V.D. Surgery for Crohn’s disease and anti-TNF agents: The changing scenario. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 689–700. [Google Scholar] [CrossRef]
- Sorrentino, D. State-of-the-art medical prevention of postoperative recurrence of Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D.; Lipton, D.S.; Fiest, K.M.; Negrón, M.E.; Dykeman, J.; Debruyn, J.; Jette, N.; Frolkis, T.; Rezaie, A.; Seow, C.H.; et al. Cumulative incidence of second intestinal resection in Crohn’s disease: A systematic review and meta-analysis of population-based studies. Am. J. Gastroenterol. 2014, 109, 1739–1748. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Geboes, K.; Vantrappen, G.; Kerremans, R.; Coenegrachts, J.L.; Coremans, G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut 1984, 25, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutgeerts, P.; Geboes, K.; Vantrappen, G.; Beyls, J.; Kerremans, R.; Hiele, M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990, 99, 956–963. [Google Scholar] [CrossRef]
- Bolwell, J.G.; Wild, D. Indications, Contraindications, and Considerations for Video Capsule Endoscopy. Gastrointest Endosc. Clin. N. Am. 2021, 31, 267–276. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.R.; Geboes, K.; Peeters, M.; Baert, F.; Penninckx, F.; Rutgeerts, P. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998, 114, 262–267. [Google Scholar] [CrossRef]
- Nguyen, V.; Kanth, R.; Gazo, J.; Sorrentino, D. Management of post-operative Crohn’s disease in 2017: Where do we go from here? Expert Rev. Gastroenterol. Hepatol. 2016, 10, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Connelly, T.M.; Messaris, E. Predictors of recurrence of Crohn’s disease after ileocolectomy: A review. World J. Gastroenterol. 2014, 20, 14393–14406. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Hida, N.; Nogami, K.; Iimuro, M.; Ohda, Y.; Yokoyama, Y.; Kamikozuru, K.; Tozawa, K.; Kawai, M.; Ogawa, T.; et al. Prospective postsurgical capsule endoscopy in patients with Crohn’s disease. World J. Gastrointest. Endosc. 2014, 6, 88–98. [Google Scholar] [CrossRef]
- Yamamoto, T. Factors affecting recurrence after surgery for Crohn’s disease. World J. Gastroenterol. 2005, 11, 3971–3979. [Google Scholar] [CrossRef]
- Ryan, W.R.; Allan, R.N.; Yamamoto, T.; Keighley, M.R. Crohn’s disease patients who quit smoking have a reduced risk of reoperation for recurrence. Am. J. Surg. 2004, 187, 219–225. [Google Scholar] [CrossRef]
- Buisson, A.; Chevaux, J.B.; Allen, P.B.; Bommelaer, G.; Peyrin-Biroulet, L. Review article: The natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol. Ther. 2012, 35, 625–633. [Google Scholar] [CrossRef]
- Sachar, D.B.; Wolfson, D.M.; Greenstein, A.J.; Goldberg, J.; Styczynski, R.; Janowitz, H.D. Risk factors for postoperative recurrence of Crohn’s disease. Gastroenterology 1983, 85, 917–921. [Google Scholar] [CrossRef]
- Raab, Y.; Bergström, R.; Ejerblad, S.; Graf, W.; Påhlman, L. Factors influencing recurrence in Crohn’s disease. An analysis of a consecutive series of 353 patients treated with primary surgery. Dis Colon Rectum. 1996, 39, 918–925. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Jiang, D.; Hoffman, S.N.; Guntaka, S.; Mays, J.L.; Wang, A.; Gomes, J.; Sorrentino, D. Impact of Diagnostic Delay and Associated Factors on Clinical Outcomes in a U.S. Inflammatory Bowel Disease Cohort. Inflamm. Bowel Dis. 2017, 23, 1825–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chongthammakun, V.; Fialho, A.; Fialho, A.; Lopez, R.; Shen, B. Correlation of the Rutgeerts score and recurrence of Crohn’s disease in patients with end ileostomy. Gastroenterol. Rep. (Oxf) 2017, 5, 271–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorn, S.D.; Abad, J.F.; Panagopoulos, G.; Korelitz, B.I. Clinical characteristics of familial versus sporadic Crohn’s disease using the Vienna Classification. Inflamm. Bowel Dis. 2004, 10, 201–206. [Google Scholar] [CrossRef]
- Bruining, D.H.; Oliva, S.; Fleisher, M.R.; Fischer, M.; Fletcher, J.G. Panenteric capsule endoscopy versus ileocolonoscopy plus magnetic resonance enterography in Crohn’s disease: A multicentre, prospective study. BMJ Open Gastroenterol. 2020, 7, e000365. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, D.; Nguyen, V.Q. Clinically Significant Small Bowel Crohn’s Disease Might Only be Detected by Capsule Endoscopy. Inflamm. Bowel Dis. 2018, 24, 1566–1574. [Google Scholar] [CrossRef]
- Hausmann, J.; Schmelz, R.; Walldorf, J.; Filmann, N.; Zeuzem, S.; Albert, J.G. Panintestinal capsule endoscopy in patients with postoperative Crohn’s disease: A pilot study. Scand. J. Gastroenterol. 2017, 52, 840–845. [Google Scholar] [CrossRef]
- Bourreille, A.; Jarry, M.; D’Halluin, P.N.; Ben-Soussan, E.; Maunoury, V.; Bulois, P.; Sacher-Huvelin, S.; Vahedy, K.; Lerebours, e.; Heresbach, D.; et al. Wireless capsule endoscopy versus ileocolonoscopy for the diagnosis of postoperative recurrence of Crohn’s disease: A prospective study. Gut 2006, 55, 978–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusaka, J.; Shiga, H.; Kuroha, M.; Kimura, T.; Kakuta, Y.; Endo, K.; Kinouchi, Y.; Shimosegawa, T. Residual Lesions on Capsule Endoscopy Is Associated with Postoperative Clinical Recurrence in Patients with Crohn’s Disease. Dig. Dis. Sci. 2018, 63, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Defranchis, R.; Seidman, E.; Leighton, J.A.; Legnani, P.; Lewis, B.S. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment. Pharmacol. Ther. 2007, 27, 146–154. [Google Scholar] [CrossRef]
- Han, Z.-M.; Qiao, W.-G.; Ai, X.-Y.; Li, A.-M.; Chen, Z.-Y.; Feng, X.-C.; Zhang, J.; Wan, T.-M.; Xu, Z.-M.; Bai, Y.; et al. Impact of capsule endoscopy on prevention of postoperative recurrence of Crohn’s disease. Gastrointest. Endosc. 2018, 87, 1489–1498. [Google Scholar] [CrossRef]
- Panes, J.; Bouhnik, Y.; Reinisch, W.; Stoker, J.; Taylor, S.; Baumgart, D.; Danese, S.; Halligan, S.; Marincek, B.; Matos, C.; et al. Imaging techniques for assessment of inflammatory bowel disease: Joint ECCO and ESGAR evidence-based consensus guidelines. J. Crohn’s Coliti. 2013, 7, 556–585. [Google Scholar] [CrossRef] [Green Version]
- Park, M.J.; Lim, J.S. Computed Tomography Enterography for Evaluation of Inflammatory Bowel Disease. Clin. Endosc. 2013, 46, 327–366. [Google Scholar] [CrossRef]
- Bruining, D.H.; Zimmermann, E.; M Loftus, E.V.J.; Sandborn, W.J.; Sauer, C.G.; Strong, S.A.; Society of Abdominal Radiology Crohn’s Disease-Focused Panel. Consensus Recommendations for Evaluation, Interpretation, and Utilization of Computed Tomography and Magnetic Resonance Enterography in Patients with Small Bowel Crohn’s Disease. Radiology 2018, 286, 776–799. [Google Scholar] [CrossRef] [Green Version]
- González-Suárez, B.; Rodriguez, S.; Ricart, E.; Ordas, I.; Rimola, J.; Díaz-González, Á.; Romero, C.; De Miguel, C.R.; Jáuregui, A.; Araujo, I.K.; et al. Comparison of Capsule Endoscopy and Magnetic Resonance Enterography for the Assessment of Small Bowel Lesions in Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 775–780. [Google Scholar] [CrossRef]
- Mitselos, I.V.; Christodoulou, D.K.; Katsanos, K.H.; Tsianos, E.V. Role of wireless capsule endoscopy in the follow-up of inflammatory bowel disease. World J. Gastrointest Endosc. 2015, 7, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Eliakim, R. The impact of panenteric capsule endoscopy on the management of Crohn’s disease. Ther. Adv. Gastroenterol. 2017, 10, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Spada, C.; Spera, G.; Riccioni, M.E.; Biancone, L.; Petruzziello, L.; Tringali, A.; Familiari, P.; Marchese, M.; Onder, G.; Mutignani, M.; et al. A Novel Diagnostic Tool for Detecting Functional Patency of the Small Bowel: The Given Patency Capsule. Endosc 2005, 37, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Chami, G.; Raza, M.; Bernstein, C.N. Usefulness and impact on management of positive and negative capsule endoscopy. Can. J. Gastroenterol. 2007, 21, 577–581. [Google Scholar] [CrossRef]
- Le Berre, C.; Trang-Poisson, C.; Bourreille, A. Small bowel capsule endoscopy and treat-to-target in Crohn’s disease: A systematic review. World J. Gastroenterol. 2019, 25, 4534–4554. [Google Scholar] [CrossRef] [PubMed]
- Enns, R.A.; Hookey, L.; Armstrong, D.; Bernstein, C.N.; Heitman, S.J.; Teshima, C.; Leontiadis, G.I.; Tse, F.; Sadowski, D. Clinical Practice Guidelines for the Use of Video Capsule Endoscopy. Gastroenterol 2017, 152, 497–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
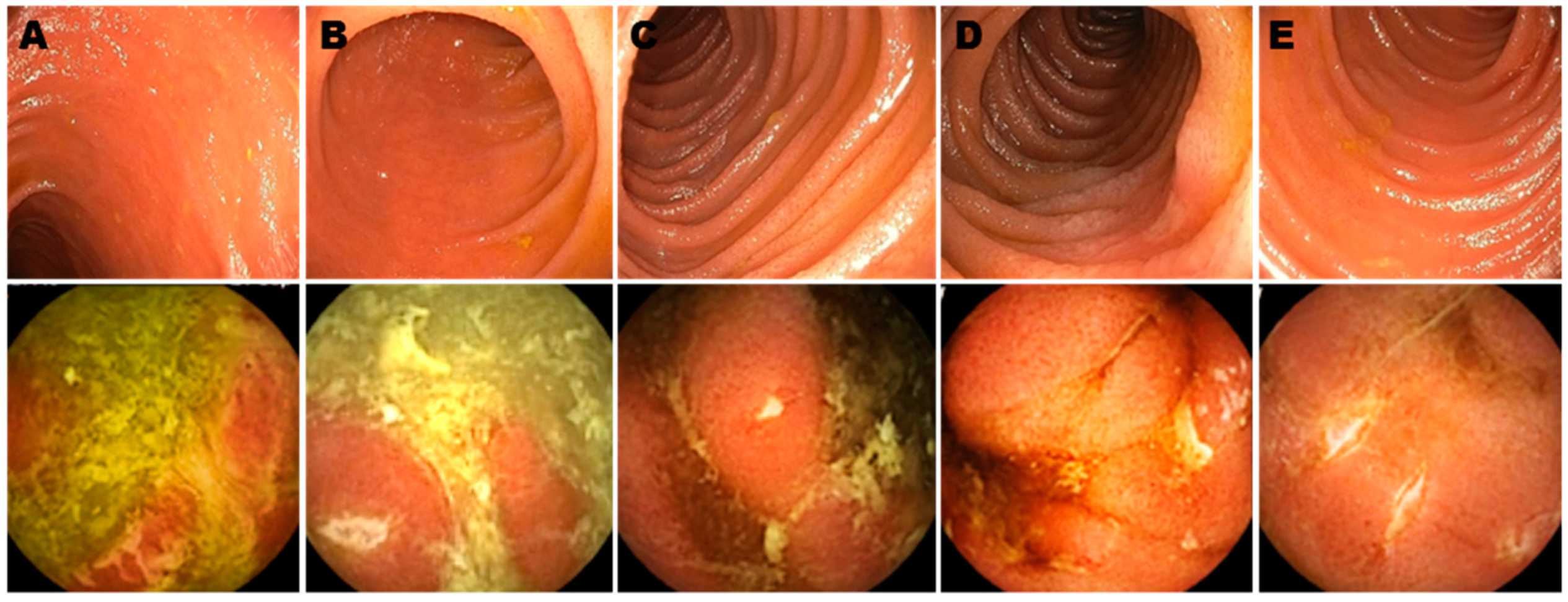
| Rutgeerts Score | Endoscopic Findings at IC | |
|---|---|---|
| Grade i0 | Endoscopic Post-operative Remission | Normal mucosa |
| Grade i1 | <5 Aphthous ulcers | |
| Grade i2 | Endoscopic Post-operative Recurrence (EPOR) | >5 Aphthous ulcers with normal intervening mucosa or large lesions confined to the anastomosis |
| Grade i3 | Diffusely inflamed mucosa with aphthous ileitis | |
| Grade i4 | Diffuse inflammation, large ulcers/nodules/narrowing |
| Study | Sample Size | Study Design | Comparisons | Results | Comments |
|---|---|---|---|---|---|
| Bruining et al. [24] | 99 | Multicenter prospective cohort | WCE * vs. MRE ^ | For proximal bowel inflammation, sensitivity of WCE and MRE were 97% and 71%. For inflammation in terminal ileum and colon, sensitivity of WCE was similar to MRE and/or IC | 3 adverse events were reported with WCE. Only patients with suspected strictures at MRE underwent patency capsule. |
| Sorrentino et al. [25] | 43 | Retrospective cohort | WCE vs. IC ± and/or MRE/CTE ∞ | WCE detected inflammation undetected by IC and imaging in 59% and 75% of patients, respectively. | WCE changed management in 52% of the cases resulting in clinical and biochemical improvement in 83% of them at follow up (up to 18 months) |
| Hausmann et al. [26] | 16 | Multicenter prospective cohort | WCE vs. IC | WCE detected inflammatory lesions early at 4–8 weeks after surgery. WCE detected 1 additional patient (out of 6) with inflammation compared to IC at 4–8 months after surgery. | WCE use changed management in 3 patients at 4–8 weeks and in 1 patient at 4–8 months. |
| Yung et al. [5] | 5 studies including 76 patients | Systematic review and meta-analysis | WCE vs. IC | Pooled sensitivity for WCE was 100%, pooled specificity was 69%. | The definition of recurrence varied in different studies. The included studies were cross sectional. |
| Bourreille et al. [27] | 32 | Prospective cohort | WCE vs. IC | Sensitivity and specificity for WCE for post-operative recurrence at neo-terminal ileum were 62–76% and 90–100%. For IC they were 90% and 100%. | In 2/3 of patients, WCE detected inflammatory lesions in the small bowel proximal to the reach of the colonoscope. |
| Kusaka et al. [28] | 25 | Prospective cohort | WCE only | 21/25 patients had EPOR β within 3 months after surgery. | The severity of inflammatory lesions in the distal small intestine was associated with CPOR ∑. |
| Han et al. [30] | 37 | Retrospective cohort | IC + WCE vs. IC only | WCE detected EPOR undetected by IC in 11 patients. Total CPOR was 2.7% (IC+WCE group) vs. 21.7% (IC only) at 1 year follow up. | The authors concluded that if recurrence was detected by WCE, starting pharmacologic therapy would result in lower risk of CPOR. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mir, A.; Nguyen, V.Q.; Soliman, Y.; Sorrentino, D. Wireless Capsule Endoscopy for Diagnosis and Management of Post-Operative Recurrence of Crohn’s Disease. Life 2021, 11, 602. https://doi.org/10.3390/life11070602
Mir A, Nguyen VQ, Soliman Y, Sorrentino D. Wireless Capsule Endoscopy for Diagnosis and Management of Post-Operative Recurrence of Crohn’s Disease. Life. 2021; 11(7):602. https://doi.org/10.3390/life11070602
Chicago/Turabian StyleMir, Adil, Vu Q. Nguyen, Youssef Soliman, and Dario Sorrentino. 2021. "Wireless Capsule Endoscopy for Diagnosis and Management of Post-Operative Recurrence of Crohn’s Disease" Life 11, no. 7: 602. https://doi.org/10.3390/life11070602
APA StyleMir, A., Nguyen, V. Q., Soliman, Y., & Sorrentino, D. (2021). Wireless Capsule Endoscopy for Diagnosis and Management of Post-Operative Recurrence of Crohn’s Disease. Life, 11(7), 602. https://doi.org/10.3390/life11070602






